Introduction
Intracranial capillary hemangiomas (ICHs) are rare
benign vascular tumors that may occur at birth or in early infancy
(1,2). Primary hemangiomas of the skull are
also rare, accounting for 0.2% of all benign tumors of the skull
and 0.7% of all osseous neoplasms (3). A total of three intraosseous ICH cases
have been published in the English literature to date (4–6). The
frontal and parietal bones have been reported to be the most common
sites of involvement in the skull, while temporal bone involvement
is extremely rare (7). Facial
paralysis and hemifacial spasms are common presentations for
intratemporal hemangiomas, however, auditory and vestibular
dysfunction may also result from these lesions (8). Intratemporal hemangiomas mimic other
more common skull base lesions, which makes them difficult to
diagnose pre-operatively (9). The
current study presents a case of a large capillary hemangioma of
the temporal bone with a dural tail sign. This case was believed to
be a meningioma pre-operatively due to a dural tail sign and the
lack of classical symptoms. Patient provided written informed
consent.
Case report
A 57-year-old female presented with pulsatile
tinnitus and episodic vertigo associated with a ten-year history of
intermittent faint headaches. There was no history of facial
twitching or weakness. There was no evidence of facial nerve
dysfunction upon physical examination. Pure tone audiometry
revealed a hearing level of 28 dB on average in the right ear and a
normal hearing level on the left. There was no history of trauma or
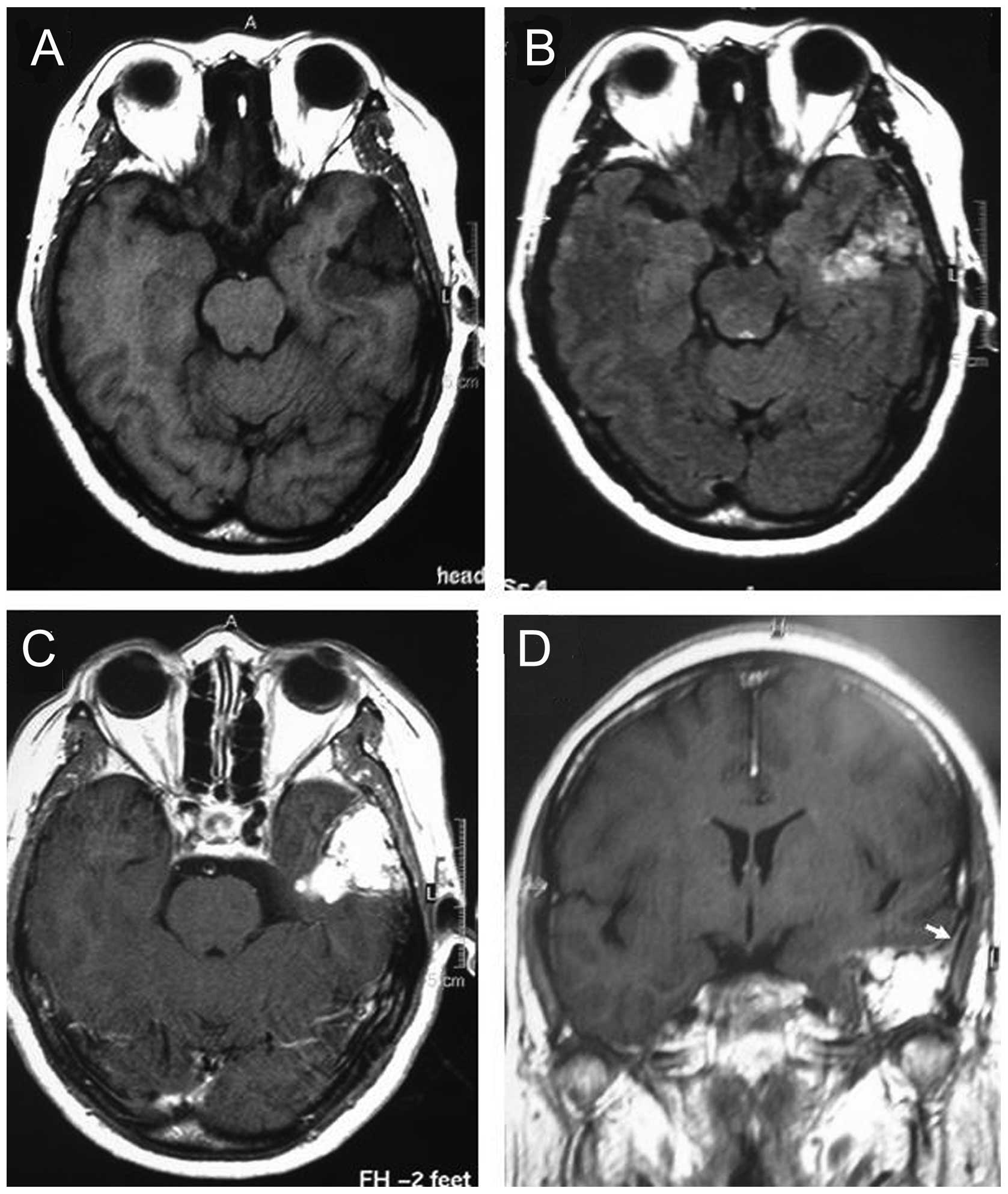
neurological disturbances. Gadolinium-enhanced magnetic resonance
imaging showed a mass measuring 42×36×35 mm in the right petrous
bone, which was hypointense on T1-weighted images and
heterogeneously hyperintense on T2-weighted images. Additionally, a
dural tail sign was shown following gadopentetate dimeglumine
administration (Fig. 1).
Surgery was performed using a modified pterional
approach. Abnormal vascular soft tissue was identified in the skull
base, with skull invasion and involvement of the dura. The invaded
temporal bone had cavernous blood-filled spaces within the bony
trabeculae. The adjacent dura mater was involved with thickening,
but remained intact. The tumor did not affect the bony walls of the
horizontal semicircular canal and the facial nerve canal. The
patient’s post-operative course was uneventful, and the tinnitus
and vertigo disappeared completely in the ensuing weeks. The
symptoms did not recur, even after one year of follow-up
examinations.
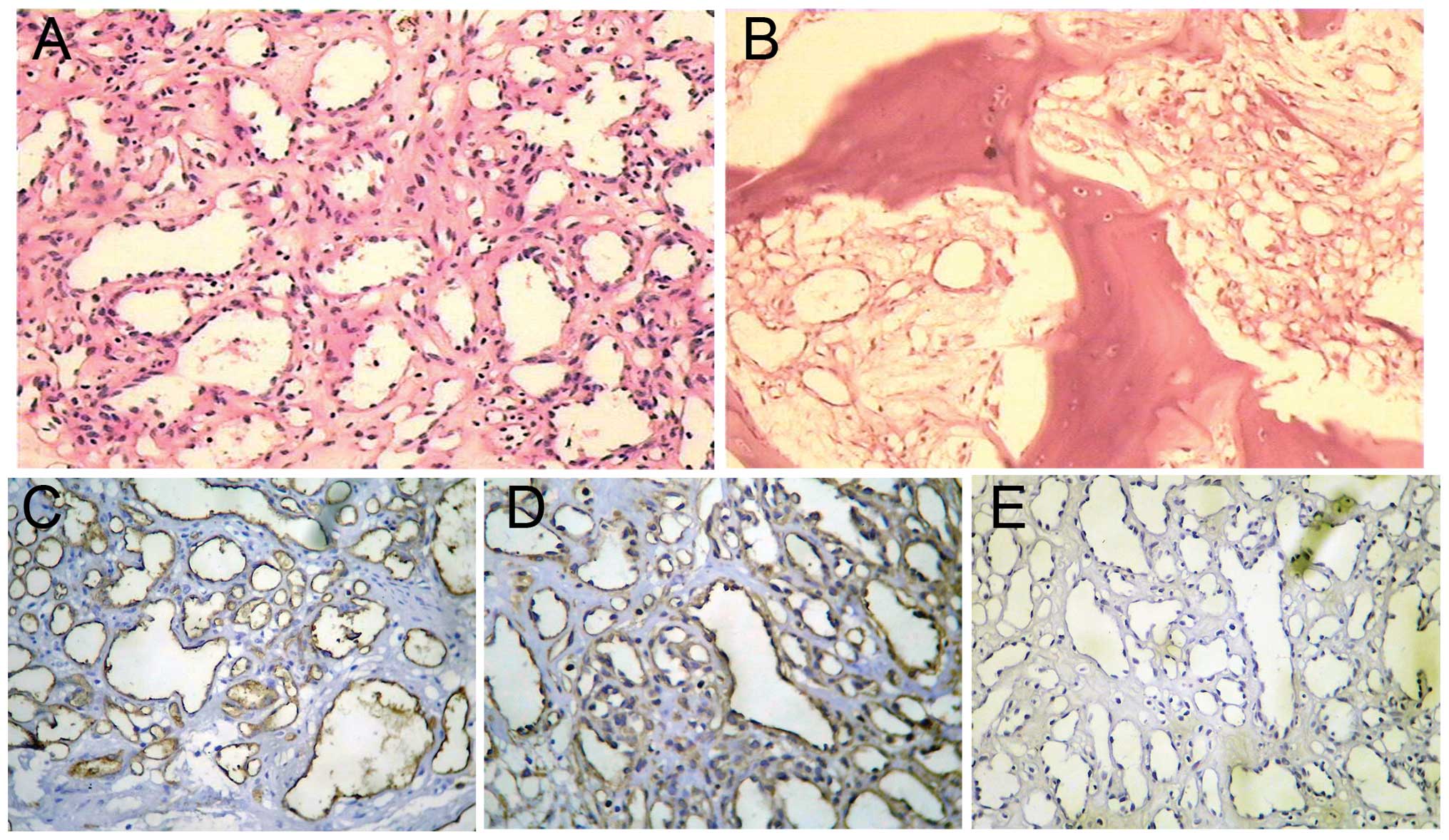
The histological examination revealed aggregates of
primarily capillary-sized microvessels in a vaguely lobular
arrangement, with diffusely fibrosed intrabecular spaces containing
numerous proliferated, partially small and dilated, thin-walled
blood vessels. Factor VIII, vimentin and cluster of differentiation
31 was detected in the blood vessel walls by immunohistochemistry,
while epithelial membrane antigen was not (Fig. 2). These findings were consistent
with an intraosseous capillary hemangioma.
Discussion
Intraosseous hemangioma most commonly affects the
vertebral column or skull (3,10), but
rarely involves the temporal bone. To the best of our knowledge,
the present study is the fourth case of intraosseous ICH to date.
The majority of the intratemporal vascular tumors reported in the
literature have been small (11–13),
although Fierek et al (7)
reported the case of a large 32-mm intratemporal hemangioma. In the
present study, the tumor size was larger than any other
intratemporal hemangiomas described in the previously published
literature.
Histopathology classifies intraosseous hemangiomas
into the venous, cavernous and capillary types (14,15).
Capillary-type hemangiomas are composed of densely packed loops of
fine vessels. Certain studies have described a mixed variety of
osseous hemangiomas that contain elements of each of the capillary
and cavernous types (16,17). The majority of intraosseous
hemangiomas arising from the skull base are cavernous, and only few
are capillary (16). Notably,
subsequent to reviewing the English-language literature, it may be
observed that, although intraosseous capillary hemangiomas are
rare, they most frequently affect the skull base (6,7,10,11,18,19),
in particular the geniculate ganglion and the fundus of the
internal auditory canal, possibly due to the rich vascular network
existing around the geniculate fossa and Scarpa’s ganglion
(7,16).
Intraosseous hemangiomas are benign tumors that are
slow growing and mostly asymptomatic (15). Hemangiomas can cause a variety of
symptoms depending on their location and size (14). The characteristic features of
intratemporal hemangiomas at the two most frequent sites of
occurrence, the geniculate ganglion and the internal auditory
canal, include facial nerve paralysis, hemifacial spasms and
auditory or vestibular symptoms (10,20,21).
Vascular tumors arising in the area of the geniculate ganglion most
commonly cause facial paralysis (8). The tumors involving the cochlear otic
capsule may cause pulsatile tinnitus or hearing loss (21,22).
In the present case, angiomatous erosion of the cochlea or
vestibular apparatus was not found, and the symptoms disappeared
gradually in the weeks following the surgery; the vertigo and
pulsatile tinnitus may have therefore been a result of the
compression of these structures.
In the present study, although the hemangioma
intruded into the brain tissue, the adjacent dural mater remained
intact, revealing no tumorous invasion of the nerve sheath. This is
consistent with other previous studies on hemangioma and indicates
a compression neuropathy rather than direct invasion (9,18).
More commonly however, hemangiomas produce an intense perineural
reaction that precludes the establishment of an oncologically sound
cleavage plane between the tumor and the affected nerve (16).
Hemangiomas of the temporal bone may mimic other
more common cranial base tumors, including acoustic tumors, facial
neuromas, meningiomas, cholesteatomas, glomus tumors and metastatic
tumors (9,16,23,24).
The pre-operative diagnosis of ICH is challenging, and angiography
may raise the possibility of a diagnosis (25). In the present study,
pre-operatively, meningioma was considered to be the most likely
diagnosis, as the lesion showed strong enhancement following
gadopentetate dimeglumine administration and had a dural tail sign.
However, a dural tail may also be present in association with other
intraaxial and extraaxial lesions. Although this sign was highly
indicative, it was not specific for the diagnosis of meningioma
(26). Direct tumor invasion or
reactive meningeal changes may cause the dural tail sign, and it is
present in neoplastic and non-neoplastic lesions (27,28).
Politi et al (27), also
described a patient with hemangioma of the frontal bone with a
dural tail sign. The case was similar to that of the present
patient as the hemangioma was large and the dura mater remained
intact. The dural tail may be therefore be attributed to the
proliferation of the connective tissue, hypervascularity or
vascular dilatation within the dura adjacent to the cranial
masses.
The treatment of ICH remains empirical (25). The majority of capillary hemangiomas
exhibit a self-limited course and spontaneously regress (1,2,29).
However, surgery remains an option for symptomatic ICH, and total
resection should be the goal (1).
As a complete resection is extremely difficult for hemangiomas of
the skull base, a successful excision requires the appropriate
surgical approach and technique (16). The preferred modality of treating
intratemporal hemangiomas is complete surgical excision, with
radiotherapy reserved for unresectable lesions (1,9).
Capillary hemangiomas are associated with a high recurrence rate of
43.5% following incomplete resection (1,21).
However, in the present patient, a repeat computed tomography scan
two years after the surgery revealed no recurrence.
In conclusion, the present study reports the fourth
case of intraosseous ICH, but the largest intratemporal hemangioma
thus far. ICH may be considered as a likely diagnosis when the
tumor involves the skull with a dural tail sign.
References
|
1
|
Morace R, Marongiu A, Vangelista T,
Galasso V, Colonnese C, Giangaspero F, Innocenzi G, et al:
Intracranial capillary hemangioma: a description of four cases.
World Neurosurg. 78:191.E15–191.E21. 2012.
|
|
2
|
Zheng SP, Ju Y and You C: Giant
intracranial capillary hemangioma in a 3-year-old child: case
report and literature review. Clin Neurol Neurosurg. 114:1270–1273.
2012.
|
|
3
|
Heckl S, Aschoff A and Kunze S: Cavernomas
of the skull: review of the literature 1975–2000. Neurosurg Rev.
25:56–62; discussion 66–57. 2002.
|
|
4
|
Shah ZK, Peh WC, Shek TW, Wong JW and
Chien EP: Hemangioendothelioma with an epithelioid phenotype
arising in hemangioma of the fibula. Skeletal Radiol. 34:750–754.
2005.
|
|
5
|
Frei-Jones M, McKinstry RC, Perry A,
Leonard JR, Park TS and Rubin JB: Use of thalidomide to diminish
growth velocity in a life-threatening congenital intracranial
hemangioma. J Neurosurg Pediatr. 2:125–129. 2008.
|
|
6
|
Suss RA, Kumar AJ, Dorfman HD, Miller NR
and Rosenbaum AE: Capillary hemangioma of the sphenoid bone.
Skeletal Radiol. 11:102–107. 1984.
|
|
7
|
Fierek O, Laskawi R and Kunze E: Large
intraosseous hemangioma of the temporal bone in a child. Ann Otol
Rhinol Laryngol. 113:394–398. 2004.
|
|
8
|
Friedman O, Neff BA, Willcox TO, Kenyon LC
and Sataloff RT: Temporal bone hemangiomas involving the facial
nerve. Otol Neurotol. 23:760–766. 2002.
|
|
9
|
Glasscock ME 3rd, Smith PG, Schwaber MK
and Nissen AJ: Clinical aspects of osseous hemangiomas of the skull
base. Laryngoscope. 94:869–873. 1984.
|
|
10
|
Mangham CA, Carberry JN and Brackmann DE:
Management of intratemporal vascular tumors. Laryngoscope.
91:867–876. 1981.
|
|
11
|
Lo WW, Horn KL, Carberry JN, Solti-Bohman
LG, Wade CT, Brackmann DD and Waluch V: Intratemporal vascular
tumors: evaluation with CT. Radiology. 159:181–185. 1986.
|
|
12
|
Lo WW, Shelton C, Waluch V, Solti-Bohman
LG, Carberry JN, Brackmann DE and Wade CT: Intratemporal vascular
tumors: detection with CT and MR imaging. Radiology. 171:445–448.
1989.
|
|
13
|
Martin N, Sterkers O and Nahum H:
Haemangioma of the petrous bone: MRI. Neuroradiology. 34:420–422.
1992.
|
|
14
|
Gottfried ON, Gluf WM and Schmidt MH:
Cavernous hemangioma of the skull presenting with subdural
hematoma. Case report. Neurosurg Focus. 17:ECP12004.
|
|
15
|
Reis BL, Carvalho GT, Sousa AA, Freitas WB
and Brandão RA: Primary hemangioma of the skull. Arq
Neuropsiquiatr. 66:569–571. 2008.
|
|
16
|
Liu JK, Burger PC, Harnsberger HR and
Couldwell WT: Primary intraosseous skull base cavernous hemangioma:
Case report. Skull Base. 13:219–228. 2003.
|
|
17
|
Tsao MN, Schwartz ML, Bernstein M,
Halliday WC, Lightstone AW, Hamilton MG, Jaywant S and Laperriere
N: Capillary hemangioma of the cavernous sinus. Report of two
cases. J Neurosurg. 98:169–174. 2003.
|
|
18
|
Eby TL, Fisch U and Makek MS: Facial nerve
management in temporal bone hemangiomas. Am J Otol. 13:223–232.
1992.
|
|
19
|
Hsueh PJ, Chen WY, Chiang YC and Lee FP:
Capillary hemangioma of the middle ear. Otolaryngol Head Neck Surg.
136:666–667. 2007.
|
|
20
|
Burton L, Burton EM, Welling DB, Marks SD
and Binet EF: Hemangioma of the temporal bone in a patient presumed
to have Ménière’s syndrome. South Med J. 90:736–739. 1997.
|
|
21
|
Tokyol C and Yilmaz MD: Middle ear
hemangioma: a case report. Am J Otolaryngol. 24:405–407. 2003.
|
|
22
|
Verret DJ, Spencer Cochran C, Defatta RJ
and Samy RN: External auditory canal hemangioma: case report. Skull
Base. 17:141–143. 2007.
|
|
23
|
Malde R, Moss T, Malcolm G, Whittlestone T
and Bahl A: Multiple intraosseous calvarial hemangiomas mimicking
metastasis from renal cell carcinoma. Adv Urol. 2008.DOI:
10.1155/2008/176392
|
|
24
|
Simon SL, Moonis G, Judkins AR, Scobie J,
Burnett MG, Riina HA and Judy KD: Intracranial capillary
hemangioma: case report and review of the literature. Surg Neurol.
64:154–159. 2005.
|
|
25
|
Mirza B, Shi WY, Phadke R, Holton JL,
Turner C, Plant GT, Brew S, et al: Strawberries on the brain -
intracranial capillary hemangioma: two case reports and systematic
literature review in children and adults. World Neurosurg.
900.e13–900.e21. 2013.
|
|
26
|
Rokni-Yazdi H, Azmoudeh Ardalan F,
Asadzandi Z, Sotoudeh H, Shakiba M, Adibi A, Ayatollahi H and
Rahmani M: Pathologic significance of the ‘dural tail sign’. Eur J
Radiol. 70:10–16. 2009.
|
|
27
|
Politi M, Romeike BF, Papanagiotou P,
Nabhan A, Struffert T, Feiden W and Reith W: Intraosseous
hemangioma of the skull with dural tail sign: radiologic features
with pathologic correlation. AJNR Am J Neuroradiol. 26:2049–2052.
2005.
|
|
28
|
Rokni-Yazdi H and Sotoudeh H: Prevalence
of ‘dural tail sign’ in patients with different intracranial
pathologies. Eur J Radiol. 60:42–45. 2006.
|
|
29
|
Phi JH, Kim SK, Cho A, Kim DG, Paek SH,
Park SH and Wang KC: Intracranial capillary hemangioma: extra-axial
tumorous lesions closely mimicking meningioma. J Neurooncol.
109:177–185. 2012.
|