Introduction
The class, Chilopoda contains ~3,500 described
species of centipedes, distributed among five living orders, which
are predators characterized by a dorsoventrally flattened body
bearing ≥15 pairs of legs; one pair per trunk segment (1). Centipedes are predators that use venom
to primarily arrest or subdue their prey and have been used,
particularly Scolopendra subspinipes mutilans (S.
subspinipes mutilans), in Eastern medicine to treat a variety
of conditions, including spasms, childhood convulsions, seizures,
poisonous nodules and diphtheria (2,3). Venom
is a key element in the predatory behavior of centipedes, however,
analysis of such has only been performed on large scolopendromorphs
known to have significant importance in medical treatment. The
composition and structure of centipede venom remains largely
unknown, however, previous studies have revealed that serotonin,
histamine, lipids, polysaccharides and polypeptides have been
identified in the crude extracts of centipede venom glands
(4,5). The ethanol extract of S.
subspinipes mutilans has also been reported to exhibit marked
cytotoxic activity against human cancer cells (2).
Cell cycle deregulation, resulting in uncontrolled
cell proliferation, is one of the most common alterations that
occurs during tumor development. Furthermore, cell cycle arrest is
considered to be an effective strategy for eliminating cancer cells
(6). Two major checkpoints, one at
the G1/S transition and one at the G2/M transition, regulate the
cell cycle and, therefore, the modulated expression of cell cycle
regulatory molecules on antiproliferation or apoptosis has been
investigated in numerous cell types (7). A general critical event associated
with DNA damage is the activation of cell cycle checkpoints, and
cyclins and cyclin-dependent kinases (cdks) are evolutionarily
conserved proteins that are essential for cell cycle control
(8). Distinct pairs of cyclins and
cdks regulate the progression through the various stages of the
cell cycle; cdk activity is regulated by cyclins, which bind to and
activate cdks (9). The present
study investigated whether AECS-induced antiproliferation or
apoptosis are associated with an uncontrolled cell cycle.
Apoptosis, which effectively reduces the size of
tumors and prevents further tumor growth, is a predominant type of
cell death, which is characterized by a series of stereotypic
molecular features, including the expression and translocation of
the Bcl-2 family proteins, release of cytochrome c and
activation of caspases (7). The
human Bcl-2 homologs comprise the major apoptosis regulatory gene
family and the Bcl-2 family of proteins may be divided into two
groups; apoptosis suppressors (including Bcl-2, Bcl-xl and Mcl-1)
and apoptosis activators (including Bax, Bak, Bid and Bad)
(10). A variety of theories
regarding the mechanism of action of the Bcl-2 family have been
presented and the accumulating data indicates that these proteins
function at numerous stages of the signaling cascade, which results
in apoptosis (11).
Therefore, the present study evaluated the antitumor
activity of the alcohol extracts of the centipede S. subspinipes
mutilans (AECS) and investigated the mechanism of AECS inducing
cell cycle arrest and apoptosis, for use in cancer treatment.
Materials and methods
Chemicals and reagents
Adult specimens of the centipede S. subspinipes
mutilans were purchased from LaoBaiXing Pharmacy (Xi’an, China)
and identification of the specimens was performed at the
Pharmacology Laboratory, Xi’an Jiaotong University (Xi’an, China)
where a voucher specimen was deposited. RPMI-1640 medium, dimethyl
sulfoxide (DMSO) and trypsin were purchased from Sigma-Aldrich (St.
Louis, MO, USA) and 3-(4,
5-dimethylthiazol-2-yl)-2.5-diphenyl-2H-tetrazolium bromide (MTT)
was purchased from Nanjing Sunshine Biotechnology Ltd. (Nanjing,
China). The Annexin V-fluorescein isothiocyanate (FITC) apoptosis
detection and Hoechst 33258 staining kits were purchased from
Beyotime Institute of Biotechnology (Shanghai, China). RNase and
propidium iodide (PI) were purchased from Sigma-Aldrich, and
protease inhibitor and phosphatase inhibitor cocktails were
purchased from Roche Technology (Basel, Switzerland). The
anti-CDC2, -CCNB1, -cyclin D1, -cyclin E, -Bad, -Bak, -Bax, -Mcl-1
and -Bcl-2 antibodies were purchased from Cell Signaling
Technology, Inc. (Danvers, MA, USA). The rabbit anti-GAPDH was
purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA)
and rabbit anti-mouse immunoglobulin G, bicinchoninic acid protein
assay reagent kit and SuperSignal® West Pico
Chemiluminescent substrate were all purchased from Pierce
Biotechnology, Inc (Rockford, IL, USA).
Cell culture
Human A375 melanoma cells, obtained from the
Shanghai Institute of Cell Biology in the Chinese Academy of
Sciences, were maintained in RPMI-1640 and supplemented with 10%
(v/v) fetal bovine serum (FBS) at 37°C in a 5% CO2
incubator with saturated humidity.
Centipede S. genus extract. The centipede
S. subspinipes mutilans was shattered into a fine powder and
50 g of the centipede S. subspinipes mutilans was decocted
in 1,500 ml ethanol solution [3/2 (v/v); ethanol/water] for 1 h.
The solution was filtered and the filtrate was collected. The
filtered residue was subsequently added to 750 ml ethanol solution
[3/2 (v/v); ethanol/water] and the above steps were repeated. The
collected filtrates were merged and filtered again. Finally, the
extract was concentrated under a rotary evaporator (RE-5220,
Shanghai Beilun Equipment Co., Ltd., Shanghai, China) (12).
Cell proliferation assay
The effects of AECS on cell viability were evaluated
by MTT assay. The exponentially growing A375 cells were plated in
96-well plates (Costar, Corning, NY, USA) at a density of
2×104 cells/well in RPMI-1640 complete medium and
following 24 h, the cells were treated with AECS at various
concentrations for 24, 48 and 72 h. Fresh cell culture medium
containing 10% FBS and 20 μl MTT solution (5 mg/ml) was added to
each well and incubated for an additional 4 h at 37°C. Next, the
medium was removed and 150 μl DMSO was added to each well. The
absorbance was recorded at a wavelength of 490 nm using a
microplate reader (Bio-Rad, Hercules, CA, USA) and the inhibition
ratio was calculated.
Cell cycle assay
For cell cycle analysis, A375 cells were treated
with AECS at various concentrations for 48 h. Following treatment,
the cells were trypsinized and fixed in ice-cold 70% ethanol
overnight at 4°C, washed with phosphate-buffered saline (PBS) and
stained with RNase and PI for 30 min in the dark. The cell cycle
was analyzed by flow cytometry (BD FACSCalibur, Becton-Dickinson,
Franklin Lakes, NJ, USA).
Hoechst staining assay
The A375 cells were treated with various
concentrations of AECS in 6-well plates for 48 h and incubated with
Hoechst 33258 stain for 10 min at 37°C according to the
manufacturer’s instructions. The cells were examined under a
fluorescence microscope (DM505, Nikon Co., Ltd., Otawara, Tochigi,
Japan).
Flow cytometric analysis of
apoptosis
The A375 cells were treated with various
concentrations of AECS for 48 h, collected, washed and resuspended
in PBS. The apoptotic cell death rate was examined by Annexin
V-FITC and PI double staining using the Annexin V-FITC apoptosis
detection kit, according to the manufacturer’s instructions.
Following the staining of cells with Annexin V-FITC/PI, flow
cytometry was performed and the results were analyzed using
CellQuest software (BD Biosciences, Franklin Lakes, NJ, USA).
Western blot analysis
The cells were harvested and lysed in
radioimmunoprecipitation assay lysis buffer, supplemented with
protease inhibitor and phosphatase inhibitor cocktail tablets. The
cell lysates were centrifuged (TGL-16B, Shanghai Anting Scientific
Instrument Factory, Shanghai, China) at 12,000 × g at 4°C for 10
min. Equivalent amounts of protein were subsequently resolved by
10% SDS-PAGE and transferred to polyvinylidene fluoride membranes
(Millipore, Billerica, MA, USA). The membranes were blocked with
Tris-buffered saline containing 0.05% Tween-20 (TBST) and 5%
non-fat powdered milk for 2 h, followed by blocking with a
solution, which contained the primary antibody (1:1,000 dilution)
overnight at 4°C. Following three washes with TBST for 10 min, the
blot was incubated with the secondary antibody (1:20,000 dilution)
and washed three times with TBST prior to exposure to the
SuperSignal® West Dura Extended Duration substrate. The
band intensity was quantified by densitometric analysis using an
image quantitative analysis system (Image-Pro Plus 5.1, Media
Cybernetics, Inc., Rockville, MD, USA).
Statistical analysis
Data are presented as the mean ± standard error of
the mean and statistical analysis was performed using analysis of
variance. P<0.05 was considered to indicate a statistically
significant difference.
Results
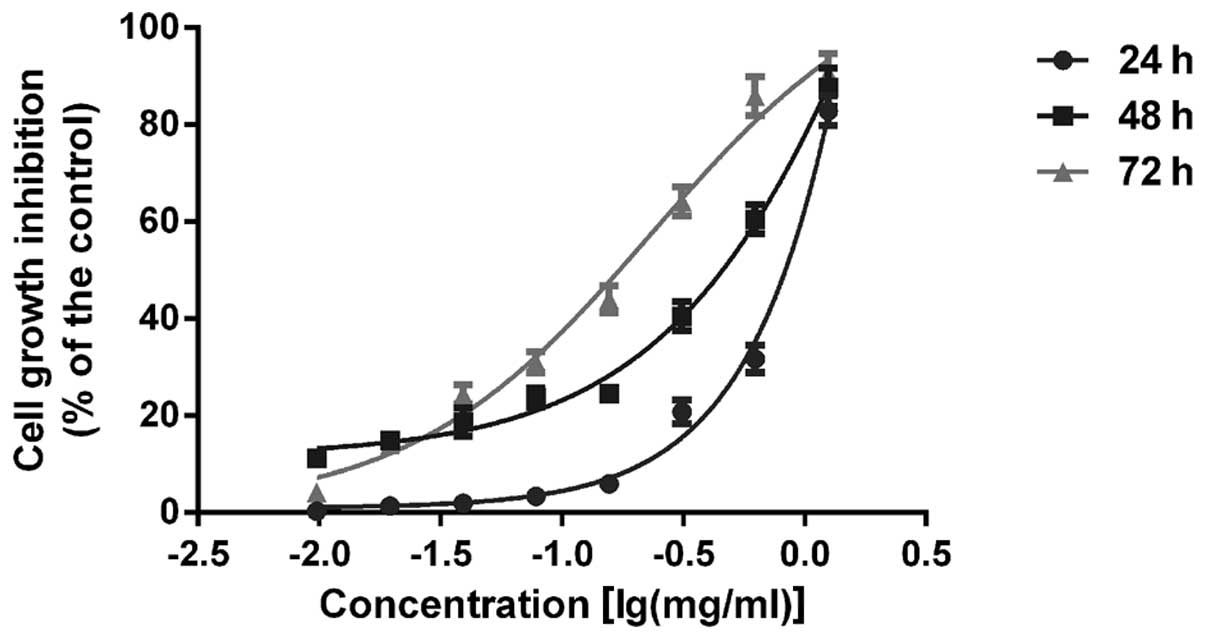
AECS suppresses A375 cell growth
To assess the effects of AECS on cell growth, A375
cells were treated with AECS at concentrations of 0.01, 0.02, 0.04,
0.08, 0.16, 0.31, 0.63 and 1.25 mg/ml. AECS was found to inhibit
the growth of A375 cells in a dose- and time-dependent manner by
MTT assay (Fig. 1). In addition,
the 50% growth inhibitory concentrations of AECS in A375 cells were
0.77, 0.29 and 0.15 mg/ml for 24, 48 and 72 h, respectively.
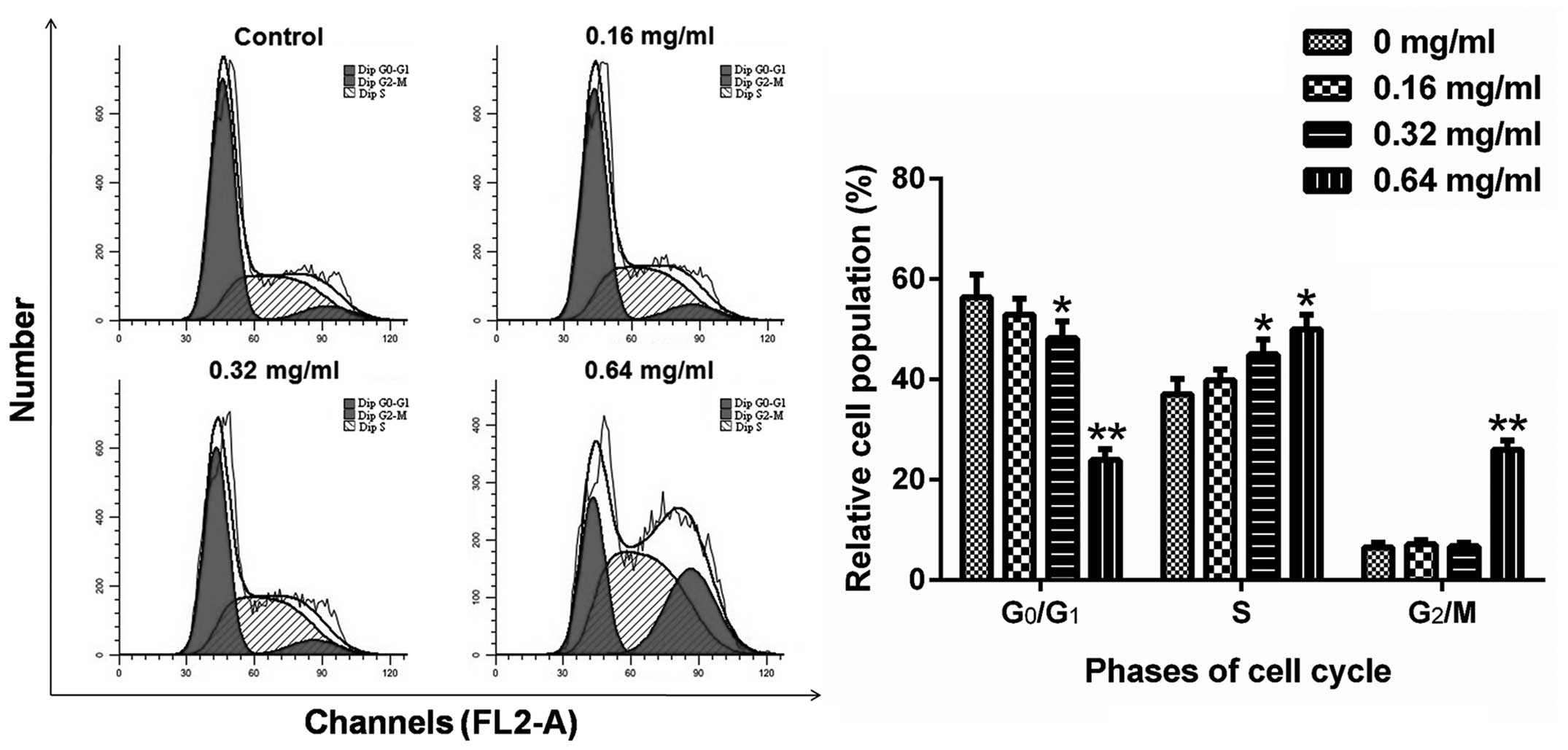
AECS induces A375 cell S-phase
arrest
To further investigate the effects of AECS on the
cell cycle, the cell cycle profiles of A375 cells were analyzed
using flow cytometry. The cells were treated with AECS at
concentrations of 0, 0.16, 0.32 and 0.64 mg/ml for 48 h and stained
with PI. Next, the cells were analyzed by flow cytometry to detect
their DNA content. AECS treatment resulted in a significant
increase in the percentage of cells in the S phase and a
significant decrease in the percentage of cells in the G0/G1 phase
(Fig. 2). The percentage of cells
accumulated in the S phase was 37.07, 39.85, 45.00 and 49.96%
following treatment with AECS concentrations of 0, 0.16, 0.32 and
0.64 mg/ml, respectively. The accumulation of G0/G1 phase cells was
maximal in the control group and declined with increasing
concentrations. The decrease in the number of G0/G1 phase cells was
56.37, 52.98, 48.26 and 23.99% with AECS concentrations of 0, 0.16,
0.32 and 0.64 mg/ml, respectively. These results indicated that
AECS mediates A375 cell growth by inducing partial S phase cell
cycle arrest.
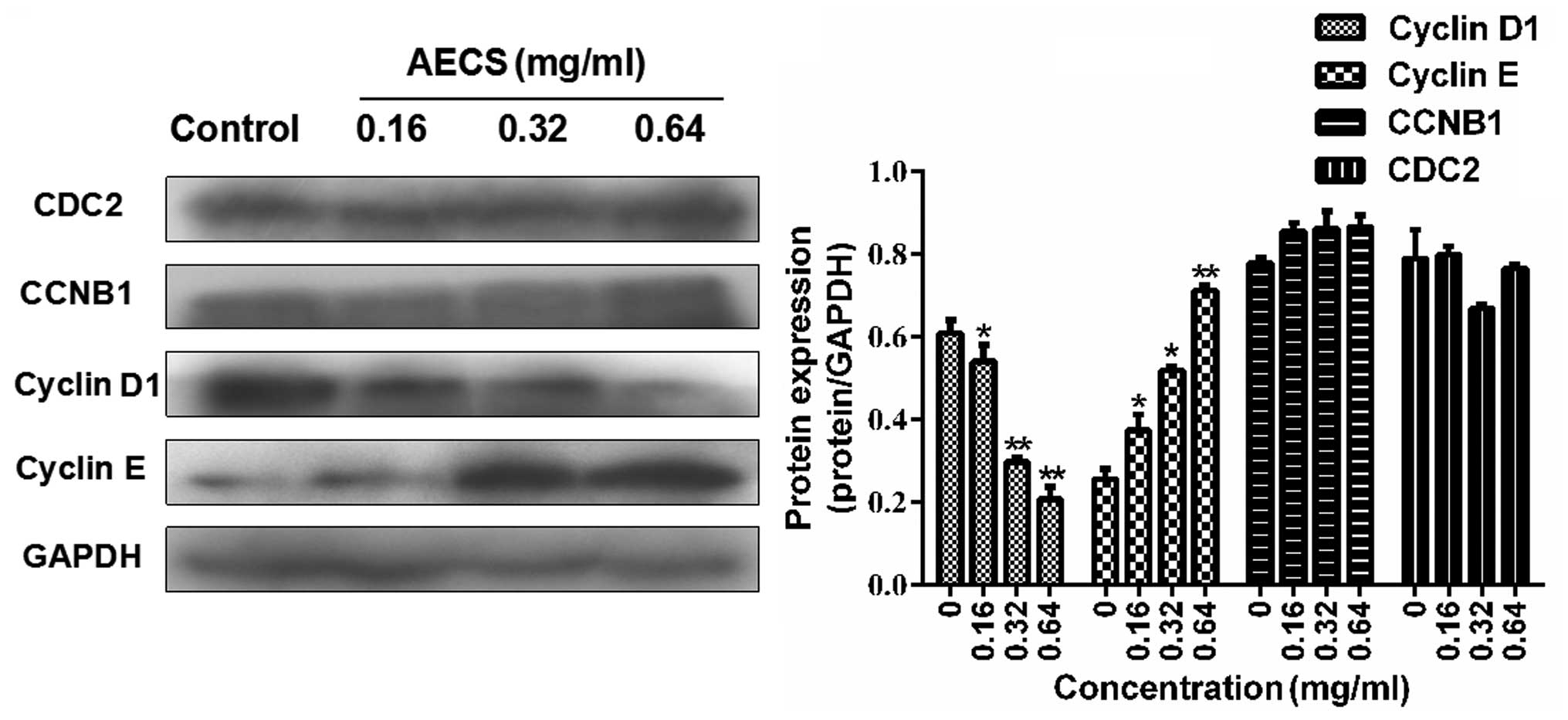
Effects of AECS on cell cycle regulatory
molecules
Since AECS-induced S phase arrest was observed in
the A375 cells following treatment with AECS for 48 h, the
expression of cell cycle regulatory protein molecules was detected
during treatment with AECS for 48 h. AECS did not affect the levels
of CDC2 and CCNB1 (Fig. 3),
however, treatment with AECS resulted in a subsequent increase in
cyclin E expression and a significant decrease in cyclin D1
expression in a dose-dependent manner (Fig. 3). These results indicated that the
cell cycle regulatory molecules are involved in AECS-induced
changes in cell cycle progression.
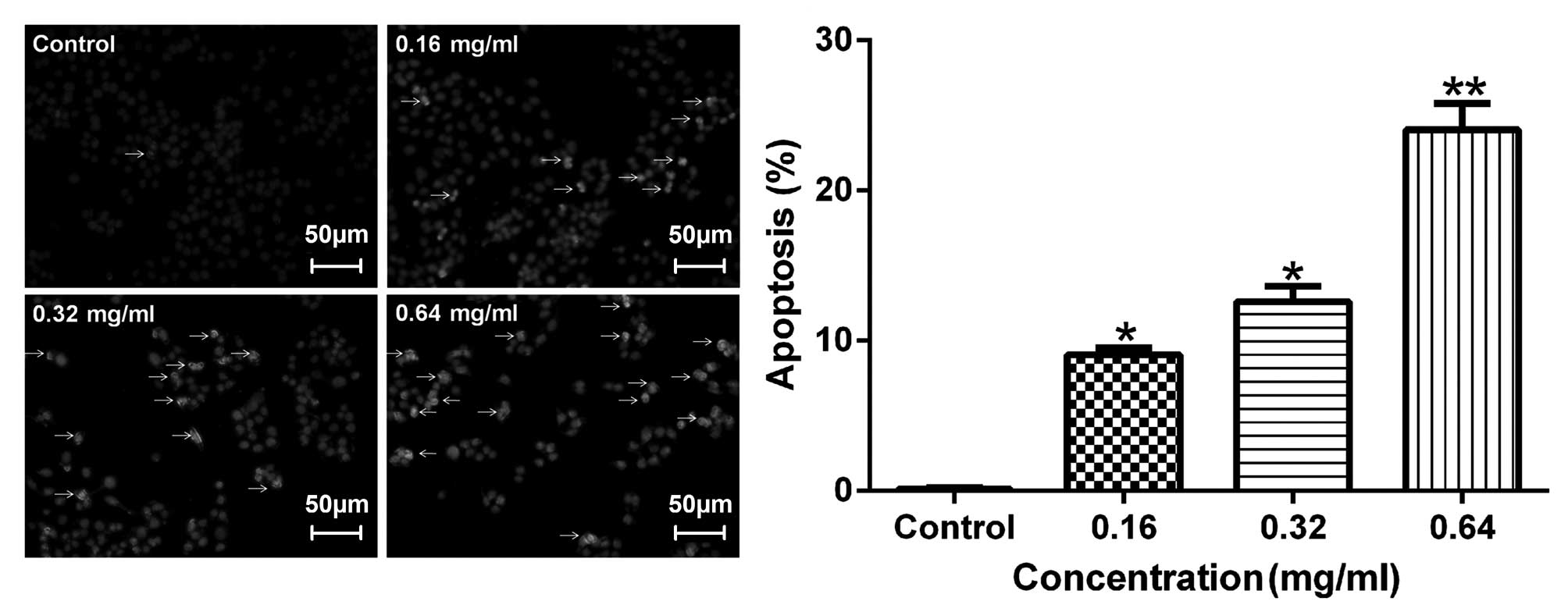
Effects of AECS on apoptosis
To detect apoptotic changes induced by AECS, the
A375 cells were incubated with Hoechst 33258 dye, which is commonly
used to stain genomic DNA. The Hoechst staining of the A375 cells
(Fig. 4) revealed that AECS
treatment induced apoptotic events characteristic of chromatin
condensation. Microscopic observation (Fig. 4) demonstrated typical morphology of
the apoptotic nuclei stained with Hoechst 33258, in which the
chromatin was observed to be condensed and aggregated at the
nuclear membrane, as indicated by a bright fluorescence at the
periphery.
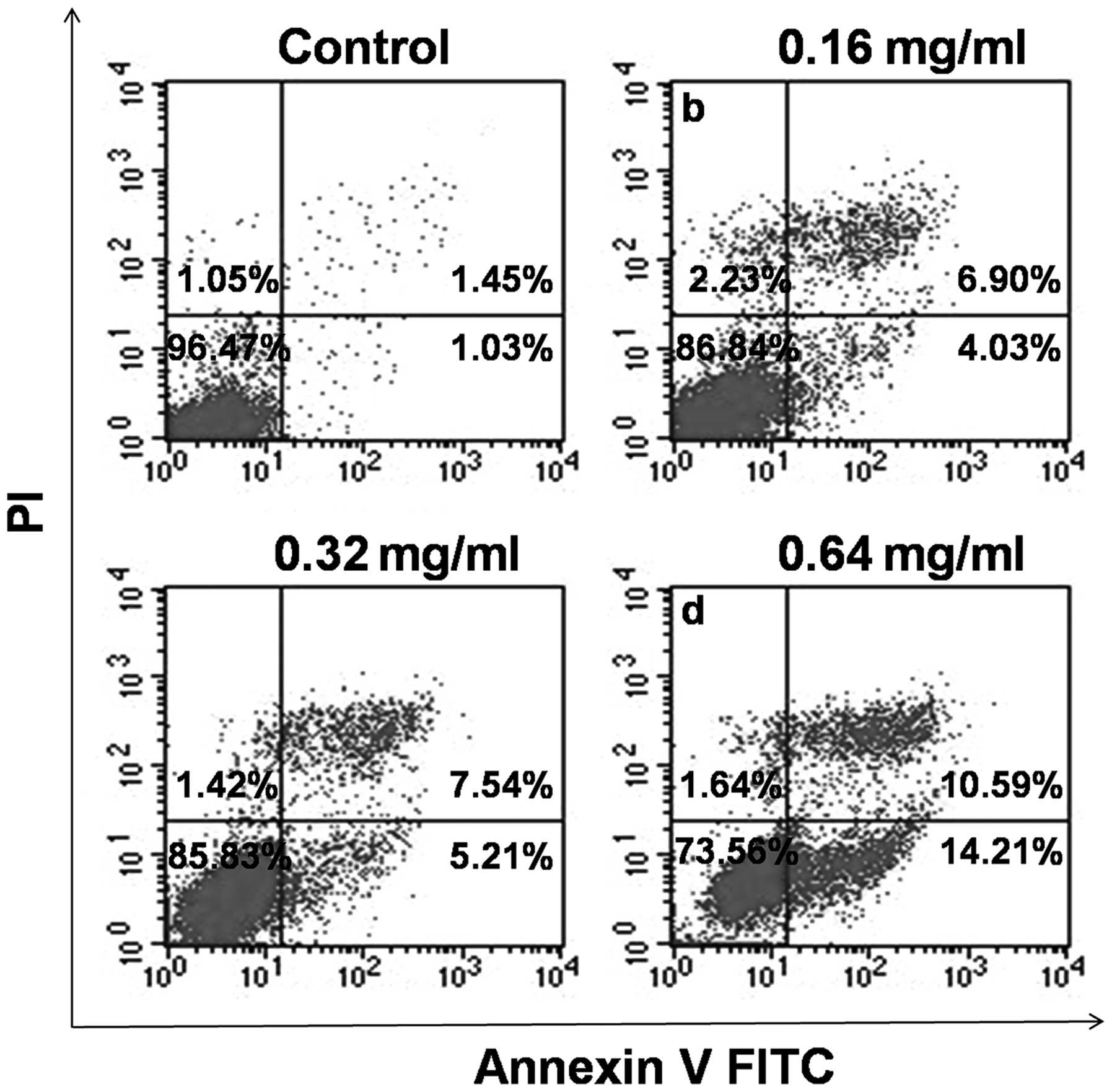
To verify the effect of AECS on cell apoptosis, the
treated cells were stained with Annexin V-FITC and PI and analyzed
by flow cytometry. As shown in Fig.
5, the A375 cells treated with AECS demonstrated a significant
increase in the early- and late-stage apoptotic fractions in a
dose-dependent manner, which indicated that the cell growth
suppression by AECS was partly due to increased apoptosis. The
percentage of apoptotic cells was 2.48, 10.93, 12.75 and 24.80% in
A375 cells following treatment with 0, 0.16, 0.32 and 0.64 mg/ml
AECS, respectively, for 48 h.
Effects of AECS on apoptosis regulatory
molecules
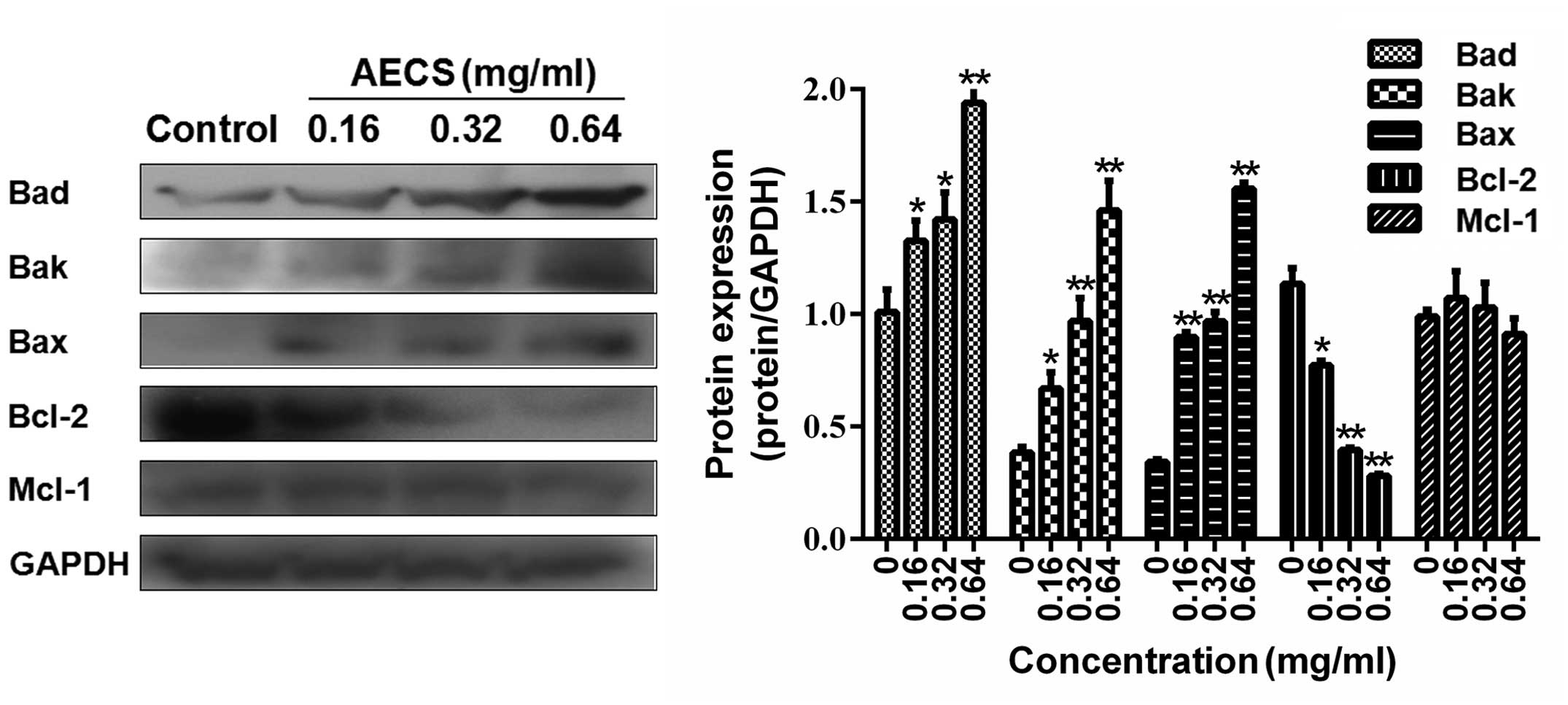
It was hypothesized that the AECS-induced apoptosis
observed may result from the effect on the Bcl-2 family members.
Therefore, the expression of the Bad, Bak, Bax, Bcl-2 and Mcl-1
proteins in the A375 cells treated with increasing concentrations
of AECS for 48 h were investigated by western blot analysis. AECS
did not affect the expression levels of Mcl-1 (Fig. 6), however, treatment with AECS
significantly decreased Bcl-2 expression and increased Bad, Bak and
Bax expression in a dose-dependent manner in the A375 cells
(Fig. 6).
Discussion
In the present study, it was demonstrated that AECS
was involved in inhibiting A375 cell proliferation via the
induction of cell cycle arrest and apoptosis. In addition, the
effect of AECS on A375 cell growth was investigated using an MTT
assay. The results revealed that AECS exhibits a significant
inhibition of A375 cell growth in a dose- and time-dependent
manner. As cell cycle arrest and apoptosis represent two effective
mechanisms involved in the induction of cell death (13), further investigation of the effect
of AECS on cell cycle arrest and apoptosis was performed in the
present study, in addition, the associated underlying molecular
mechanism of AECS-induced cell cycle arrest and apoptosis in A375
cells was investigated.
Eukaryotic cell proliferation is primarily regulated
by the cell cycle, which consists of four phases: The S phase, DNA
synthesis phase; the M phase, mitosis; the G1 phase, prophase DNA
synthesis; and the G2 phase, anaphase DNA synthesis (14). Furthermore, it is well established
that the loss of key cell cycle checkpoints is a hallmark of cancer
cells, which leads to abnormal proliferation and facilitates
oncogenic transformation (15). The
G1/S transition is one of the two predominant checkpoints of the
cell cycle, and is responsible for the initiation and completion of
DNA replication. The majority of studies have reported perturbation
of the S/G2 phase transition with a decrease of cells in the G0/G1
phase of the cell cycle and an increase of cells in the S phase
(15,16). In the present study, FACS analysis
with PI staining revealed that the percentage proportion was
increased in the S phase cells and reduced in the G0/G1 phase cells
following AECS treatment in a dose-dependent manner, indicating
that the inhibitory effect of AECS on A375 cell proliferation is
mediated by S phase cell cycle arrest.
Progression through the cell cycle is regulated by
the coordinated action of cdks and their associated regulatory
subunits, cyclins. In addition, studies have demonstrated that
progression through the G1/S transition is regulated by cyclin E
(17), which is expressed in late
G1, preceding cyclin A expression, with maximal expression observed
at the G1/S boundary. Cyclin E-cdk2 activity is maximal near the
G1/S boundary and is required for the G1 to S phase transition
(18). In the current study, the
expression of these important regulatory proteins was analyzed
following the treatment of A375 cells with AECS and the results
were consistent with previous observations that S phase arrest is
accompanied by the increased expression of cyclin E (19) and the decreased expression of cyclin
D1. The modifications of these cell cycle-associated proteins
induced by AECS appear to perturb the cell progression through the
S phase.
Clear evidence exists that tumor growth is a result
of uncontrolled proliferation and reduced apoptosis, thus, inducing
cancer cell apoptosis is a key strategy in anticancer therapy
(20). Inducing apoptosis
contributes to cancer treatment through various mechanisms,
including preventing growth-factor-independent cell survival,
inhibiting resistance to immune-based cytotoxicity and interfering
with the bypassing of cell cycle checkpoints (11). Thus, the current study performed
flow cytometric and Hoechst 33258 staining assays to observe the
apoptotic effects of AECS on A375 cells. It was revealed that AECS
treatment induces apoptotic events, which are characteristic of
chromatin condensation and significantly increase the apoptotic
fraction of A375 cells in a dose-dependent manner; this indicated
that the inhibitory effect on tumor cell proliferation by AECS was
partially due to the effect of inducing apoptosis.
To gain further insight into the mechanism of
AECS-induced cell apoptosis, its effect on the protein levels of
the Bcl-2 family was determined by western blot analysis. The
members of the Bcl-2 family have an important function in the
regulation of cell survival/apoptosis by serving as antiapoptotic
(for example Bcl-2 and Bcl-xl) or proapoptotic (such as Bax and
Bad) proteins. The balance between these two classes of proteins is
critical for determining whether a cell undergoes apoptosis
(21). Therefore, the current study
detected the protein expression of Bcl-2, Mcl-1, Bak, Bax and Bad
in A375 cells using western blot analysis, which revealed that AECS
induces the downregulation of Bcl-2 expression, correlating with
the upregulation of Bak, Bax and Bad expression. The results showed
that the apoptosis-inducing effect of AECS in A375 cells is
significantly associated with the effects on the Bcl-2 family and
that A375 cell apoptosis by AECS contributes to the inhibition of
cell growth.
In conclusion, the present study demonstrated that
AECS inhibits the growth of A375 cells by arresting the cell cycle
at the S phase and inducing cell apoptosis. Therefore, the use of
AECS may present a potential strategy for the treatment of human
melanoma cancer.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81370088 and
81227802), the Fundamental Research Funds for the Central
Universities, the Project of Shaanxi Star of Science and Technology
(grant no. 2012KJXX-06) and the Supporting Plan of Education
Ministry’s New Century Excellent Talents (grant no.
NCET-13-0467).
References
|
1
|
Dugon MM and Arthur W: Prey orientation
and the role of venom availability in the predatory behaviour of
the centipede Scolopendra subspinipes mutilans (Arthropoda:
Chilopoda). J Insect Physiol. 58:874–880. 2012.
|
|
2
|
Moon SS, Cho N, Shin J, Seo Y, Lee CO and
Choi SU: Jineol, a cytotoxic alkaloid from the centipede
Scolopendra subspinipes. J Nat Prod. 59:777–779. 1996.
|
|
3
|
Undheim EA and King GF: On the venom
system of centipedes (Chilopoda), a neglected group of venomous
animals. Toxicon. 57:512–524. 2011.
|
|
4
|
Gomes A, Datta A, Sarangi B, Kar PK and
Lahiri SC: Isolation, purification and pharmacodynamics of a toxin
from the venom of the centipede Scolopendra subspinipes
dehaani Brandt. Indian J Exp Biol. 21:203–207. 1983.
|
|
5
|
Liu ZC, Zhang R, Zhao F, Chen ZM, Liu HW,
Wang YJ, Jiang P, Zhang Y, Wu Y, Ding JP, et al: Venomic and
transcriptomic analysis of centipede Scolopendra subspinipes
dehaani. J Proteome Res. 11:6197–6212. 2012.
|
|
6
|
Cho JH, Lee JG, Yang YI, Kim JH, Ahn JH,
Baek NI, Lee KT and Choi JH: Eupatilin, a dietary flavonoid,
induces G2/M cell cycle arrest in human endometrial cancer cells.
Food Chem Toxicol. 49:1737–1744. 2011.
|
|
7
|
Lee HZ, Leung HW, Lai MY and Wu CH:
Baicalein induced cell cycle arrest and apoptosis in human lung
squamous carcinoma CH27 cells. Anticancer Res. 25:959–964.
2005.
|
|
8
|
He G, Kuang J, Khokhar AR and Siddik ZH:
The impact of S- and G2-checkpoint response on the fidelity of
G1-arrest by cisplatin and its comparison to a non-cross-resistant
platinum (IV) analog. Gynecol Oncol. 122:402–409. 2011.
|
|
9
|
Morgan DO: Principles of CDK regulation.
Nature. 374:131–134. 1995.
|
|
10
|
Nomura M, Shimizu S, Ito T, Narita M,
Matsuda H and Tsujimoto Y: Apoptotic cytosol facilitates Bax
translocation to mitochondria that involves cytosolic factor
regulated by Bcl-2. Cancer Res. 59:5542–5548. 1999.
|
|
11
|
Tamm I, Schriever F and Dörken B:
Apoptosis: implications of basic research for clinical oncology.
Lancet Oncol. 2:33–42. 2001.
|
|
12
|
Liu X and Zhong D: Study on the mechanisms
of tumor inhibitory effect of centipede extraction on heterotopic
grafting hepatocellular carcinoma in nude mice. Zhong Guo Pu Tang
Wai Ke Za Zhi. 2:164–168. 2010.(In Chinese).
|
|
13
|
King KL and Cidlowski JA: Cell cycle
regulation and apoptosis. Annu Rev Physiol. 60:601–617. 1998.
|
|
14
|
Lin W, Zheng L, Zhuang Q, Shen A, Liu L,
Chen Y, Sferra TJ and Peng J: Spica prunellae extract
inhibits the proliferation of human colon carcinoma cells via the
regulation of the cell cycle. Oncol Lett. 6:1123–1127. 2013.
|
|
15
|
Pan J, She M, Xu ZX, Sun L and Yeung SC:
Farnesyltransferase inhibitors induce DNA damage via reactive
oxygen species in human cancer cells. Cancer Res. 65:3671–3681.
2005.
|
|
16
|
Wolter F, Akoglu B, Clausnitzer A and
Stein J: Downregulation of the cyclin D1/Cdk4 complex occurs during
resveratrol-induced cell cycle arrest in colon cancer cell lines. J
Nutr. 131:2197–2203. 2001.
|
|
17
|
Park JW, Choi YJ, Jang MA, Lee YS, Jun DY,
Suh SI, Baek WK, Suh MH, Jin IN and Kwon TK: Chemopreventive agent
resveratrol, a natural product derived from grapes, reversibly
inhibits progression through S and G2 phases of the cell cycle in
U937 cells. Cancer Lett. 163:43–49. 2001.
|
|
18
|
Longley DB, Ferguson PR, Boyer J, Latif T,
Lynch M, Maxwell P, Harkin DP and Johnston PG: Characterization of
a thymidylate synthase (TS)-inducible cell line: a model system for
studying sensitivity to TS- and non-TS-targeted chemotherapies.
Clin Cancer Res. 7:3533–3539. 2001.
|
|
19
|
Shreeram S, Sparks A, Lane DP and Blow JJ:
Cell type-specific responses of human cells to inhibition of
replication licensing. Oncogene. 21:6624–6632. 2002.
|
|
20
|
Zhou QM, Wang XF, Liu XJ, Zhang H, Lu YY,
Huang S and Su SB: Curcumin improves MMC-based chemotherapy by
simultaneously sensitising cancer cells to MMC and reducing
MMC-associated side-effects. Eur J Cancer. 47:2240–2247. 2011.
|
|
21
|
Rasul A, Ding C, Li X, Khan M, Yi F, Ali M
and Ma T: Dracorhodin perchlorate inhibits PI3K/AKT and NF-κβ
activation, up-regulates the expression of p53, and enhances
apoptosis. Apoptosis. 17:1104–1119. 2012.
|