Introduction
Colorectal cancer (CRC) is a serious health problem
worldwide and recent evidence indicates that obesity and metabolic
syndrome (Mets), both of which are also global health problems,
closely correlate with an increased risk of CRC development
(1–5). Several pathophysiological mechanisms,
such as the emergence of insulin resistance, state of chronic
inflammation and the induction of oxidative stress, may be involved
in colorectal carcinogenesis in patients with Mets (3–5). For
example, diabetic patients, who frequently present with Mets in
addition to insulin resistance, are considered as a high-risk group
for CRC development (1–5). However, several rodent studies have
demonstrated that targeting insulin resistance and chronic
inflammation is effective for preventing obesity- and
diabetes-related colorectal carcinogenesis (4,6–9).
In addition to diabetes, epidemiological studies
have revealed that hypertension, which is an additional component
of Mets, may increase the risk of CRC (1,2).
Hypertension is critically involved in the early stage of
colorectal carcinogenesis via the activation of the
renin-angiotensin system and subsequent induction of oxidative
stress and chronic inflammation (10). The renin-angiotensin system is
important in the regulation of blood pressure and hydromineral
balance, and its activation is one of the key factors in the
etiology of Mets, particularly hypertension (11). Angiotensin-converting enzyme (ACE)
cleaves angiotensin (AT)-I to AT-II, which is the active product of
the renin-angiotensin system and exerts a physiological effect
through binding to its receptor, AT-II type 1 receptor (AT-1R)
(12,13). Therefore, the renin-angiotensin
system inhibitors, including ACE inhibitors and AT-1R blockers
(ARB), are used widely for the treatment of hypertension.
Furthermore, ACE inhibitors have been shown to exert beneficial
effects on cardiovascular disease and reduce mortality as a result
of hypertension (14,15).
In addition to the regulation of cardiovascular
function, the renin-angiotensin system, which exists in multiple
tissues, including the colon, exhibit functions in effecting tissue
angiogenesis and chronic inflammation, as well as controlling
cellular proliferation and apoptosis. Furthermore, abnormalities in
the renin-angiotensin system closely correlate with the enhancement
of cancer cell migration, invasion and metastasis, which are
correlated with poor prognosis (16–18).
The levels of gene expression and enzymatic activity of ACE are
increased in human colon adenocarcinoma tissues (19). These aforementioned studies indicate
that dysregulation of the renin-angiotensin system may be
significant in Mets-related colorectal carcinogenesis and,
therefore, an effective target for the chemoprevention of CRC,
specifically in patients with Mets.
SHRSP.Z-Leprfa/IzmDmcr
(SHRSP-ZF) rats were established as a new model of human Mets by
crossing SHRSP rats, which have a higher blood pressure, with obese
and diabetic Zucker fatty rats (20,21).
In our previous study, a new Mets-related colorectal carcinogenesis
model was established using SHRSP-ZF rats and a colonic carcinogen,
azoxymethane (AOM) (10). In this
model, the activation of the renin-angiotensin system and
subsequent augmentation of chronic inflammation and oxidative
stress enhanced the development of AOM-induced colonic premalignant
lesions, aberrant crypt foci (ACF), which indicated that the model
was useful to test the potential efficacy of renin-angiotensin
system inhibitors in preventing CRC development in patients with
Mets (10). The objective of the
present study was to examine the preventive effects of captopril, a
widely used ACE inhibitor in hypertensive patients, on the
development of AOM-induced ACF in diabetic and hypertensive
SHRSP-ZF rats.
Materials and methods
Animals and chemicals
Five-week-old male SHRSP-ZF rats (n=20) were
obtained from the Japan SLC (Shizuoka, Japan) and humanely
maintained at Gifu University Life Science Research Center (Gifu,
Japan) in accordance with the Institutional Animal Care Guidelines.
AOM was purchased from Wako Pure Chemical Industries, Ltd. (Osaka,
Japan) and captopril was obtained from Sigma-Aldrich (St. Louis,
MO, USA). The study was approved by the ethics committee of Gifu
University Life Science Research Center (Gifu, Japan).
Experimental procedure
After one week of acclimatization, the rats were
separated into two groups of 10 rats each. All rats received an
intraperitoneal injection of AOM (20 mg/kg body weight) once a week
for two weeks. One week following the second injection of AOM, the
rats were administered water with or without captopril (8
mg/kg/day) for two weeks. The intake of captopril was maintained by
adjusting its concentration in the drinking water, the volume of
which was measured three times a week (22). At the end of the experiment, when
the rats were 10 weeks of age, systolic and diastolic blood
pressures were measured non-invasively using a tail cuff (Softron
BP98A; Softron, Tokyo, Japan) and all rats were sacrificed by
CO2 asphyxiation for colon resection. The third portion
of the excised colons (cecum side) was used to extract RNA, and the
remaining portion was used to determine the number of ACF (10,23).
Number of ACF
The frequency of ACF was determined as previously
described (10,23). The colon samples were fixed with 10%
buffered formalin, stained with methylene blue (0.5% in distilled
water; Wako Pure Chemical Industries, Ltd.) for 20 sec and then
placed on microscope slides to count the number of ACF using a BH2
Olympus microscope (Olympus, Tokyo, Japan). The number of ACF was
recorded along with the number of aberrant crypts (ACs) in each
focus. Data are presented as per unit area (cm2).
RNA extraction and quantitative
polymerase chain reaction (qPCR)
The isolation of epithelial crypts, extraction of
total RNA from isolated epithelial crypts, amplification of cDNA
from total RNA and qPCR analysis were performed as previously
described (10,23). The sequences of specific primers
that amplify tumor necrosis factor α (TNF-α), interleukin 18
(IL-18), monocyte chemoattractant protein 1 (MCP-1),
inducible nitric oxide synthase (iNOS), ACE,
AT-1R, vascular endothelial growth factor (VEGF),
catalase (CAT), proliferating cell nuclear antigen
(PCNA) and glyceraldehyde-3-phosphate dehydrogenase
(GAPDH) genes were obtained from Primer-BLAST (http://www.ncbi.nlm.nih.gov/tools/primer-blast/;
Table I). Each sample was analyzed
on a LightCycler Nano (Roche Diagnostics, Basel, Switzerland) with
FastStart Essential DNA Green Master (Roche Diagnostics). Parallel
amplification of GAPDH was used as the internal control.
 | Table IPrimer sequences. |
Table I
Primer sequences.
| Target gene | Direction | Primer sequence
(5′-3′) |
|---|
| TNF-α | Forward |
AACACACGAGACGCTGAAGT |
| Reverse |
TCCAGTGAGTTCCGAAAGCC |
| IL-18 | Forward |
ACAGCCAACGAATCCCAGAC |
| Reverse |
ATAGGGTCACAGCCAGTCCT |
| MCP-1 | Forward |
TGGGCCTGTTGTTCACAGTT |
| Reverse |
ACCTGCTGCTGGTGATTCTC |
| iNOS | Forward |
GTGGTGACAAGCACATTTGG |
| Reverse |
GGCTGGACTTTTCACTCTGC |
| ACE | Forward |
CTTGACCCTGGATTGCAGCC |
| Reverse |
GTTTCGTGAGGAAGCCAGGA |
| AT-1R | Forward |
TCGTGGCTTGAGTCCTGTTC |
| Reverse |
CGCGCACACTGTGATATTGG |
| VEGF | Forward |
TCCACCGTGTATGCCTTCTCC |
| Reverse |
CCTGCTGTATCTGCGCACTGGA |
| CAT | Forward |
GAGGCAGTGTACTGCAAGTTCC |
| Reverse |
GGGACAGTTCACAGGTATCTGC |
| PCNA | Forward |
AAGACCTCGCTCCCCTTACA |
| Reverse |
ATCAGGCGTGCCTCAAACAT |
| GAPDH | Forward |
CCTTCATTGACCTCAACTACATGGT |
| Reverse |
TCATTGTCATACCAGGAAATGAGCT |
Clinical chemistry
The blood samples, which were collected at the time
of sacrifice after 6 h of fasting, were used for chemical analyses.
The serum levels of insulin (Shibayagi, Gunma, Japan), glucose
(BioVision Research Products, Mountain View, CA, USA), leptin
(Shibayagi), triglyceride (Wako Pure Chemical Industries, Ltd.),
non-esterified fatty acid (NEFA; Wako Pure Chemical Industries,
Ltd.) and AT-II (Phoenix Pharmaceuticals, Inc., Burlingame, CA,
USA) were determined by an enzyme-linked immunosorbent assay
(ELISA) kit according to the manufacturer’s protocols (NIKKEN SEIL
Co. Ltd., Shizuoka, Japan).
Oxidative stress analysis
Urine 8-hydroxy-2′-deoxyguanosine (8-OHdG) levels
were determined using the ELISA kit (NIKKEN SEIL Co. Ltd.). Serum
levels of hydroperoxide, a marker for oxidative stress, were
evaluated using the derivatives of reactive oxygen metabolites
(d-ROM) test (FREE Carpe Diem; Diacron International s.r.l.,
Grosseto, Italy) (10,24).
Statistical analysis
All data are presented as the mean ± standard
deviation and were analyzed using JMP 9 (Statistical Analysis
System Institute, Inc., Cary, NC, USA) for Windows. Student’s
t-test was performed to compare the mean values among the groups.
P<0.05 was considered to indicate a statistically significant
difference.
Results
General observations
Irrespective of captopril administration, no
significant differences were observed in the mean body weights of
experimental rats at the termination of the experiment (10 weeks of
age; Table II). The mean adipose
tissue weights increased in the rats treated with captopril
(P<0.05) and treatment with captopril effectively lowered the
systolic and diastolic blood pressures (P<0.05).
Histopathological examinations of the liver, kidney and spleen
confirmed the absence of toxicity from captopril (data not
shown).
 | Table IIBody weights, adipose tissue weights
and blood pressure of the experimental rats. |
Table II
Body weights, adipose tissue weights
and blood pressure of the experimental rats.
| Group | n | Treatment | Body weight, g | Relative adipose
tissue weight, g/100 g body weighta | Blood pressure,
mmHg |
|---|
|
|---|
| Systolic | Diastolic |
|---|
| 1 | 10 | AOM | 270.7±20.1b | 1.67±0.16 | 170±13.1 | 130±8.6 |
| 2 | 10 | AOM +
captopril | 261.4±4.1 | 1.97±0.24c | 146±15.4c | 112±14.2c |
Effects of captopril on AOM-induced ACF
and colonic epithelial expression of PCNA mRNA in SHRSP-ZF
rats
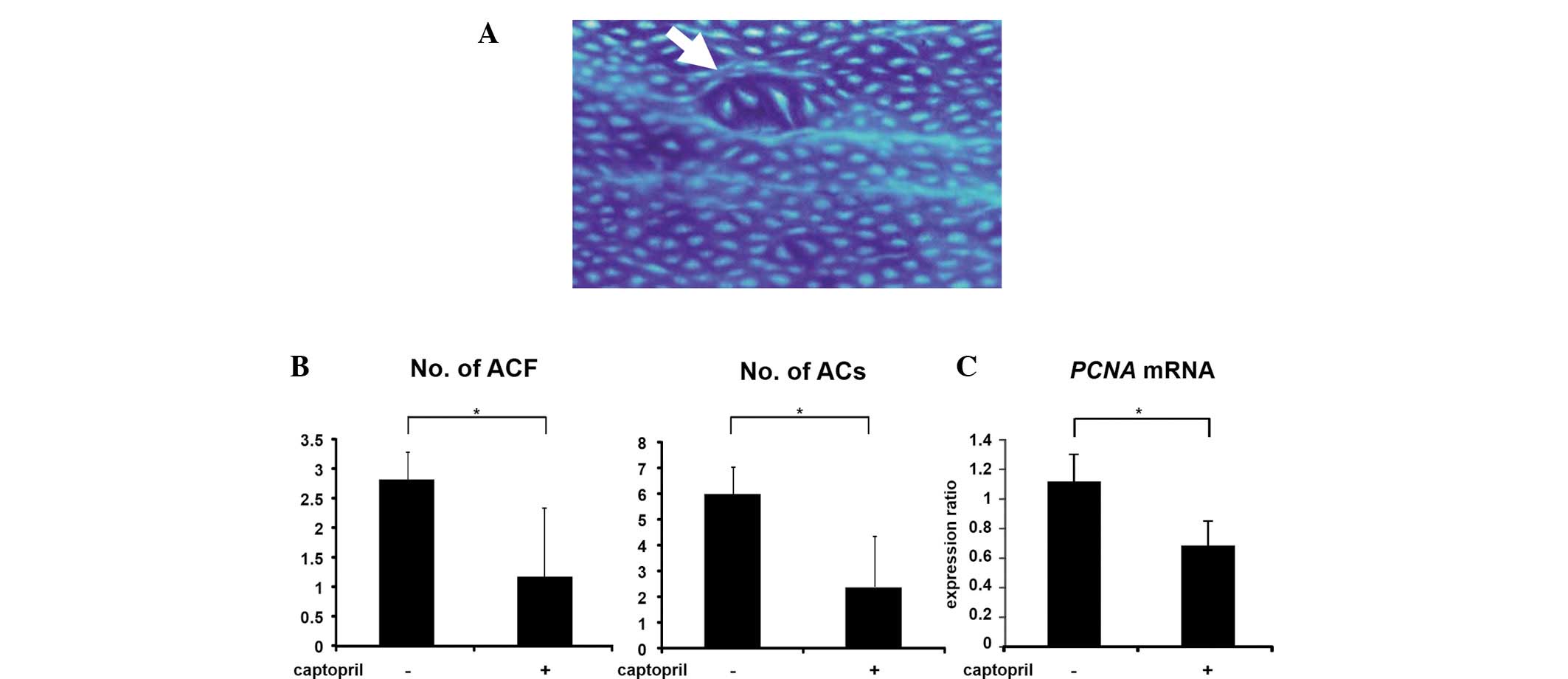
At the end of the study, ACF (Fig. 1A) were observed in the colons of all
rats that received AOM. However, captopril treatment significantly
reduced the number and size (ACs per cm2) of the ACF in
these rats (Fig. 1B; P<0.05). In
addition, the colonic epithelial expression of PCNA mRNA
decreased significantly with captopril administration (Fig. 1C; P<0.05). These observations
suggested that captopril inhibits the early stage of colorectal
carcinogenesis in obese and hypertensive rats, at least in part,
through the suppression of cell proliferation.
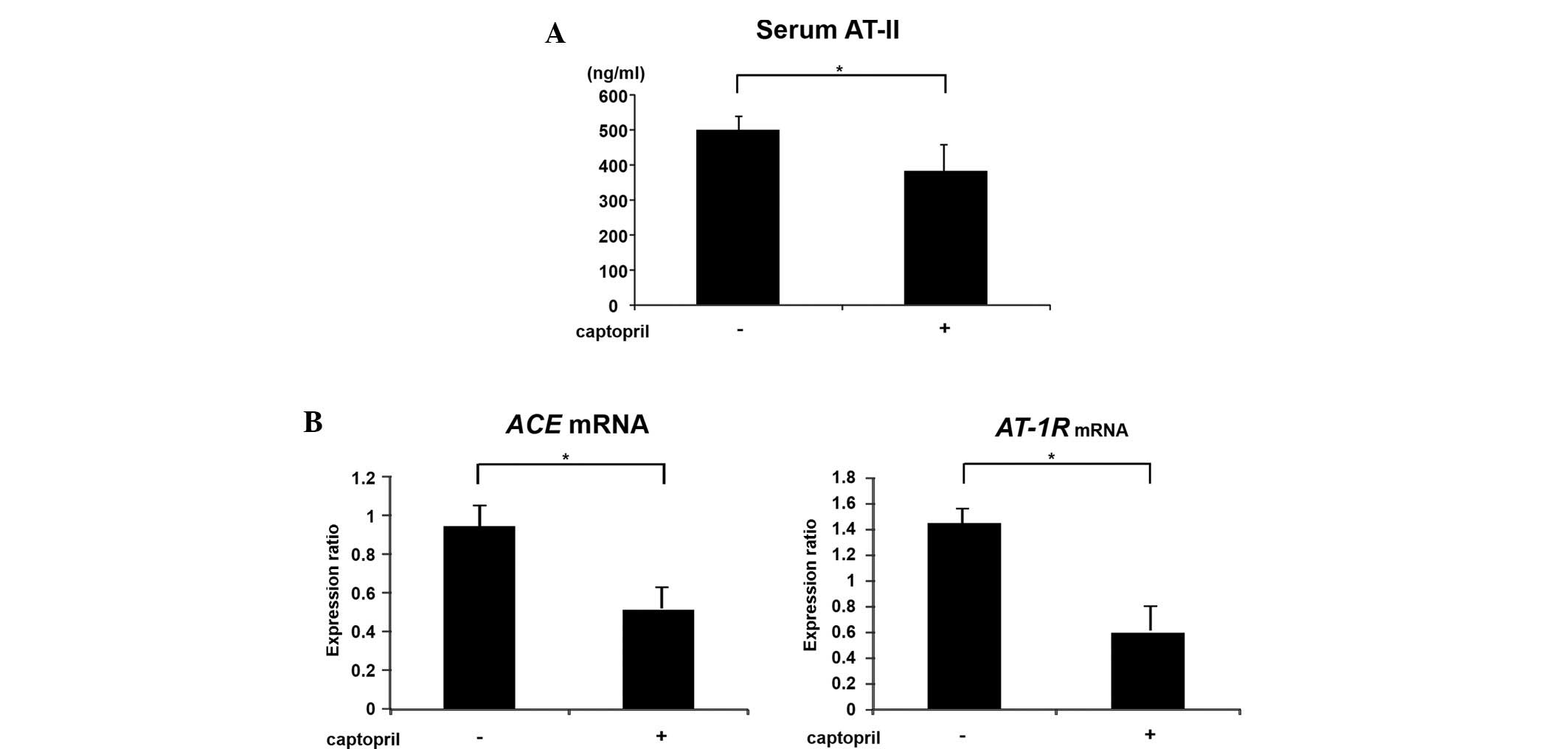
Effects of captopril on serum AT-II and
colonic epithelial expression of ACE and AT-1R mRNA
in SHRSP-ZF rats
Hyperactivity of the renin-angiotensin system is
implicated in the etiology of Mets and closely correlates with the
development and the progression of CRC (16–18).
Therefore, the current study investigated the effects of captopril
on the serum levels of AT-II and the expression levels of
renin-angiotensin system components, including ACE and
AT-1R mRNA in the colonic epithelium. Administration of
captopril significantly reduced the levels of serum AT-II (Fig. 2A; P<0.01), and the expression
levels of ACE and AT-IR mRNA in the colonic
epithelium were also decreased with captopril treatment (Fig. 2B; P<0.01). These observations
indicated that the local level (colonic epithelium), in addition to
the systemic level (serum), of renin-angiotensin system activation
in diabetic and hypertensive SHRSP-ZF rats was significantly
inhibited by captopril.
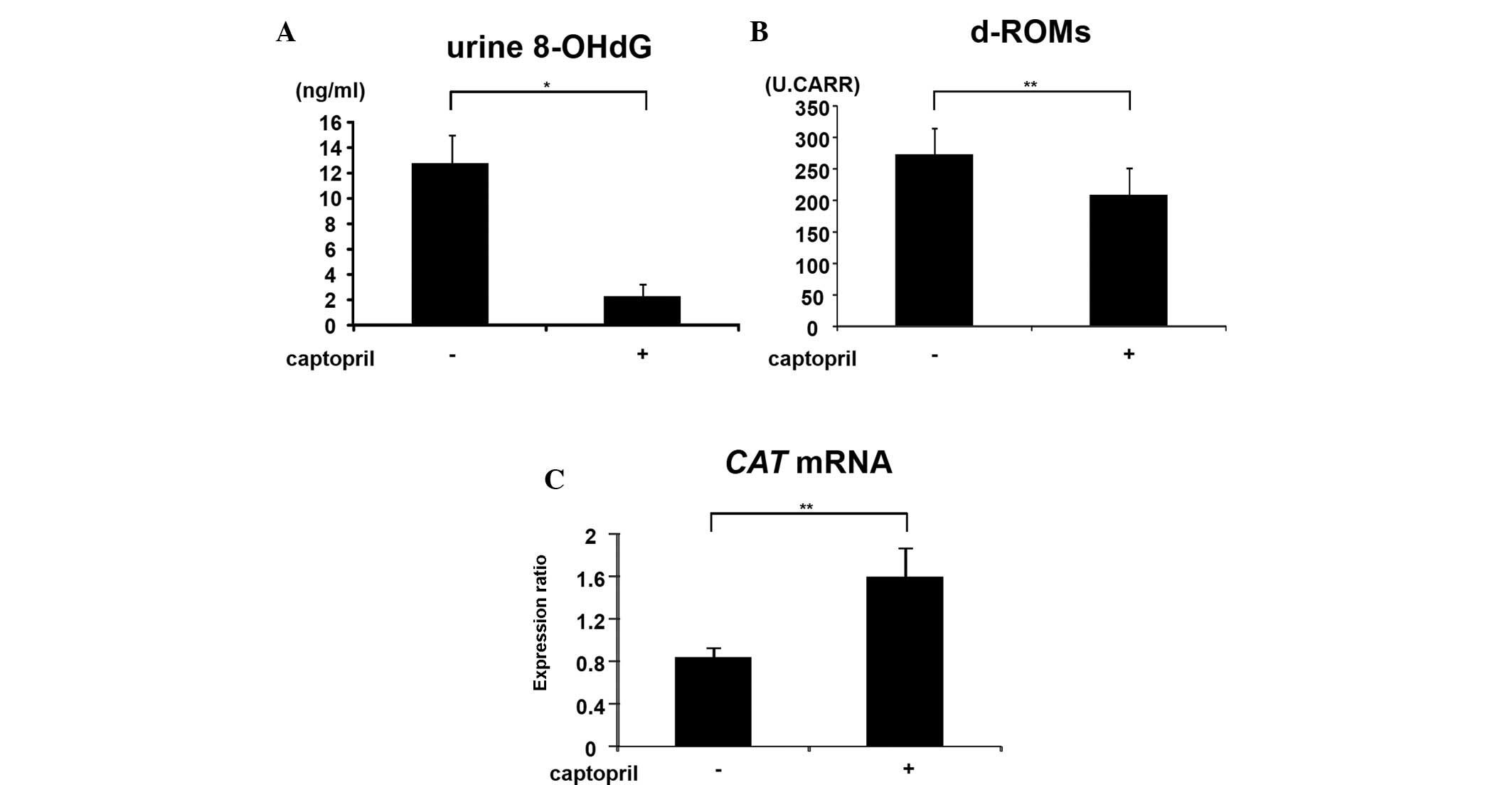
Effects of captopril on systemic
oxidative stress and colonic epithelial expression of CAT mRNA in
SHRSP-ZF rats
Oxidative stress is key in Mets-related colorectal
tumorigenesis (3,4). Therefore, the current study examined
whether captopril administration effects the levels of oxidative
stress and antioxidant biomarkers in experimental rats. Captopril
administration significantly decreased the levels of urine 8-OHdG
(Fig. 3A; P<0.001), a marker of
DNA damage induced by oxidative stress and serum d-ROM (Fig. 3B; P<0.01), which reflects serum
hydroperoxide levels, in SHRSP-ZF rats. By contrast, in
captopril-treated rats, a significant increase was identified in
the colonic epithelial expression of CAT mRNA, which encodes
an antioxidant enzyme (Fig. 3C;
P<0.01). These observations suggested that captopril attenuates
the systemic and colonic epithelial oxidative stress.
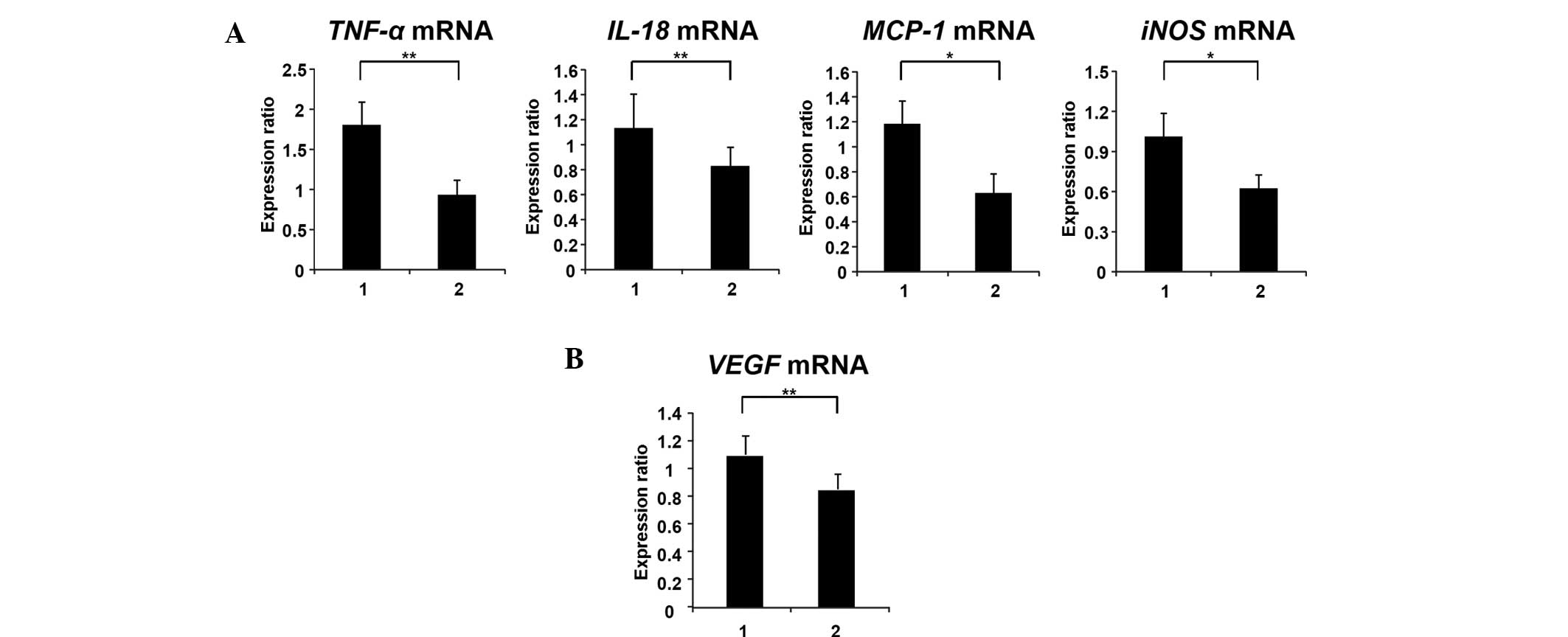
Effects of captopril on colonic
epithelial expression of TNF-α, IL-18, MCP-1, iNOS and VEGF mRNA in
SHRSP-ZF rats
Chronic inflammation is associated with Mets and CRC
development (3–5). Therefore, the current study examined
the effects of captopril on the colonic expression levels of
inflammatory mediators in SHRSP-ZF rats. Captopril treatment
significantly decreased the colonic epithelial expression of
TNF-α (P<0.05), IL-18 (P<0.05), MCP-1
(P<0.01) and iNOS mRNA (P<0.01) in the experimental
rats (Fig. 4A). In addition, the
expression levels of VEGF mRNA, which are upregulated by the
AT-II/AT-1R axis (25), were also
significantly decreased by captopril treatment (Fig. 4B; P<0.05).
Effects of captopril on serum levels of
glucose, insulin, leptin, NEFA and triglycerides in SHRSP-ZF
rats
Insulin resistance and adipokine imbalance are
associated with Mets-related colorectal carcinogenesis (3). SHRSP-ZF rats are hyperglycemic,
hyperinsulinemic, hyperleptinemic and hypertriglyceridemic compared
with their genetic control (10).
Therefore, whether captopril treatment alters the serum levels of
glucose, insulin, leptin, NEFA and triglycerides in SHRSP-ZF rats
was investigated in this study. It was found that captopril
treatment did not improve these metabolic parameters in the
experimental rats (Table III).
The value of QUICKI, a useful index of insulin sensitivity
(26), was also not affected by
captopril treatment.
 | Table IIISerum parameters of the experimental
rats. |
Table III
Serum parameters of the experimental
rats.
| Group | n | Glucose, mg/dl | Insulin,
μIU/ml | Quicki | Leptin, pg/ml | NEFA, mEq/ml | Triglyceride,
mg/dl |
|---|
| 1 | 10 | 120.0±14.2a | 25.6±9.0 | 0.29±0.01 | 102.7±30.6 | 537.9±30.0 | 257.1±79.4 |
| 2 | 10 | 118.5±15.4 | 25.5±7.2 | 0.28±0.02 | 101.2±27.7 | 555.0±27.8 | 234.7±64.5 |
Discussion
Mets and its associated metabolic abnormalities,
including diabetes mellitus and hypertension, are significant risk
factors for the development of CRC (1–3). Among
pathophysiological disorders associated with Mets, in particular
hypertension, activation of the renin-angiotensin system is
considered to be critical in the early events of colorectal
carcinogenesis (10). Dysregulation
of the renin-angiotensin system is involved in cancer cell
migration and invasion, as well as metastasis in malignant tumors,
including CRC (13,16–18).
AT-II, which is a main effector peptide in the renin-angiotensin
system, has been known to enhance cell proliferation, invasion and
survival of CRC cells (27). The
gene expression and enzymatic activity of ACE are also increased in
human CRC tissues (19). These
reports indicated that the activated renin-angiotensin system is
mechanistically fundamental in Mets-related colorectal
carcinogenesis and, therefore, may be a promising target for the
prevention of CRC.
The results of the present study clearly indicated
that the administration of captopril, a renin-angiotensin system
inhibitor, effectively suppresses the development of AOM-induced
colonic preneoplastic lesions in diabetic and hypertensive SHRSP-ZF
rats by decreasing the serum levels of AT-II and colonic epithelial
expression levels of ACE and AT-1R mRNA. These
observations are consistent with those of a previous study
demonstrating that the treatment with an ACE inhibitor and ARB
inhibits chemically induced colorectal carcinogenesis in obese and
diabetic mice (22). In a human
trial, long-term use of an ACE inhibitor also reduced the incidence
and size of colorectal adenomas (28). These studies (22,28),
together with the results of the present study, markedly suggest
that renin-angiotensin system inhibitors, including ACE inhibitors
and ARBs, may be useful for the prevention of CRC development in
patients with Mets, particularly those with hypertension.
A recent study showed, even without obesity and
diabetes, that hypertension per se enhances colorectal
carcinogenesis and is associated with the elevated levels of
oxidative stress (10). Increased
levels of AT-II activate the renin-angiotensin system and lead to
an increase in oxidative stress (17,29),
which is involved in the production of DNA damage and mutations
associated with colorectal carcinogenesis (3,30). In
this study, captopril administration lowered the blood pressure,
decreased the levels of urine 8-OHdG and serum d-ROM, which are
implicated in increased oxidative stress (24,31),
and increased the expression of CAT, an antioxidant enzyme, thus
suppressing the development of AOM-induced ACF in SHRSP-ZF rats,
which are subjected to strong oxidative stress (10). These observations are consistent
with previous studies (22,32) that have reported the cancer
preventive effects of renin-angiotensin system inhibitors via the
reduction of oxidative stress.
In addition to oxidative stress, renin-angiotensin
system activation is also implicated in the induction of chronic
inflammation (16,17,33),
which is a key factor for Mets and CRC development (3–5).
Activation of AT-1R by AT-II induces a number of molecules that
participate in inflammatory responses (16,17,34).
AT-II also induces the expression of iNOS, an inflammatory marker,
along with 8-OHdG in cancer cells through the activation of AT-1R
(32), suggesting a cross-link
between renin-angiotensin system-related inflammation and oxidative
stress in cancer tissue. In addition, AT-II stimulates the
expression of VEGF through the activation of AT-1R and the
induction of chronic inflammation (25). In the present study, captopril
administration decreased the expression levels of TNF-α,
IL-18, MCP-1, iNOS and VEGF mRNA in the
colonic epithelium of AOM-treated SHRSP-ZF rats. Therefore, in
addition to the reduction of oxidative stress, the chemopreventive
effect of captopril on Mets-related colorectal carcinogenesis is
most likely associated with the attenuation of systemic
inflammation.
Pathological conditions implicated in Mets, such as
insulin resistance, hyperleptinemia and dyslipidemia, may be
critical therapeutic targets in the prevention of obesity- and
diabetes-related colorectal carcinogenesis (4,6–9).
However, in the present study, captopril treatment did not improve
these metabolic disorders. These observations, together with the
results of a recent study (10),
may suggest that the renin-angiotensin system is a promising target
for preventing early-phase colorectal carcinogenesis associated
with Mets, in particular, hypertension. To confirm this hypothesis,
experiments of longer duration are required to determine whether
renin-angiotensin system inhibitors actually suppress Mets-related
CRC development by suppressing the activation of the system. In
addition, the possibility of combination chemoprevention using
renin-angiotensin system inhibitors and specific drugs for Mets
(such as antidiabetic drugs, which improve insulin resistance) for
preventing Mets-related colorectal carcinogenesis must also be
explored.
In conclusion, targeting Mets-related metabolic
abnormalities, particularly the activation of the renin-angiotensin
system and subsequent induction of oxidative stress and
inflammation, may be an effective strategy to prevent the
development of CRC in patients with Mets. Renin-angiotensin system
inhibitors, including ACE inhibitors, appear to be potentially
effective and viable candidates for this purpose since these agents
reduce oxidative stress while also attenuating chronic
inflammation.
References
|
1
|
Ahmed RL, Schmitz KH, Anderson KE,
Rosamond WD and Folsom AR: The metabolic syndrome and risk of
incident colorectal cancer. Cancer. 107:28–36. 2006.
|
|
2
|
Stocks T, Van Hemelrijck M, Manjer J, et
al: Blood pressure and risk of cancer incidence and mortality in
the Metabolic Syndrome and Cancer Project. Hypertension.
59:802–810. 2012.
|
|
3
|
Ishino K, Mutoh M, Totsuka Y and Nakagama
H: Metabolic syndrome: A novel high-risk state for colorectal
cancer. Cancer Lett. 2012.
|
|
4
|
Shimizu M, Kubota M, Tanaka T and Moriwaki
H: Nutraceutical approach for preventing obesity-related colorectal
and liver carcinogenesis. Int J Mol Sci. 13:579–595. 2012.
|
|
5
|
Donohoe CL, Pidgeon GP, Lysaght J and
Reynolds JV: Obesity and gastrointestinal cancer. Br J Surg.
97:628–642. 2010.
|
|
6
|
Shimizu M, Shirakami Y, Iwasa J, et al:
Supplementation with branched-chain amino acids inhibits
azoxymethane-induced colonic preneoplastic lesions in male
C57BL/KsJ-db/db mice. Clin Cancer Res. 15:3068–3075. 2009.
|
|
7
|
Shimizu M, Shirakami Y, Sakai H, et al:
(−)-Epigallocatechin gallate suppresses azoxymethane-induced
colonic premalignant lesions in male C57BL/KsJ-db/db mice. Cancer
Prev Res (Phila). 1:298–304. 2008.
|
|
8
|
Kubota M, Shimizu M, Sakai H, et al:
Preventive effects of curcumin on the development of
azoxymethane-induced colonic preneoplastic lesions in male
C57BL/KsJ-db/db obese mice. Nutr Cancer. 64:72–79. 2012.
|
|
9
|
Yasuda Y, Shimizu M, Shirakami Y, et al:
Pitavastatin inhibits azoxymethane-induced colonic preneoplastic
lesions in C57BL/KsJ-db/db obese mice. Cancer Sci. 101:1701–1707.
2010.
|
|
10
|
Kochi T, Shimizu M, Ohno T, et al:
Enhanced development of azoxymethane-induced colonic preneoplastic
lesions in hypertensive rats. Int J Mol Sci. 14:14700–14711.
2013.
|
|
11
|
de Kloet AD, Krause EG and Woods SC: The
renin angiotensin system and the metabolic syndrome. Physiol Behav.
100:525–534. 2010.
|
|
12
|
Chrysant SG, Chrysant GS, Chrysant C and
Shiraz M: The treatment of cardiovascular disease continuum: focus
on prevention and RAS blockade. Curr Clin Pharmacol. 5:89–95.
2010.
|
|
13
|
Fyhrquist F and Saijonmaa O:
Renin-angiotensin system revisited. J Intern Med. 264:224–236.
2008.
|
|
14
|
Fox KM; EURopean trial On reduction of
cardiac events with Perindopril in stable coronary Artery disease
Investigators. Efficacy of perindopril in reduction of
cardiovascular events among patients with stable coronary artery
disease: randomised, double-blind, placebo-controlled, multicentre
trial (the EUROPA study). Lancet. 362:782–788. 2003.
|
|
15
|
van Vark LC, Bertrand M, Akkerhuis KM, et
al: Angiotensin-converting enzyme inhibitors reduce mortality in
hypertension: a meta-analysis of randomized clinical trials of
renin-angiotensin-aldosterone system inhibitors involving 158,998
patients. Eur Heart J. 33:2088–2097. 2012.
|
|
16
|
Deshayes F and Nahmias C: Angiotensin
receptors: a new role in cancer? Trends Endocrinol Metab.
16:293–299. 2005.
|
|
17
|
George AJ, Thomas WG and Hannan RD: The
renin-angiotensin system and cancer: old dog, new tricks. Nat Rev
Cancer. 10:745–759. 2010.
|
|
18
|
Ager EI, Neo J and Christophi C: The
renin-angiotensin system and malignancy. Carcinogenesis.
29:1675–1684. 2008.
|
|
19
|
Bernardi S, Zennaro C, Palmisano S, et al:
Characterization and significance of ACE2 and Mas receptor in human
colon adenocarcinoma. J Renin Angiotensin Aldosterone Syst.
13:202–209. 2012.
|
|
20
|
Hiraoka-Yamamoto J, Nara Y, Yasui N, et
al: Establishment of a new animal model of metabolic syndrome:
SHRSP fatty (fa/fa) rats. Clin Exp Pharmacol Physiol. 31:107–109.
2004.
|
|
21
|
Ueno T, Takagi H, Fukuda N, et al:
Cardiovascular remodeling and metabolic abnormalities in SHRSP.
Z-Lepr(fa)/IzmDmcr rats as a new model of metabolic syndrome.
Hypertens Res. 31:1021–1031. 2008.
|
|
22
|
Kubota M, Shimizu M, Sakai H, et al:
Renin-angiotensin system inhibitors suppress azoxymethane-induced
colonic preneoplastic lesions in C57BL/KsJ-db/db obese mice.
Biochem Biophys Res Commun. 410:108–113. 2011.
|
|
23
|
Ogawa K, Hara T, Shimizu M, et al:
Suppression of azoxymethane-induced colonic preneoplastic lesions
in rats by 1-methyltryptophan, an inhibitor of indoleamine
2,3-dioxygenase. Cancer Sci. 103:951–958. 2012.
|
|
24
|
Suzuki Y, Imai K, Takai K, et al:
Hepatocellular carcinoma patients with increased oxidative stress
levels are prone to recurrence after curative treatment: A
prospective case series study using the d-ROM test. J Cancer Res
Clin Oncol. in press. 2013.
|
|
25
|
Tamarat R, Silvestre JS, Durie M and Levy
BI: Angiotensin II angiogenic effect in vivo involves vascular
endothelial growth factor- and inflammation-related pathways. Lab
Invest. 82:747–756. 2002.
|
|
26
|
Chen H, Sullivan G, Yue LQ, Katz A and
Quon MJ: QUICKI is a useful index of insulin sensitivity in
subjects with hypertension. Am J Physiol Endocrinol Metab.
284:E804–812. 2003.
|
|
27
|
Shimomoto T, Ohmori H, Luo Y, et al:
Diabetes-associated angiotensin activation enhances liver
metastasis of colon cancer. Clin Exp Metastasis. 29:915–925.
2012.
|
|
28
|
Kedika R, Patel M, Pena Sahdala HN, et al:
Long-term use of angiotensin converting enzyme inhibitors is
associated with decreased incidence of advanced adenomatous colon
polyps. J Clin Gastroenterol. 45:e12–16. 2011.
|
|
29
|
Cassis P, Conti S, Remuzzi G and Benigni
A: Angiotensin receptors as determinants of life span. Pflugers
Arch. 459:325–332. 2010.
|
|
30
|
Tudek B and Speina E: Oxidatively damaged
DNA and its repair in colon carcinogenesis. Mutat Res. 736:82–92.
2012.
|
|
31
|
Valavanidis A, Vlachogianni T and Fiotakis
C: 8-hydroxy-2′-deoxyguanosine (8-OHdG): A critical biomarker of
oxidative stress and carcinogenesis. J Environ Sci Health C Environ
Carcinog Ecotoxicol Rev. 27:120–139. 2009.
|
|
32
|
Uemura H, Ishiguro H, Ishiguro Y, Hoshino
K, Takahashi S and Kubota Y: Angiotensin II induces oxidative
stress in prostate cancer. Mol Cancer Res. 6:250–258. 2008.
|
|
33
|
Smith GR and Missailidis S: Cancer,
inflammation and the AT1 and AT2 receptors. J Inflamm (Lond).
1:32004.
|
|
34
|
Yvan-Charvet L, Massiera F, Lamande N, et
al: Deficiency of angiotensin type 2 receptor rescues obesity but
not hypertension induced by overexpression of angiotensinogen in
adipose tissue. Endocrinology. 150:1421–1428. 2009.
|