Introduction
Gliomas originate from glial cells and are the most
common type of primary brain tumors accounting for 80% of all
malignant primary brain tumors (1).
According to the pathological and clinical criteria established by
the World Health Organization, gliomas are classified as grades
I–IV (2,3). Grade IV tumors, such as glioblastomas
(GBMs), are the most devastating and aggressive form comprising
>50% of gliomas, and have a poor prognosis (4). The current treatment standard of GBMs
is surgical resection to a feasible extent, followed by
radiotherapy and chemotherapy. Among the currently available
chemotherapy agents, temozolomide is the most popular; doctors and
patients favor it as it is administrated orally and efficiently
crosses the blood-brain barrier (BBB) (5). However, 67.2–76% of patients are
resistant to this agent and therefore do not benefit from it
(6). Regardless of systemic
therapeutic strategies, including surgery, temozolomide and
radiotherapy, patient median survival is only 14.6 months and the
five-year survival rate is ~9.8% (7,8). The
poor prognosis of glioma fuels the requirement for identifying
therapeutic agents with the merits of temozolomide, such as high
lipophilicity and strong anti-glioma activity.
Steroid hormones are generally divided into the
following five groups: Estrogens, androgens, progestogens,
glucocorticoids and mineralocorticoids (9,10). The
natural steroid hormones are predominantly synthesized from
cholesterol in the gonads and adrenal glands, and readily diffuse
through the cell membrane due to their lipophilic properties
(11,12). Accumulating evidence has indicated
that certain steroid hormones possess antitumor activities, such as
the 17β-estradiol metabolite, 2-methoxyestradiol (2ME), which
exerts the strongest activity. 2ME inhibits proliferation and
induces apoptosis of various types of cancer, including gliomas,
and breast and gastric cancer, independently of estrogen receptors
α and β (13–15). However, findings from a clinical
trial identified low oral bioavailability of 2ME, which prevents
the transfer of this promising agent from bench to bed side
(16). The example of 2ME implies
the application potential of steroid hormones and provides the
basis for the subsequent investigation of other endogenous steroid
hormones, such as pregnenolone (Fig.
1).
The present study aimed to investigate the
pharmacological effects of the endogenous steroid, pregnenolone, on
GBM cells, and the mechanisms underlying its pro-apoptotic activity
via the extrinsic and intrinsic apoptotic pathways. Pregnenolone
may be a leading compound in the treatment of gliomas and may be
modified and developed for clinical application.
Materials and methods
Antibodies and reagents
Antibodies against tubulin, B-cell lymphoma 2
(Bcl-2) and Fas ligand (L) were obtained from Cell Signaling
Technology, Inc. (Beverly, MA, USA). Bak and Bax antibodies were
purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA)
and cluster of differentiation (CD)95/Fas antibody was purchased
from Epitomics, Inc. (Burlingame, CA, USA). Pregnenolone, Hoechst
33342 and methyl-thiazolyl-tetrazolium (MTT) were purchased from
Sigma-Aldrich (St. Louis, MO, USA). The general caspase inhibitor,
Z-VAD-FMK, was obtained from EMD Millipore Corp. (Billerica, MA,
USA). The Caspase-Glo® 8, 9 and 3/7 Activity assay kits
were purchased from Promega Corp. (Madison, WI, USA).
Cell culture and drug treatment
The C6 rat glioma and U-87 MG and LN-18 human glioma
cell lines were obtained from the American Type Culture Collection
(Manassas, VA, USA). The cells were cultured in Dulbecco’s modified
Eagle’s medium (DMEM; Gibco, Grand Island, NY, USA) supplemented
with fetal bovine serum (FBS; Invitrogen Life Technologies,
Carlsbad, CA, USA), 100X MEM Non-Essential Amino Acid, GlutaMax™
(Gibco), penicillin (Gibco) and streptomycin (Gibco) in a
humidified atmosphere of 5% CO2 at 37°C. Pregnenolone
was dissolved in ethanol to obtain a 10-mM stock solution and
stored at −20°C. For the drug treatment, pregnenolone was diluted
in DMEM and added at different concentrations. Ethanol at
corresponding concentrations served as the vehicle control.
Cell viability and cytotoxicity
assays
Cell viability was determined via MTT assay. The
cells that were growing in logarithmic phase were seeded in 96-well
plates and treated with pregnenolone. MTT (10 μl; 0.5 mg/ml) was
added to each well, which was subsequently incubated at 37°C for 4
h to allow the yellow dye to transform into blue crystals. The
medium was removed and 100 μl dimethyl sulfoxide (DMSO;
Sigma-Aldrich) was added to each well to dissolve the dark blue
crystals. The optical density was measured with a microplate reader
(iMark™, Bio-Rad, Hercules, CA, USA) at 490 nm. Five replicates
were prepared for each condition.
Lactate dehydrogenase (LDH) release was quantified
with a CytoTox 96® Non-Radioactive Cytotoxicity assay
kit (Promega Corp.) according to the manufacturer’s instructions.
Plates were incubated with an LDH substrate at room temperature for
30 min in the dark and absorbance was measured at 490 nm with a
microplate reader (iMark™, Bio-Rad).
Caspase 3/7, 8 and 9 activities
assay
The glioma cells were seeded into 96-well plates at
3,000–5,000 cells/well and incubated for 24 h. The cells were
treated with pregnenolone at different concentrations. After 24 h
of treatment, the enzymatic activity of caspase 8, 9 and 3/7 was
determined using the Caspase-Glo® 8 and 9 Activity assay
kit and the Caspase-Glo® 3/7 Activity assay kit,
respectively, according to the manufacturer’s instructions.
Simultaneously, the cells were plated in 96-well plates and treated
with pregnenolone for 24 h. The relative cell number was measured
by MTT assay and evaluated as follows: Caspase activities = total
enzymatic activity values of caspase/cell number.
Hoechst 33342 staining and terminal
deoxynucleotidyl transferase dUTP nick end labeling (TUNEL)
assay
The glioma cells were stained with Hoechst 33342 (5
μg/ml) at 37°C for 20 min in the dark and photographed under a
fluorescent microscope (IX71, Olympus Corp., USA) with a 340-nm
excitation filter. Apoptotic cells were characterized as
demonstrating condensed nuclei and cell shrinkage. The proportion
of apoptotic cells in total cells was quantified in three randomly
selected microscopic fields.
For the TUNEL assay (Roche Diagnostics GmbH,
Mannheim, Germany), the glioma cells were fixed in 4%
paraformaldehyde solution at room temperature for 1 h. The cell
samples were detected via an In Situ Cell Death Detection
kit, TMR red (Roche Diagnostics GmbH) according to the
manufacturers’ instructions and analyzed under a fluorescent
microscope (Olympus Corp.) with a 540-nm excitation filter. A red
fluorescence signal was observed in the apoptotic cells.
Immunoblotting
Immunoblotting was performed as previously described
(17). Briefly, following cell
lysis and measurement of protein concentrations, the cells were
dissolved in sodium dodecyl sulfate (SDS) sample buffer (Beyotime
Institute of Biotechnology, Shanghai, China). Equal amounts of
protein were analyzed by SDS-PAGE on 12% polyacrylamide gels
(Bio-Rad) and the proteins were electroblotted onto polyvinylidene
fluoride membranes (Roche Diagnostics GmbH). The membranes were
incubated in 5% non-fat dry milk in Tris-buffered saline
(NaCl/Tris; Boster Biological Engineering Co., Ltd., Wuhan, China)
containing 0.1% Tween-20 (Sigma-Aldrich) for 1 h at room
temperature overnight at 4°C, and subsequently incubated with
primary antibodies. Following incubation with a horseradish
peroxidase-labeled secondary antibody, protein exposure was
achieved with a molecular imager (ChemiDOC™ XRS+, Bio-Rad).
Small interfering (si)RNA-mediated
knockdown of Fas expression
Fas-siRNA (si-Fas) and negative control (NC)
oligonucleotides were purchased from Guangzhou RiboBio Co., Ltd.
(Guangzhou, China). The C6 cells of 30–50% confluence were
transfected by Lipofectamine® RNAiMAX (Invitrogen Life
Technologies) according to the manufacturer’s instructions and
siRNA inhibitory efficacy was examined by immunoblotting.
Statistical analysis
Data are expressed as means ± standard deviation of
the three separate experiments. One-way analysis of variance and
Student’s t-test were used to determine the differences between the
the two groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
Pregnenolone decreases cell viability of
malignant glioma cells
First, the effect of pregnenolone on the growth of
U-87 MG human GBM cells was investigated. The cell numbers were
greatly reduced following treatment with 100 μM pregnenolone for 48
h (Fig. 2A) and the growth
inhibitory efficacy was comparable with 2-ME. To further
investigate the dose-dependent effect of pregnenolone on the
anti-glioma spectrum, a series of glioma cell lines, including C6,
LN-18 and T98, were treated with pregnenolone at different doses
(0, 12.5, 25, 50, 75 and 100 μM) for 48 h. Notably, pregnenolone
dose-dependently decreased the cell viability in all the glioma
cell lines at varying degrees (Fig.
2B). The dose-dependent decline of cell viability in the
majority of the cell lines was more sensitive between 12.5 and 50
μM pregnenolone compared with between 50 and 100 μM pregnenolone.
Therefore, the glioma cells were treated with doses between 12.5
and 50 μM pregnenolone to further investigate the detailed
mechanisms of action. Exogenous cholesterol may completely prevent
the pregnenolone-induced cell loss in U-87MG cells (Fig. 2C).
Pregnenolone induces glioma cell
apoptosis
To investigate the mechanisms underlying the loss of
cell viability induced by pregnenolone, further experiments in the
C6, U-87 MG and LN-18 glioma cell lines were conducted. The glioma
cells were incubated with pregnenolone at different concentrations
for 48 h, and examined via cytotoxicity assay, Hoechst 33342
staining and TUNEL assay. No significant changes in LDH release
were observed in the pregnenolone-treated glioma cells, indicating
that pregnenolone did not result in cell necrosis (Fig. 2D). Compared with the control group,
the condensed chromatin in the nucleus of the 50 μM
pregnenolone-treated group appeared smaller and brighter under the
fluorescent microscope (Fig. 2E).
The apoptosis rate in the pregnenolone-treated groups increased in
a dose-dependent manner (Fig. 2F).
Following the TUNEL assay, there were more positive signals
observed in the cells that were treated with pregnenolone (Fig. 2E). These findings support the
conclusion that pregnenolone induced glioma cell apoptosis in a
dose-dependent manner.
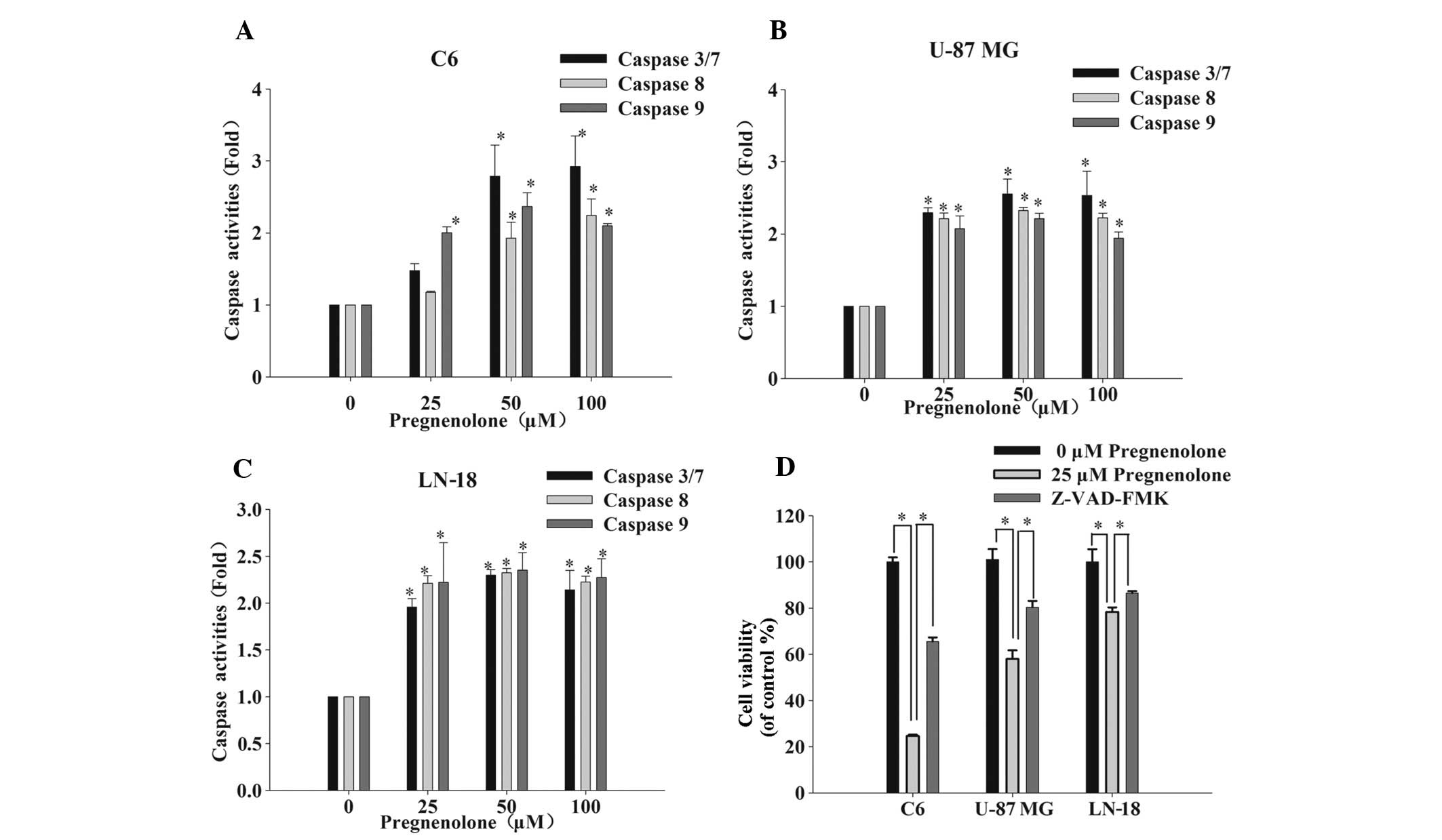
Pregnenolone leads to caspase-dependent
apoptosis via extrinsic and intrinsic apoptotic pathways
Previous studies have reported that anticancer
therapies eventually result in the activation of caspases, a family
of cysteine proteases that act as common death effector molecules
in the apoptotic pathway (18–20).
To determine whether the apoptotic pathway is induced by
pregnenolone, the activity of caspase 8 and 9, and 3/7 in the death
receptor (extrinsic) and mitochondria-dependent (intrinsic)
pathways, respectively, was investigated. Caspase 9 is an important
intracellular amplifier of caspase signaling that is downstream of
mitochondria and caspase 8 is an apical caspase in death receptor
signaling, which has been well-established (21). After 24 h of exposure to
pregnenolone, the activity of caspase 8 and 9 markedly increased
compared with the control group. The activity of the downstream
effector caspase 3/7 increased 2–3-fold following a 48-h exposure
to pregnenolone (Fig. 3A–C). To
determine whether apoptosis was due to the activation of caspases,
the general caspase inhibitor, Z-VAD-FMK, was applied to the
pregnenolone-treated glioma cells and cell viability was measured
via an MTT assay. As expected, 50 μM Z-VAD-FMK prevented the loss
of cell viability that was caused by pregnenolone (Fig. 3D).
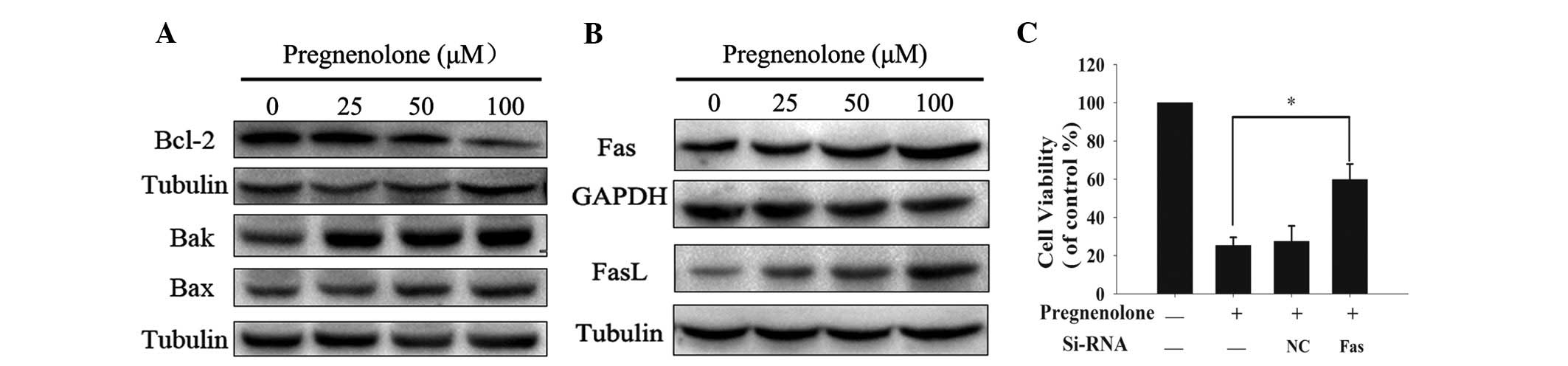
Pregnenolone induces apoptosis by
decreasing Bcl-2 and increasing Fas/FasL activity
The present study demonstrated that pregnenolone
triggers the intrinsic and extrinsic apoptotic pathways by
activating caspase 8 and 9. To investigate the mechanisms
underlying pregnenolone-induced cell apoptosis, the upstream
targets in the mitochondria- and death receptor-mediated pathways
were analyzed. First, the protein levels of the Bcl-2 family
members, which are responsible for the stability of the
mitochondrial membrane, were examined. The data demonstrated that
the anti-apoptotic protein, Bcl-2, was markedly downregulated,
while the pro-apoptotic factors, Bax and Bak, were upregulated in
the pregnenolone-treated C6 cells (Fig.
4A). In addition, pregnenolone treatment resulted in a marked
increment of the activity of Fas and FasL, an important death
receptor and ligand, respectively that are involved in the
extrinsic pathway (Fig. 4B). To
confirm whether pregnenolone-induced apoptosis was mediated by Fas
and FasL, Fas was silenced in the C6 cells, using siRNA, and the
cell viability was measured (Fig.
4D). Compared with the cell viability loss that was observed in
the control group, Fas knockdown significantly decreased the cell
apoptosis, which was induced by pregnenolone. These findings
indicated that pregnenolone may trigger the intrinsic and extrinsic
apoptotic pathways by regulating the Bcl-2 family, Fas and
FasL.
Discussion
Pregnenolone, a neurosteroid, is synthesized in the
nervous system (22,23), arises from cholesterol and is
metabolized into important steroid hormones (24). Due to its chemical structure,
pregnenolone is highly lipid-soluble and rapidly crosses the BBB
(25). The concentration of
pregnenolone in overall brain tissue is 74-fold higher compared
with that in plasma (26). Notably,
the findings of the present study indicated that this endogenous
steroid hormone is able to induce glioma cell apoptosis. The
antitumor efficacy of pregnenolone is as strong as 2-ME, however,
whether it may be developed into an anti-glioma agent requires
further investigation, as does its bioavailability.
In addition to pregnenolone and 2-ME, previous
studies have reported that a number of other steroid hormones
originating from cholesterol exhibit antineoplastic properties,
such as dehydroepiandrosterone (DHEA) (27) and cholesteryl sulfate (28), the underlying mechanisms of which
are distinct. Furthermore, DHEA induced apoptotic and necrotic
death in cervical cancer cells (29). In the present study, pregnenolone
induced glioma cell apoptosis, but not necrotic death.
Additionally, 2-ME upregulated death receptor 5 and induced
apoptosis through activation of the extrinsic apoptotic pathway
(13). However, the present study
demonstrated that pregnenolone upregulated Fas and FasL, and
induced apoptosis through the activation of the extrinsic pathway.
Moreover, the intrinsic apoptotic pathway was activated by
pregnenolone, which was characterized by downregulation of
anti-apoptotic Bcl-2 expression, upregulation of pro-apoptotic Bax
and Bak expression, and activation of caspase 9.
Notably, cholesterol blocked the loss of cell
viability that was induced by pregnenolone (Fig. 2C), demonstrating the critical role
of cholesterol in apoptosis. As a neutral lipid, cholesterol is
indispensable in the regulation of cell membrane properties in
mammalian cells, contributes to the unique biophysical properties
of the lipid raft microdomain and is mechanistically important for
signal transduction by raft proteins (30). Lipid rafts, incorporating distinct
classes of proteins, are comprised of cholesterol and sphingolipids
in the exoplasmic leaflets of the bilayer (31,32).
Disruption of lipid rafts by dispersion or extraction of membrane
cholesterol results in inhibition of raft-dependent signaling
events and eventually affects cell survival. It was reported that
simvastatin, a cholesterol synthesis inhibitor, lowered raft
cholesterol content and induced apoptosis in prostate cancer cells;
however, replenishing cell membranes with cholesterol reversed
these inhibitory and pro-apoptotic effects (33). In addition, previous studies have
shown that ginsenoside Rh2, containing a cholesterol backbone with
hydroxyl groups, induced ligand-independent Fas activation and
activated caspase 8 via lipid raft disruption (34,35).
Similar to Rh2, other cholesterol derivatives, such as desmosterol
treatment, resulted in the disappearance of caveolae and blocked
insulin receptor activation via lipid raft disruption (36). As a cholesterol metabolite,
pregnenolone has a similar chemical structure to cholesterol, and
may interrupt normal lipid rafts and trigger apoptosis.
The Fas/FasL and mitogen-activated protein kinase
(MAPK) signaling pathways are two key cell death and survival
signaling pathways, which are influenced by the alteration of lipid
rafts (37). In the present study,
Fas/FasL signaling was dramatically potentiated (Fig. 4), while EGFR/Ras/MAPK signaling
remained stable (data not shown). Moreover, an elevation in
Fas/FasL signaling was confirmed as a critical cause for
pregnenolone-induced apoptosis by si-Fas. The mechanisms by which
pregnenolone upregulates Fas and FasL, and whether pregnenolone
disrupts lipid rafts require further investigation.
In conclusion, the present study demonstrated that
pregnenolone, an endogenous steroid hormone, exerts a strong
anti-glioma effect by inducing apoptosis. Pregnenolone treatment
resulted in caspase-dependent apoptosis via the extrinsic and
intrinsic apoptotic pathways in glioma cells. Based on this
anti-glioma activity and high BBB permeability, pregnenolone may be
a promising compound for, and aid in the development of, novel
agents for anti-glioma therapy.
Acknowledgements
The present study was supported by the Natural
Science Foundation of China (grant nos. 81202554 and 81202555) and
the Guangdong Natural Science Foundation (grant nos.
S10451008901004893 and S2012010009237).
References
|
1
|
Dolecek TA, Propp JM, Stroup NE and
Kruchko C: CBTRUS statistical report: primary brain and central
nervous system tumors diagnosed in the United States in 2005–2009.
Neuro Oncol. 14(Suppl 5): v1–v49. 2012.
|
|
2
|
Louis DN, Ohgaki H, Wiestler OD, et al:
The 2007 WHO classification of tumours of the central nervous
system. Acta Neuropathol. 114:97–109. 2007.
|
|
3
|
Yan H, Parsons DW, Jin G, et al: IDH1 and
IDH2 mutations in gliomas. N Engl J Med. 360:765–773. 2009.
|
|
4
|
Rousseau A, Mokhtari K and Duyckaerts C:
The 2007 WHO classification of tumors of the central nervous system
- what has changed? Curr Opin Neurol. 21:720–727. 2008.
|
|
5
|
Mutter N and Stupp R: Temozolomide: a
milestone in neuro-oncology and beyond? Expert Rev Anticancer Ther.
6:1187–1204. 2006.
|
|
6
|
Chamberlain MC: Temozolomide: therapeutic
limitations in the treatment of adult high-grade gliomas. Expert
Rev Neurother. 10:1537–1544. 2010.
|
|
7
|
Stupp R, Mason WP, van den Bent MJ, et al:
Radiotherapy plus concomitant and adjuvant temozolomide for
glioblastoma. N Engl J Med. 352:987–996. 2005.
|
|
8
|
Wirth T, Samaranayake H, Pikkarainen J,
Määttä AM and Ylä-Herttuala S: Clinical trials for glioblastoma
multiforme using adenoviral vectors. Curr Opin Mol Ther.
11:485–492. 2009.
|
|
9
|
Gorski J and Gannon F: Current models of
steroid hormone action: a critique. Annu Rev Physiol. 38:425–450.
1976.
|
|
10
|
Stocco DM: StAR protein and the regulation
of steroid hormone biosynthesis. Annu Rev Physiol. 63:193–213.
2001.
|
|
11
|
Falkenstein E, Tillmann HC, Christ M,
Feuring M and Wehling M: Multiple actions of steroid hormones - A
focus on rapid, nongenomic effects. Pharmacol Rev. 52:513–555.
2000.
|
|
12
|
Rupprecht R: The
neuropsychopharmacological potential of neuroactive steroids. J
Psychiatr Res. 31:297–314. 1997.
|
|
13
|
LaVallee TM, Zhan XH, Johnson MS, et al:
2-methoxyestradiol up-regulates death receptor 5 and induces
apoptosis through activation of the extrinsic pathway. Cancer Res.
63:468–475. 2003.
|
|
14
|
LaVallee TM, Zhang XGH, Herbstritt CJ,
Kough EC, Green SJ and Pribluda VS: 2-methoxyestradiol inhibits
proliferation and induces apoptosis independently of estrogen
receptors alpha and beta. Cancer Res. 62:3691–3697. 2002.
|
|
15
|
Lin HL, Liu TY, Wu CW and Chi CW:
2-Methoxyestradiol-induced caspase-3 activation and apoptosis
occurs through G(2)/M arrest dependent and independent pathways in
gastric carcinoma cells. Cancer. 92:500–509. 2001.
|
|
16
|
Kirches E and Warich-Kirches M:
2-methoxyestradiol as a potential cytostatic drug in gliomas?
Anticancer Agents Med Chem. 9:55–65. 2009.
|
|
17
|
Tang YC, Williams BR, Siegel JJ and Amon
A: Identification of aneuploidy-selective antiproliferation
compounds. Cell. 144:499–512. 2011.
|
|
18
|
Fulda S and Debatin KM: Extrinsic versus
intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene.
25:4798–4811. 2006.
|
|
19
|
Chandrasekar B, Vemula K, Surabhi RM, et
al: Activation of intrinsic and extrinsic proapoptotic signaling
pathways in interleukin-18-mediated human cardiac endothelial cell
death. J Biol Chem. 279:20221–20233. 2004.
|
|
20
|
Johnstone RW, Frew AJ and Smyth MJ: The
TRAIL apoptotic pathway in cancer onset, progression and therapy.
Nat Rev Cancer. 8:782–798. 2008.
|
|
21
|
Degterev A, Boyce M and Yuan J: A decade
of caspases. Oncogene. 22:8543–8567. 2003.
|
|
22
|
Dubrovsky BO: Steroids, neuroactive
steroids and neurosteroids in psychopathology. Prog
Neuropsychopharmacol Biol Psychiatry. 29:169–192. 2005.
|
|
23
|
Mellon SH and Griffin LD: Neurosteroids:
biochemistry and clinical significance. Trends Endocrinol Metab.
13:35–43. 2002.
|
|
24
|
Marx CE, Bradford DW, Hamer RM, et al:
Pregnenolone as a novel therapeutic candidate in schizophrenia:
emerging preclinical and clinical evidence. Neuroscience.
191:78–90. 2011.
|
|
25
|
Tsutsui K, Matsunaga M and Ukena K:
Biosynthesis and biological actions of neurosteroids in the avian
brain. Avian Poultry Biol Rev. 14:63–78. 2003.
|
|
26
|
Lacroix C, Fiet J, Benais JP, et al:
Simultaneous radioimmunoassay of progesterone, androst-4-enedione,
pregnenolone, dehydroepiandrosterone and 17-hydroxyprogesterone in
specific regions of human brain. J Steroid Biochem. 28:317–325.
1987.
|
|
27
|
Liu S, Ishikawa H, Li FJ, et al:
Dehydroepiandrosterone can inhibit the proliferation of myeloma
cells and the interleukin-6 production of bone marrow mononuclear
cells from patients with myeloma. Cancer Res. 65:2269–2276.
2005.
|
|
28
|
Ishimaru C, Yonezawa Y, Kuriyama I,
Nishida M, Yoshida H and Mizushina Y: Inhibitory effects of
cholesterol derivatives on DNA polymerase and topoisomerase
activities, and human cancer cell growth. Lipids. 43:373–382.
2008.
|
|
29
|
Girón RA, Montaño LF, Escobar ML and
López-Marure R: Dehydroepiandrosterone inhibits the proliferation
and induces the death of HPV-positive and HPV-negative cervical
cancer cells through an androgen- and estrogen-receptor independent
mechanism. FEBS J. 276:5598–5609. 2009.
|
|
30
|
Simons K and Ikonen E: How cells handle
cholesterol. Science. 290:1721–1726. 2000.
|
|
31
|
Lingwood D and Simons K: Lipid rafts as a
membrane-organizing principle. Science. 327:46–50. 2010.
|
|
32
|
Anderson RG and Jacobson K: A role for
lipid shells in targeting proteins to caveolae, rafts, and other
lipid domains. Science. 296:1821–1825. 2002.
|
|
33
|
Zhuang L, Kim J, Adam RM, Solomon KR and
Freeman MR: Cholesterol targeting alters lipid raft composition and
cell survival in prostate cancer cells and xenografts. J Clin
Invest. 115:959–968. 2005.
|
|
34
|
Shieh PC, Tsao CW, Li JS, et al: Role of
pituitary adenylate cyclase-activating polypeptide (PACAP) in the
action of ginsenoside Rh2 against beta-amyloid-induced inhibition
of rat brain astrocytes. Neurosci Lett. 434:1–5. 2008.
|
|
35
|
Yi JS, Choo HJ, Cho BR, et al: Ginsenoside
Rh2 induces ligand-independent Fas activation via lipid raft
disruption. Biochem Biophys Res Commun. 385:154–159. 2009.
|
|
36
|
Jansen M, Pietiaïnen VM, Pölönen H, et al:
Cholesterol substitution increases the structural heterogeneity of
caveolae. J Biol Chem. 283:14610–14618. 2008.
|
|
37
|
Patra SK: Dissecting lipid raft
facilitated cell signaling pathways in cancer. Biochim Biophys
Acta. 1785:182–206. 2008.
|