Introduction
Gastric cancer is one of the most common types of
gastrointestinal cancer in China, with notably high incidence
(3.3%) and mortality (75%) rates, and is associated with a poor
prognosis. Therefore, the accurate preoperative evaluation of the
extent of tumor tissue infiltration may be extremely valuable.
Previously, the diagnosis of gastric cancer has
predominantly been based on upper gastroenterography and
gastroscopy, which directly or indirectly observes the morphology,
range and pathological changes occurring on the gastric mucosal
surface. These were the necessary methods to confirm the
identification, location and characteristic diagnosis of gastric
cancer. These methods were unable to directly reveal the stomach
structure, were limited in identifying the invasion depth of the
gastric wall, extra-stomach infiltration and metastasis, however,
they aided to a certain extent with the qualitative diagnosis of
cavity lesions (1). Recently,
diagnosis has been improved by the application of the endoscopic
ultrasound (EUS) and multislice computed tomography (MSCT), which
clearly exhibit the location, size and shape of stomach tumors, and
determine the extent of tumor invasion, lymph node metastasis and
distant organ metastasis. These methods are, therefore, considered
to aid with the preoperative staging of the tumor. However, in the
stratified diagnosis of gastric cancer, EUS and MSCT have certain
limitations, in addition, the accuracy of MSCT and EUS, with regard
to detecting the depth of gastric cancer invasion and the staging,
remains controversial.
With the utilization and development of magnetic
resonance imaging (MRI), the accuracy of preoperative TNM
Classification of Malignant Tumours (TNM) staging in gastric cancer
has gradually improved and exhibited its superiority (2). Matsushita et al (3) previously considered that the spoiled
gradient-recalled echo technique was able to display signal layers
that were lower than stomach and omentum signal layers, and that
the T3 stage of extraserous infiltration was likely to be expressed
as disappearance of the band or hyperintense lesions that entered
this band. Therefore, the use of MRI for the preoperative staging
of gastric cancer has become a predominant focus in recent years.
Few previous studies have analyzed the accuracy of MRI in the
preoperative staging of gastric cancer and in particular, studies
comparing the MRI preoperative staging of gastric cancer and
pathological results are rare. In 1994, Chin et al (4) applied the air-barium double contrast
technique to determine the histological type of gastric cancer
according to the Lauren classification system. In 1999, Rossi et
al (5) used an ordinary
computed tomography (CT) warm water filling technique for the
diagnostic study of a Lauren classification type of gastric cancer,
which was not widely recognized due to the limitations of X-ray and
ordinary CT in the diagnosis of gastric cancer. In the present
study, the patients with gastric cancer underwent preoperative
hypotonic water filling, MRI and dynamic contrast-enhanced and
high-resolution scanning detection to determine the TNM staging.
The results were compared with surgical pathology results to
evaluate the accuracy, sensitivity and specificity of the MRI scans
for the preoperative TNM staging of gastric cancer.
Patients and methods
Clinical data
In total, 30 patients who underwent surgery for
gastric cancer between June 2008 and Feb 2011 at the Fourth
Affiliated Hospital of Hebei Medical University (Shijiazhuang,
China) were included. Prior to scanning, endoscopy was performed as
the diagnostic test. The 30 patients included 19 males and 11
females (age, 50–69 years; mean age, 60 years) and there were 12
cases of gastric cardia tumor, 10 cases of gastric body tumor, four
cases of gastric antrum tumor, and four cases of gastric antrum and
body tumors. All of the cases were confirmed by pathology and
included 15 cases of poorly differentiated adenocarcinoma, 12 cases
of moderately and well-differentiated adenocarcinoma, one case of
undifferentiated adenocarcinoma, one case of signet ring cell
carcinoma and one case of mucinous adenocarcinoma. The 30 patients
received a preoperative MRI examination one week prior to surgery
and the results were assessed and identified by two experienced
radiologists. The current study was conducted in accordance with
the declaration of Helsinki and approved by the Ethics Committee of
the Handan Hospital of Jizhong Energy Fengfeng Group (Handan,
China). Written informed consent was obtained from all
patients.
Method
The Siemens 1.5T Tim Avanto MRI instrument (Siemens
AG, Munich, Germany) was used. Anisodamine (654-2; 10 mg) was
intramuscularly injected and 500 ml warm water was administered
orally 15–30 min prior to the examination. An additional 500 ml
warm water was administered prior to the patient lying on the
examination table; the patients were generally in the supine
position to make a major cross section. Conventional scanning with
T1-weighted imaging (WI), T2WI, short inversion time inversion
recovery, diffusion weighted imaging and enhanced scanning were
performed. A gadopentetate dimeglumine injection (20 ml/ampule;
Beijing Beilu Pharmaceutical Co., Ltd., Beijing, China) was used
for the enhanced scanning and the scan range was between the top of
the diaphragm and the umbilicus. Three-phase dynamic enhancement
was performed in the arterial phase (25–30 sec), the venous phase
(65–70 sec) and the equilibrium phase (3–4 min), following
initiation of the injection. A scan voltage of 120 kV and a current
of 120 mA were used.
T stage criteria
The following 2009 Union for International Cancer
Control (7th edition) TNM staging of gastric cancer was
adopted: T1 stage, no thickening of the gastric wall, with abnormal
enhancement of the stomach lining and enhanced tissues not
exceeding the intermediate layer; T2 stage, abnormal thickening of
the stomach wall, while the whole outer layer of the
multilayer-structure of the stomach wall is structurally integrated
or the serosal surface is smooth and tidy; T3 stage, the whole
stomach is infiltrated by the tumor and the external edge of the
stomach wall or peripheral-stomach adipose tissue exhibits lower or
irregular signal patterns or interruption; and T4 stage, structure
signals of the stomach-adjacent organs are changed or an abnormal
enhancement shadow of the stomach-adjacent organs appears in the
enhanced scan.
Statistical analysis
MRI diagnosis for the T stage of 30 patients with
gastric cancer was compared with the postoperative pathological
diagnosis. κ values were used as the index to measure the degree of
consistency. If the κ value was ≥0.75, this indicated that a very
satisfactory degree of consistency had been obtained. If the κ
value was <0.4, this indicated that the desired consistency
level was sufficient. The present study also examined κ values
using the Mann-Whitney U test. P<0.05 was considered to indicate
a statistically significant difference. All statistical analyses
were performed using SPSS 11.0 software (SPSS, Inc., Chicago, IL,
USA).
Results
MRI assessment of the preoperative
invasion depth of gastric cancer (T stage)
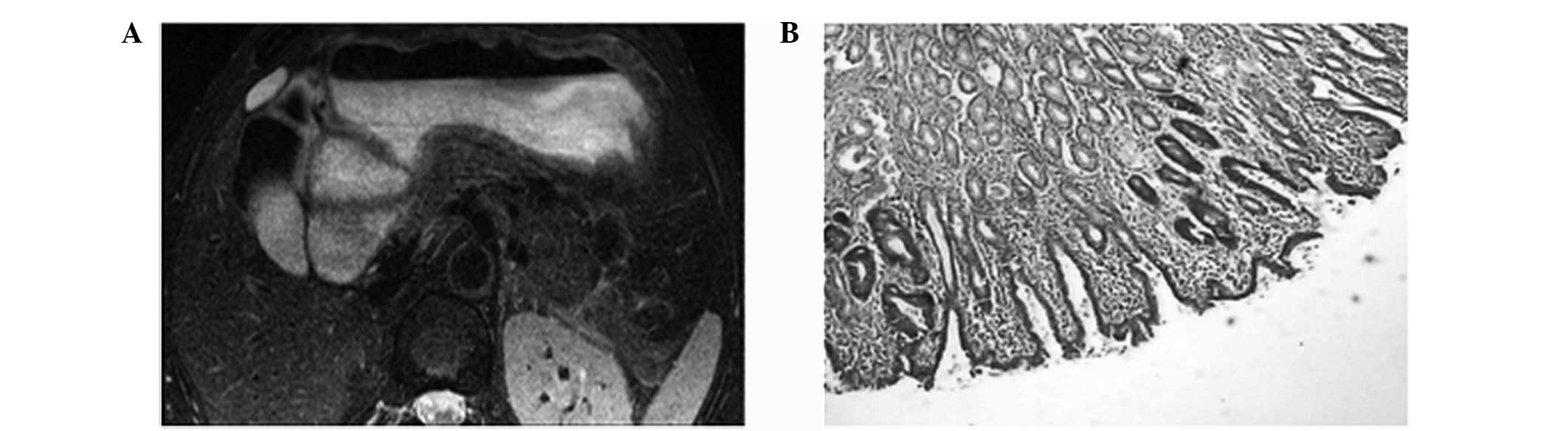
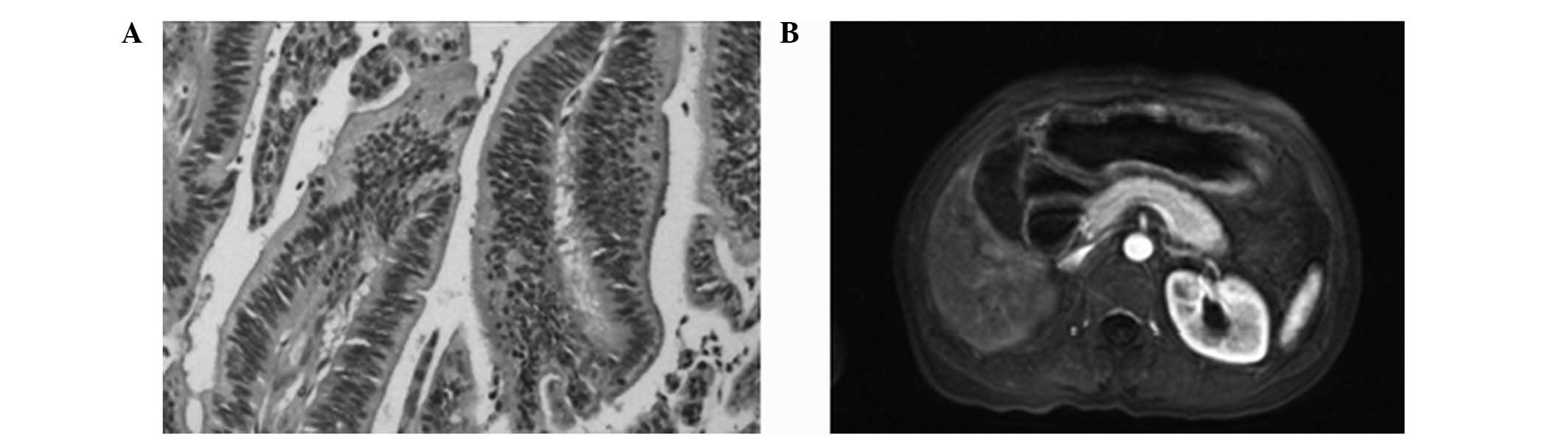
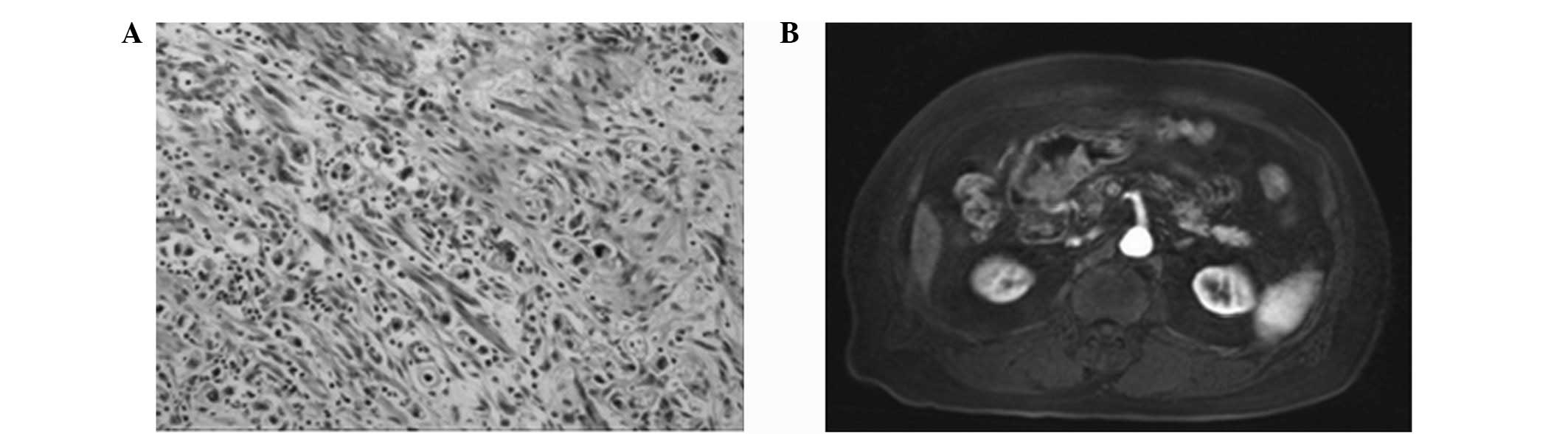
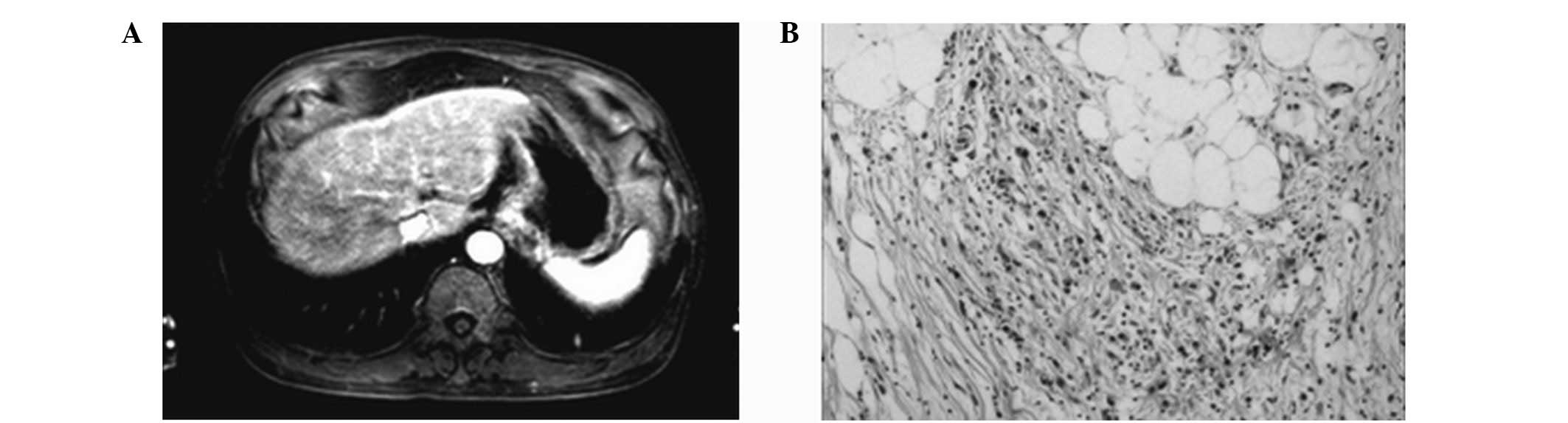
In total, four cases of T1 stage (Fig. 1), eight cases of T2 stage (Fig. 2), 11 cases of T3 stage (Fig. 3) and seven cases of T4 stage
(Fig. 4) were identified.
Confirmation of the MRI results by
postoperative pathology (T stage)
In total, five cases of T1, six cases of T2, 10
cases of T3 and eight cases of T4 stage were identified by
postoperative pathology.
Comparison between the MRI T stage
results and pathological diagnosis
Comparison of T1 stage
MRI correctly diagnosed three cases, misdiagnosed
one case and missed two cases. The MRI diagnostic accuracy of T1
stage was 90%, with a specificity of 96% and sensitivity of 60% (κ
value=0.61; P<0.05; Table
I).
 | Table IComparative analysis between the MRI
and pathological diagnosis of the depth of invasion (T stage) in 30
gastric cancer patients. |
Table I
Comparative analysis between the MRI
and pathological diagnosis of the depth of invasion (T stage) in 30
gastric cancer patients.
| MRI | Surgical
pathology | Sensitivitya, % (n) | Specificityb, % (n) | POS
predictionc value, % (n) | NEG
predictiond value, % (n) | Accuracye, % (n) | κ | P-value |
|---|
|
|---|
| POS | NEG |
|---|
| T1 | | | 60 (3/5) | 96 (24/25) | 75 (3/4) | 92.33 (24/26) | 90 (27/30) | 0.61 | <0.001 |
| POS | 3 | 1 | | | | | | | |
| NEG | 2 | 24 | | | | | | | |
| T2 | | | 83.3 (5/6) | 87.5 (21/24) | 62.5 (5/8) | 95.4 (21/22) | 86.7 (26/30) | 0.71 | <0.001 |
| POS | 5 | 3 | | | | | | | |
| NEG | 1 | 21 | | | | | | | |
| T3 | | | 90 (9/10) | 90 (18/20) | 81.8 (9/11) | 94.7 (18/19) | 90 (27/30) | 0.78 | <0.001 |
| POS | 9 | 2 | | | | | | | |
| NEG | 1 | 18 | | | | | | | |
| T4 | | | 87.5 (7/8) | 100 (22/22) | 100 (7/7) | 95.7 (22/23) | 96.7 (29/30) | 0.91 | <0.001 |
| POS | 7 | 0 | | | | | | | |
| NEG | 1 | 22 | | | | | | | |
Comparison of T2 stage
MRI correctly diagnosed five cases, misdiagnosed
three cases (overestimating two cases and underestimating one case)
and missed one case. The underestimated case was diagnosed as T3
stage due to the indistinct appearance of the external edge, while
the two overestimated cases were diagnosed as T1 stage, as the thin
wall induced stronger signals. The MRI diagnostic accuracy of T2
stage was 86.7%, with a specificity of 87.5% and sensitivity of
83.3% (κ value=0.71; P<0.05; Table
I).
Comparison of T3
MRI correctly diagnosed nine cases, misdiagnosed two
cases (overestimating one case and underestimating one case) and
missed one case. The underestimated case was diagnosed as T4 stage
due to the ambiguous expansion of the fat surrounding the lesion,
while the overestimated case was diagnosed as T2 stage due to the
integrated and continuous edge of the stomach wall. The MRI
diagnostic accuracy, specificity and sensitivity of T3 stage were
all 90% (κ value=0.78; P<0.05; Table
I).
Comparison of T4
MRI correctly diagnosed seven cases, missed one case
and no cases were misdiagnosed. The MRI diagnostic accuracy of T4
stage was 96.7%, with a specificity of 100% and sensitivity of
87.5% (κ value=0.91; P<0.05; Table
I).
These results showed that the MRI preoperative T
staging of gastric cancer exhibited statistical significance
(P<0.001) compared with the postoperative pathological
observations, particularly for the diagnosis of T3 and T4 stages.
The κ values were >0.75 for the T3 and T4 stages, which
reflected a relatively satisfactory degree of consistency between
the two diagnostic methods.
Discussion
Commonly, gastric cancer occurs in the gastric
antrum, predominantly in the lesser curvature of the stomach, which
accounts for ~75% of gastric cancers. Other types are located in
the fundus (including the cardia) or gastric body and, according to
the development of the gastric cancer, it may be categorized as
early- or advanced-type. Early gastric cancer is defined by tumor
tissues infiltrating only the lamina propria and submucosa, not the
stomach muscle layer. Minute gastric carcinoma is an early gastric
cancer, with a diameter of <5 mm; small gastric cancers exhibit
diameters of 6–10 mm. The five-year survival rate of early gastric
cancer is ≥85% and that of minute gastric cancer may be ~100%,
therefore, the early diagnosis and treatment of these types of
cancer is important (6). The
widespread use of fiberoptic endoscopy enables the early diagnosis
of gastric cancer. Furthermore, early gastric cancer may be divided
into the three types; protruded, superficial and depressed.
Advanced gastric cancer is defined by cancer invasion that is
deeper than the submucosa, reaching the stomach muscle layer or the
whole layer of the gastric wall. Prognosis worsens with the depth
of carcinoma infiltration and the five-year survival rate of
serosal invasion is significantly lower when compared with muscular
invasion. Clinically it has been found that the majority of gastric
cancers identified at the clinic were advanced. Gastric cancers may
be divided into the following types by naked-eye observations:
Mushroom, ulcer and infiltrating. Primary gastric cancer is an
adenocarcinoma, which may also be subdivided, according to the
degree of differentiation, into high and low undifferentiated
types. Additionally, according to their secretion, mucinous
carcinoma may be divided into signet ring cell carcinoma, nodular
mucinous adenocarcinoma, colloid carcinoma and other cancer types,
including squamous cell carcinoma, adenosquamous carcinoma and
carcinoid. The predominant types of carcinomatosis exhibit direct
diffusion, hematogenous metastasis and implantation metastases. The
early symptoms of gastric cancer are not clear. As the disease
progresses, stomach-related symptoms may become increasingly
significant, including abdominal pain, weight loss and loss of
appetite. The growing tumor may cause partial or complete
obstruction of the pylorus, indigestion and vomiting, usually of
gastric fluid. The appearance of carcinoma is likely to cause fecal
occult blood and, following invasion into large vessels, sudden
upper gastrointestinal bleeding may occur. In the late stage, an
upper abdominal mass and symptoms caused by tumor metastasis, such
as supraclavicular lymphadenopathy and ascites, followed by anemia,
weight loss and cachexia, are likely to appear.
The pathophysiological basis of three-stage enhanced
scanning is as follows. The contrast agent is intravenously
injected into the elbow and flows to the aortic branch via the
heart and into the systemic circulation. At this point, the
concentration of the contrast agent within the aorta increases
rapidly. In addition, the CT value increases rapidly and reaches it
peak value (A phase). Next, the contrast agent gradually flows out
of the vessel into the extravascular space (portal venous phase)
and finally achieves the equilibrium phase. The gastric blood
supply is rich, with the main blood supply from the left gastric
artery, hepatic artery and pulmonary artery, arising from the
celiac trunk. The left gastric artery supplies the lesser curvature
of the stomach, the left and right gastroepiploic arteries supply
the greater curvature of the stomach and the short gastric arteries
supply the fundus of the stomach. Angiography of gastric cancer and
microvascular studies have shown that neovascularization may be
observed in the arterial and capillary phases of the majority of
gastric cancer, with a rich blood supply, a high number of
distorted new blood vessels and an increased vascular volume
(7). Additionally, the venous phase
is likely to show positive tumor staining.
Matsushita et al (3) previously considered the spoiled
gradient-recalled echo technique to exhibit signal layers that are
lower than those of the stomach and omentum, and that the T3 stage
of extraserous infiltration was likely to be expressed as a
disappearance of the band or as hyperintense lesions entering this
band. In the present study, the delay period in the dynamic
enhanced scan revealed the blurred and disappeared fat layer in the
adjacent tissues and certain enhancement of the invaded tissues at
the interface of the tumor. This generally lasted for ~5 min, which
is marginally longer when compared with the previous literature.
Therefore, the effects of enhanced scanning are more useful in the
detection of early and advanced types of gastric cancer.
Originally, gastrointestinal motility led to
numerous limitations for MRI, however, as new MRI technologies have
emerged, it has become possible to use MRI to determine gastric
cancer (3,8,9).
In normal imaging of the stomach, MRI generally
exhibits between two and three or more layers of clear and normal
stomach structure, with a display rate of 30–70% (3). A single-layer structure of the stomach
is common, which poses a certain degree of difficulty for
diagnosis. Previously, Kang et al (10) analyzed an in vitro stomach
specimen by MRI; low signal stratum mucosum, low signal muscularis
propria and stratum mucosum, and low or high signal submucosa were
shown on T1WI. The longitudinal muscle exhibited a low signal,
while the lamina propria muscle tissues exhibited high signals on
T2WI. To reduce the quantity of gastrointestinal motility-related
artifacts, the patients were administered 654-215 mg by an
intramuscular injection and 700–1200 ml water contrast agent
orally; with a subsequent Bolus injection of iohexol 100 ml to fill
the stomach cavity, followed by a large volume of water to fully
expand the stomach. Horizontal scanning of the gastric cancer was
then performed. Kang et al (10) found that the gastric cancer is a
three-tier structure. In addition, Wang et al (7) performed dynamic enhanced MRI scanning
and revealed a three-layer stomach structure in vivo, with a
significantly enhanced mucosal layer, a low signal submucosa in the
middle and thickening in the muscularis and serosa. The MRI display
rate of the three-layer structure was 93.3% and MSCT was 53.6%
(11). This difference was found to
be significantly different (P<0.05), indicating that MRI is
significantly better than MSCT in the hierarchical description of
gastric cancer.
MRI scanning emits no radiation to the human body
and may be used to perform multifaceted and multiscan sequence
horizontal scanning, which may provide a signal comparison between
different imaging modalities. In addition, MRI can perform enhanced
scanning repeatedly during breath-holding, without using high doses
of contrast agent. MRI enables easy observation of tumor invasion
depth, extent and thickening. In recent years, MRI has been widely
used for analysis of the nervous and skeletal system.
The MRI horizontal scanning results of gastric
cancer are as follows: Low signal intensity on T1WI and moderate
but relatively low signal intensities on T2WI. When T1WI are used
in combination with fat suppression sequences, the lesions appear
as high signals, which indicates that high signals may be due to
the suppression of fat tissue around the lesion. The moderate but
relatively low signal intensity in T2WI may be due to the increased
number of fibrous tissue components in gastric cancer. The mucilage
ingredients present in mucinous adenocarcinoma demonstrate the
lesions more clearly. Therefore, MRI horizontal scanning may be
better than MSCT for the analysis of gastric cancer.
In the current study, MRI analysis of early gastric
cancer (four cases of T1 stage) exhibited a high reinforced
magnitude of the mucosal layer with a linear shape in the arterial
phase. However, this was more evident in the parenchymal phase, in
which the submucosa was continuous and complete. By contrast, the
significant enhancement effect disappeared in the equilibrium
phase. In the 26 advanced gastric cancer cases, a marked thickening
of the inner layer was observed in the arterial phase and a
gradually expanding enhancement area appeared throughout the whole
lesion in the parenchymal phase.
Previously, Kang et al (10) reported a high display rate of the
gastric submucosa, with an MRI scanning accuracy rate of >75%
for T1WI. The T1WI diagnosis accuracy of gastric cancer was 77%
(23/30) and increased to 87% when enhanced scanning was applied
(26/30). This indicated that the scanning resolution of the
enhanced MRI on the soft tissues was significantly higher than that
of the horizontal scanning.
The distinction between the T2 and T3, and the T3
and T4 stages in gastric cancer has long been a focus of MSCT
investigations. Serosal contour imaging of the fat around the
stomach may not always be able to clearly distinguish between T2
and T3 stages and T3 and T4 stages of MSCT (9,12,13).
The use of MSCT to determine T3 stage gastric cancer is more
difficult when the imaging of intestinal serosa shows irregular
protruding strips, thus, the application of MRI may be a solution
to this problem. In the MRI images of the present study, T2 stage
gastric cancer showed significant enhancement in the lag period,
while the edges of the stomach wall were smooth and intact, and the
outer layer showed a low signal intensity. In T3 stage gastric
cancer, the fat around the lesions exhibited a film strip and
showed marked enhancement following enhanced scanning. In addition,
the outer layer and adjacent tissue boundaries were blurred, and an
enhanced performance of the whole layer was observed. This imaging
difference may be adopted for the differential diagnosis of T2
stage gastric cancer. In the determination of the T2 and T3 stages,
the MRI accuracy was 86.7 and 90%, with a specificity of 87.5 and
90%, and sensitivity of 83.3 and 90%, respectively. In addition,
the κ values were 0.71 and 0.78, respectively, indicating that the
T3 stage was more compliant with the histological results than the
T2 stage, which also complied well with the histological
results.
In addition, the selection of surgical methods for
the differential diagnosis of T3 and T4 stages was important.
Previously, Matsushita et al (3,14,15)
hypothesized that the spoiled gradient-recalled echo technique was
able to exhibit signal layers that were lower than those for the
stomach and omentum. In addition, the authors considered that the
degree of extraserous infiltration could be determined by observing
whether the hyperintense lesion entered the low signal band or by
observing the disappearance of the low signal band. In the current
study, MRI showed that dynamic contrast-enhanced scanning delayed
the thickening effect at the interface of the lesions in the
invaded lesion tissue during the lag period. The abovementioned MRI
horizontal scanning showed blurred structures in the adjacent
tissues and disappearance of the fat layer. Therefore, it was
essential to enhance the scanning during the lag period (16–18),
which was generally ~5 min longer than those reported in the
previous literature. The effects of enhanced scanning may aid with
the detection of early and advanced gastric cancer. The accuracy
rates of T3 and T4 stage diagnosis compared with those of surgical
pathology were 90 and 96.7%, with a specificity of 90 and 100%, and
sensitivity of 90 and 87.5%, respectively. In addition, the κ
values were 0.78 and 0.91, respectively, indicating that T3 and T4
staging exhibited a high degree of consistency with the
pathological results. The results showed that the reason for the
higher staging was due to gastric cancer combined with
inflammation. Future studies concerning staging are required, as
the identification of the T3 and T4 stages significantly affects
the selection of the appropriate surgery (19,20),
which was shown by the current study.
The correlation between the preoperative T staging
of gastric cancer and postoperative histopathology is important for
clinical treatment as it involves numerous factors, including
whether surgical resection must be performed, selection of the
surgical procedure, comprehensive treatment plans and prognosis
assessment as well as other factors. The preoperative T staging of
gastric cancer is also associated with the survival period and the
patient quality of life, therefore, it is particularly important to
obtain an early diagnosis of gastric cancer. Regular medical
screening is significant to ensure that progression of the gastric
cancer is not overlooked, as early diagnosis and treatment may
improve patient quality of life. Furthermore, the MRI prediction of
poorly differentiated stomach carcinoma is important as the
malignancy of poorly differentiated gastric cancer is high and
requires extensive surgical resection, which as a result, has a
poor prognosis and is prone to distant metastasis. Therefore,
combined modality therapy for poorly differentiated gastric cancer
must be improved and all cases must be followed up.
In conclusion, MRI is valuable in the preoperative T
staging of gastric cancer due to its accuracy and specificity in
determining the invasion depth of gastric cancer, which may aid
with guiding the selection of treatment options and avoiding
unnecessary surgery. General MRI scanning has advantages and
disadvantages for the T staging of gastric cancer. The
disadvantages include: i) A long clinical assessment time, normally
~30–45 min; ii) a small and limited scan range; and iii) poor image
quality, as dynamic enhanced scanning requires the patients to
breathe repeatedly, which certain patients are unable to cope with,
thus producing an unclear image. However, the continued development
of MRI technology may resolve these difficulties and increase the
value of adopting MRI for the preoperative staging of gastric
cancer.
References
|
1
|
Düx M, Grenacher C, Lubienski A, Schipp A,
Richter GM and Hansmann J: Carcinoma of the stomach. Role of
imaging for primary diagnosis and preoperative tumor staging. Rofo.
172:661–669. 2000.(In German).
|
|
2
|
Bruneton JN, Francois E, Padovani B and
Raffaelli C: Primary tumor staging of gastric and colorectal
cancer. Eur Radiol. 6:140–146. 1996.
|
|
3
|
Matsushita M, Oi H, Murakami T, et al:
Extraserosal invasion in advanced gastric cancer: evaluation with
MR imaging. Radiology. 192:87–91. 1994.
|
|
4
|
Chin SY, Lee BH, Kim KH, Park ST, Do YS
and Cho KJ: Radiological prediction of the depth of invasion and
histologic type in early gastric cancer. Abdom Imaging. 19:521–526.
1994.
|
|
5
|
Rossi M, Broglia L, Graziano P, et al:
Local invasion of gastric cancer: CT findings and pathologic
correlation using 5-mm incremental scanning, hypotonia, and water
filling. AJR Am J Roentgenol. 172:383–388. 1999.
|
|
6
|
Hao Jie and Chen Wanqing: 2012 Chinese
Cancer Registry Annual Report. Military Medical Science Press;
Beijing, China: pp. 21–39. 2012
|
|
7
|
Wang CK, Kuo YT, Liu GC, Tsai KB and Huang
YS: Dynamic contrast-enhanced subtraction and delayed MRI of
gastric tumors: radiologic-pathologic correlation. J Comput Assist
Tomogr. 24:872–877. 2000.
|
|
8
|
Kondo Y, Sakaguchi H, Nakamuro M, Kawamura
J, Takami M and Kotake Y: Successful TS-1 therapy in a patient with
non-resectable gastric cancer and renal dysfunction. Gan To Kagaku
Ryoho. 27:2249–2253. 2000.(In Japanese).
|
|
9
|
Dorfman RE, Alpern MB, Gross BH and
Sandler MA: Upper abdominal lymph nodes: criteria for normal size
determined with CT. Radiology. 180:319–322. 1991.
|
|
10
|
Kang BC, Kim JH, Kim KW, et al: Value of
the dynamic and delayed MR sequence with Gd-DTPA in the T-staging
of stomach cancer: correlation with the histopathology. Abdom
Imaging. 25:14–24. 2000.
|
|
11
|
Tsuda K, Hori S, Murakami T, et al:
Intramural invasion of gastric cancer: evaluation by CT with
water-filling method. J Comput Assist Tomogr. 19:941–947. 1995.
|
|
12
|
Piasecki C and Wyatt C: Patterns of blood
supply to the gastric mucosa. J Anat. 149:21–39. 1986.
|
|
13
|
Takao M, Fukuda T, Iwanaga S, Hayashi K,
Kusano H and Okudaira S: Gastric cancer: evaluation of triphasic
spiral CT and radiologic-pathologic correlation. J Comput Assist
Tomogr. 22:288–294. 1998.
|
|
14
|
Sohn KM, Lee JM, Lee SY, Ahn BY, Park SM
and Kim KM: Comparing MR imaging and CT in the staging of gastric
carcinoma. AJR Am J Roentgenol. 174:1551–1557. 2000.
|
|
15
|
Portnoı̌ LM, Kazantseva IA, Viatchanin OV
and Stashuk GA: Cancer of the upper stomach: current problems of
its diagnosis. Vestn Rentgenol Radiol. Jan–Feb;4–22. 2003.(In
Russian).
|
|
16
|
Wada Y, Yamamoto T, Kita Y, Fukunishi S
and Ashida K: An autopsy case of encephalopathy associated with
small cell carcinoma of the stomach with nonconvulsive status
epilepticus resembling Creutzfeldt-Jakob disease. No To Shinkei.
55:423–428. 2003.(In Japanese).
|
|
17
|
Maccioni F: Current status of
gastrointestinal MRI. Abdom Imaging. 27:358–360. 2002.
|
|
18
|
Berr SS, Roche JK, El-Rifai W, Smith MF Jr
and Powell SM: Magnetic resonance imaging of gastric cancer in Tffl
knock-out mice. Magn Reson Med. 49:1033–1036. 2003.
|
|
19
|
Kwak HS, Jin GY and Lee JM: Radiologic
fingdings of multiple myeloma with gastric involvement: a case
report. Korean J Radiol. 3:133–135. 2002.
|
|
20
|
Genvresse I, Dietzmann A, Massenkeil G,
Späth-Schwalbe E and Possinger K: Subacute encephalopathy after
combination chemotherapy including moderate-dose methotrexate in a
patient with gastric cancer. Anticancer Drugs. 10:293–294.
1999.
|