Introduction
Colorectal cancer (CRC) is one of the most common
types of lethal cancer worldwide, with over a million new cases
diagnosed annually (1). In China,
the majority of patients with CRC are diagnosed at a late stage,
however, early detection of the disease significantly enhances the
probability of survival. Understanding of the mechanisms that
result in CRC and the specific biomarkers for CRC progression must
be enhanced to aid the prediction and improvement of clinical
outcomes.
Long non-coding RNAs (lncRNAs) are >200
nucleotides and do not code for proteins, but interact with
proteins (2,3). Although lncRNAs are not as well
characterised as small non-coding (nc) microRNAs, lncRNAs are
critical in the regulation of diverse cellular processes, such as
stem cell pluripotency, cell growth, cell cycle, apoptosis,
metabolism and cancer migration (4–10).
Functional lncRNAs may be used for cancer diagnosis and prognosis,
as well as serve as potential therapeutic targets. Functional
lncRNAs are considered to be particularly promising candidates for
future cancer diagnosis and therapeutic strategies (11). BRAF-activated lncRNA (BANCR) is a
recurrently overexpressed, previously unannotated 693-bp transcript
on chromosome 9 with a potential functional role in melanoma cell
migration (12,13). BANCR is closely associated with
V600EBRAF, the most frequent mutation type of the BRAF
gene. Furthermore, high frequencies of V600EBRAF
mutations are detected in malignant melanoma (70%), papillary
thyroid cancer (36–53%), and CRC (5–22%) (14). A close correlation exists between
the presence of V600EBRAF and progression to the
advanced stages of CRC (15),
however, the expression pattern and biological functions of BANCR
in CRC remain unclear.
The epithelial-mesenchymal transition (EMT) is a key
step toward cancer invasion and metastasis. From a molecular
perspective, EMT is characterised by the loss of epithelial
markers, including E-cadherin and cytokeratins, and the
upregulation of mesenchymal markers, such as N-cadherin and
vimentin (16). The overexpression
of V600EBRAF is susceptible to transforming growth
factor-β-induced EMT through an extracellular signal-regulated
kinase (ERK)-dependent mechanism (17).
The aim of the present study was to detect the
expression levels of BANCR in CRC, and to reveal the function and
molecular mechanisms of BANCR in CRC.
Materials and methods
Tissue samples and cell culture
In total, 60 specimens of human CRC tissues and
adjacent normal tissues were obtained between March 2012 and June
2013 from the First Affiliated Hospital of Nanjing Medical
University, (Nanjing, China) with written informed consent obtained
from all patients. The study was approved by the Protection of
Human Ethics Committee of the First Affiliated Hospital of Nanjing
Medical University. The diagnosis of CRC was histopathologically
confirmed and no patient received preoperative treatment. The
resected tissue samples were immediately frozen in liquid nitrogen
and stored at −80°C until RNA extraction. Of the 60 patients, 27
exhibited lymph node (LN) metastasis, while 33 did not. The data
collected from all subjects included age, gender, and CRC features,
such as tumour size, location, histological grade, depth of
invasion and serum carcinoembryonic antigen (CEA) value. The
clinical stage of CRC was evaluated based on the tumour, node,
metastasis (TNM) classification system (National Comprehensive
Cancer Network 2012) (18). The
human CRC cells were obtained from the Type Culture Collection of
the Chinese Academy of Sciences (Shanghai, China) and preserved at
the First Affiliated Hospital of Nanjing Medical University. All
cells were maintained in the recommended culture conditions and
incubated at 37°C in a 5% CO2 humidified atmosphere.
Quantitative polymerase chain reaction
(qPCR)
Total RNAs from the tissues and cells were extracted
using RNAiso Plus (Takara Bio, Inc., Dalian, China) and reverse
transcription (RT) reactions were performed using a
PrimeScriptTM RT reagent kit (Takara Bio, Inc.)
according to the manufacturer’s instructions. For qPCR, a final
volume of 20 μl reaction was performed according to a standard
protocol and the SYBR Green PCR kit (Roche Diagnostics,
Indianapolis, IN, USA) on the StepOnePlus Real-Time PCR System
(Applied Biosystems, Carlsbad, CA, USA). The qPCR was performed in
triplicate, with no template controls. The 2−ΔΔCT method
was conducted to determine the relative gene expression levels with
β-actin serving as the endogenous control to normalise the data.
The primers used were as follows: Forward, 5′-ACAGGACTCCATGGC
AAACG-3′ and reverse, 5′-ATGAAGAAAGCCTGGTGC AGT-3′ for BANCR;
forward, 5′-GTGTCATCCAACGGA ATGC-3′ and reverse,
5′-TGGCGGCATTGTAGGTGTTC-3′ for E-cadherin; forward,
5′-ATGACCGCTTCGCCAACTAC-3′ and reverse, 5′-CGGGCTTTGTCGTTGGTTAG-3′
for vimentin; and forward, 5′-AGA AAATCTGGCACCACACC-3′ and reverse,
5′-TAGCACAGCCTGGATAGCAA-3′ for β-actin. The PCR was performed using
the following cycles: 95°C for 30 sec; 40 cycles of 95°C for 5 sec
and 60°C for 31 sec; and a dissociation stage at 95°C for 15 sec,
60°C for 1 min and 95°C for 15 sec.
Generation of stably infected cell
lines
Recombinant lentiviruses containing short hairpin
(sh)RNA-323 (LV-BANCR-323), shRNA-540 (LV-BANCR-540), human
full-length BANCR cDNA (LV-BANCR) and negative control (LV-NC) were
purchased from GenePharma (Shanghai, China). The HCT116 cells were
infected with LV-BANCR-323, LV-BANCR-540 and LV-NC [multiplicity of
infection (MOI)=20] and the Caco-2 cells were infected with
LV-BANCR and LV-NC (MOI=10). The supernatant was removed after 24 h
and replaced with a fresh culture medium. The infection efficiency
was confirmed by qPCR 72 h following infection and the cells were
treated with 2 μg/ml puromycin for two weeks.
Wound healing experiments
The cells (5×105) were seeded in 6-well
plates and after 24 h, when the cells had grown by 90–100%, they
were scraped with a pipette tip to generate straight wounds. To
ensure documentation of the same region, the wells were marked
across the wounded area. The medium was replaced with a serum-free
medium (RPMI-1640, Wisent Inc., St-Bruno, QC, Canada) and the cells
were treated with a medium containing 1 mM mitomycin to inhibit
cell division. Phase-contrast images were recorded under an
inverted microscope (Nikon ECLIPSE Ti-E, Nikon, Kobe, Japan) at the
time of wounding, 0 h, and at 24 h. The untreated cells served as
controls.
Transwell migration assay
For the Transwell migration assays, 3×104
HCT116 cells and 5×104 Caco-2 cells were plated on the
non-coated membrane on the top chamber (24-well insert; 8-mm pore
size; Corning Costar Corp., Cambridge, MA, USA). The cells were
plated on a medium without serum and a medium, which was
supplemented with serum, served as a chemotactic agent in the lower
chamber. The cells were incubated for 48 h and the cells that did
not migrate through the pores were removed with a cotton swab,
while cells on the lower surface of the membrane were stained with
crystal violet (Beijing Solarbio Science & Technology Co.,
Ltd., Beijing, China). The cell numbers were determined by counting
the penetrating cells under a microscope (Nikon ECLIPSE Ti-E) in
random fields (five fields per chamber). Each experiment was
performed in triplicate.
Western blot analysis
Proteins were extracted with
radioimmunoprecipitation assay (Beyotime, Shanghai, China) and
equal amounts of protein were electrophoresed on a 6, 10 or 12%
sodium dodecyl sulphate-polyacrylamide gel and subsequently
transferred to polyvinylidene fluoride membranes (Millipore,
Billerica, MA, USA). The membranes were blocked in 5% non-fat milk
in Tris-buffered saline containing 0.1% Tween-20 (TBST) at room
temperature for 2 h. The membranes were incubated overnight with
the following primary antibodies at 4°C: E-cadherin (1:1,000; Cell
Signalling Technology, BSN, USA), vimentin (1:1,000, Cell
Signalling Technology) and GAPDH (1:10,000; Beijing Biosynthesis
Biotechnology, Beijing, China). The membranes were washed thrice
with TBST and incubated with horseradish peroxidase-conjugated
secondary antibody (1:1,000; Beijing Biosynthesis Biotechnology) at
room temperature for 2 h. Following three TBST washes, the
membranes were developed using ECL Plus (Millipore, MA, USA) and
exposed to X-ray film for visualisation of the protein bands. GAPDH
served as an internal loading control.
Statistical analysis
Data were analysed using SPSS 19.0 (IBM, Armonk, NY,
USA) and were expressed as the mean ± standard error of mean. The
significance of the differences between groups was estimated by
Student’s t-test, Pearson’s χ2-test, or one-way analysis
of variance, as appropriate. Two-sided P<0.05 was considered to
indicate a statistically significant difference.
Results
BANCR expression in tissues and cell
lines
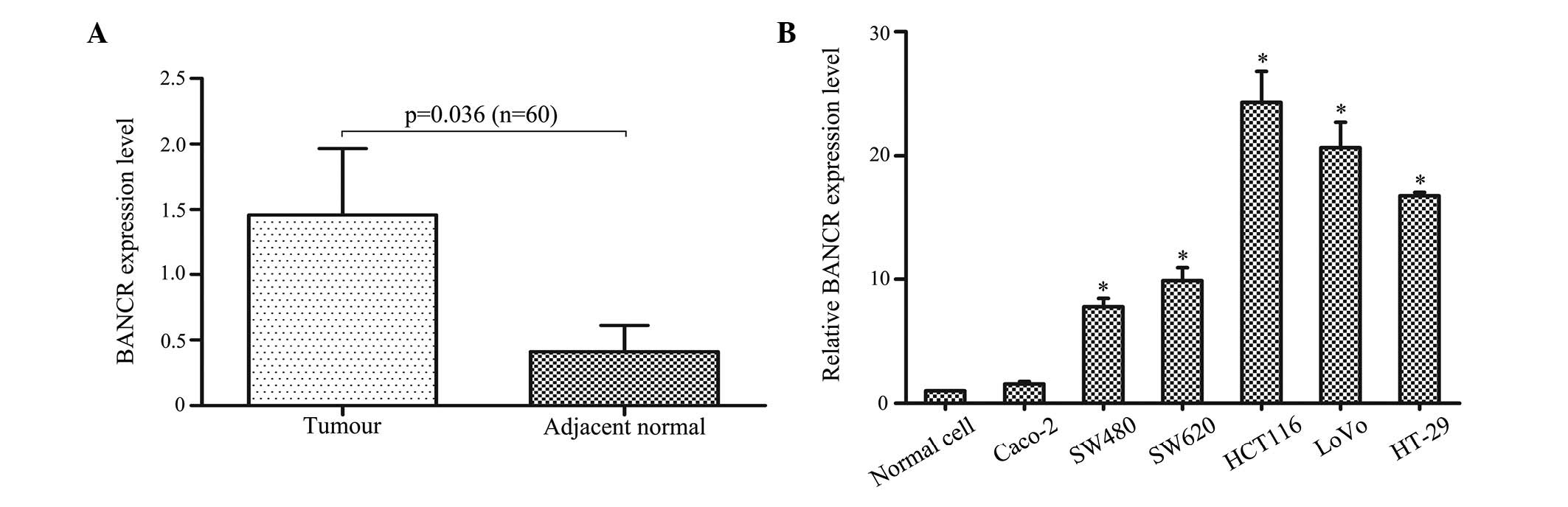
The primary aim of the present study was to
investigate whether BANCR was detectable and altered in 60 pairs of
CRC tissues compared with adjacent normal tissues. The qPCR results
demonstrated that BANCR expression was significantly higher in the
tumour tissues than in the adjacent normal tissues (P<0.05;
Fig. 1A). The qPCR assays were
further developed to quantify the BANCR expression in the CRC cell
lines. A higher BANCR expression was found in the HCT116
(P<0.01), LoVo (P<0.01), HT-29 (P<0.01), SW480 (P<0.05)
and SW620 (P<0.05) cells when compared with the CCD 841 CoN
normal intestinal mucous cell line, however, no significant
difference was identified in the Caco-2 cells (P>0.05; Fig. 1B).
Correlation between BANCR expression and
the clinicopathological parameters of patients with CRC
The 60 patients with CRC were divided into high-
(n=18) and low-BANCR expression (n=42) groups according to the mean
value of the expression levels of BANCR in tumour tissues.
Clinicopathological factors were analysed between the two groups
and the high-BANCR expression group showed more advanced LN
metastasis and tumour stage than the low-BANCR expression group
(P<0.05). However, no significant correlation was found between
the BANCR expression and other clinicopathological features, such
as age, gender, tumour size, location, histological grade, depth of
invasion and serum CEA value (P>0.05; Table I).
 | Table ICorrelation between BANCR expression
and clinicopathological parameters of colorectal cancer
patients. |
Table I
Correlation between BANCR expression
and clinicopathological parameters of colorectal cancer
patients.
| | BANCR expression | |
|---|
| |
| |
|---|
| Characteristic | n | High (n=18) | % | Low (n=42) | % | P-value |
|---|
| Age, years (mean ±
SEM) | 60 | 60.4±17.3 | | 64.6±14.5 | | 0.333 |
| Gender | | | | | | 0.253 |
| Male | 35 | 13 | 37.1 | 22 | 62.9 | |
| Female | 25 | 5 | 20.0 | 20 | 80.0 | |
| Tumour size, cm | | | | | | 0.253 |
| <4 | 25 | 5 | 20.0 | 20 | 80.0 | |
| ≥4 | 35 | 13 | 37.1 | 22 | 62.9 | |
| Location | | | | | | 0.167 |
| Colon | 32 | 7 | 21.9 | 25 | 78.1 | |
| Rectum | 28 | 11 | 39.3 | 17 | 60.7 | |
| Histological
grade | | | | | | 0.207 |
| Well/moderately | 44 | 11 | 25.0 | 33 | 75.0 | |
| Poorly/other | 16 | 7 | 43.8 | 9 | 56.3 | |
| Depth of
invasion | | | | | | 0.192 |
| T1, T2 | 15 | 2 | 13.3 | 13 | 86.7 | |
| T3, T4 | 45 | 16 | 35.6 | 29 | 64.4 | |
| Lymph node
metastasis | | | | | | 0.010b |
| Absent | 33 | 4 | 12.1 | 26 | 78.8 | |
| Present | 27 | 14 | 51.9 | 16 | 59.3 | |
| Serum CEA value,
μg/l | | | | | | 0.085 |
| <5 | 35 | 7 | 20.0 | 28 | 80.0 | |
| ≥5 | 25 | 11 | 44.0 | 14 | 56.0 | |
| Tumour stagea | | | | | | <0.001b |
| I, II | 28 | 2 | 7.0 | 26 | 93.0 | |
| III, IV | 32 | 16 | 50.0 | 16 | 50.0 | |
Modulation of BANCR expression affects
cell migration
To clarify the role of BANCR within CRC cells,
Caco-2 cells were transfected with LV-BANCR, which express
relatively low levels of endogenous BANCR within CRC cell lines,
while LV-BANCR-323 and LV-BANCR-540 were transfected with stable
BANCR-expressing HCT116 cells. As a result, the BANCR expression
was significantly downregulated in the HCT116 cells and upregulated
in the Caco-2 cells (Fig. 2A). The
wound healing experiment (Fig. 2B)
and Transwell migration assay (Fig.
2C) showed that BANCR overexpression promoted cell migration in
the Caco-2 cells, which was reduced in the BANCR-downregulated
HCT116 cells.
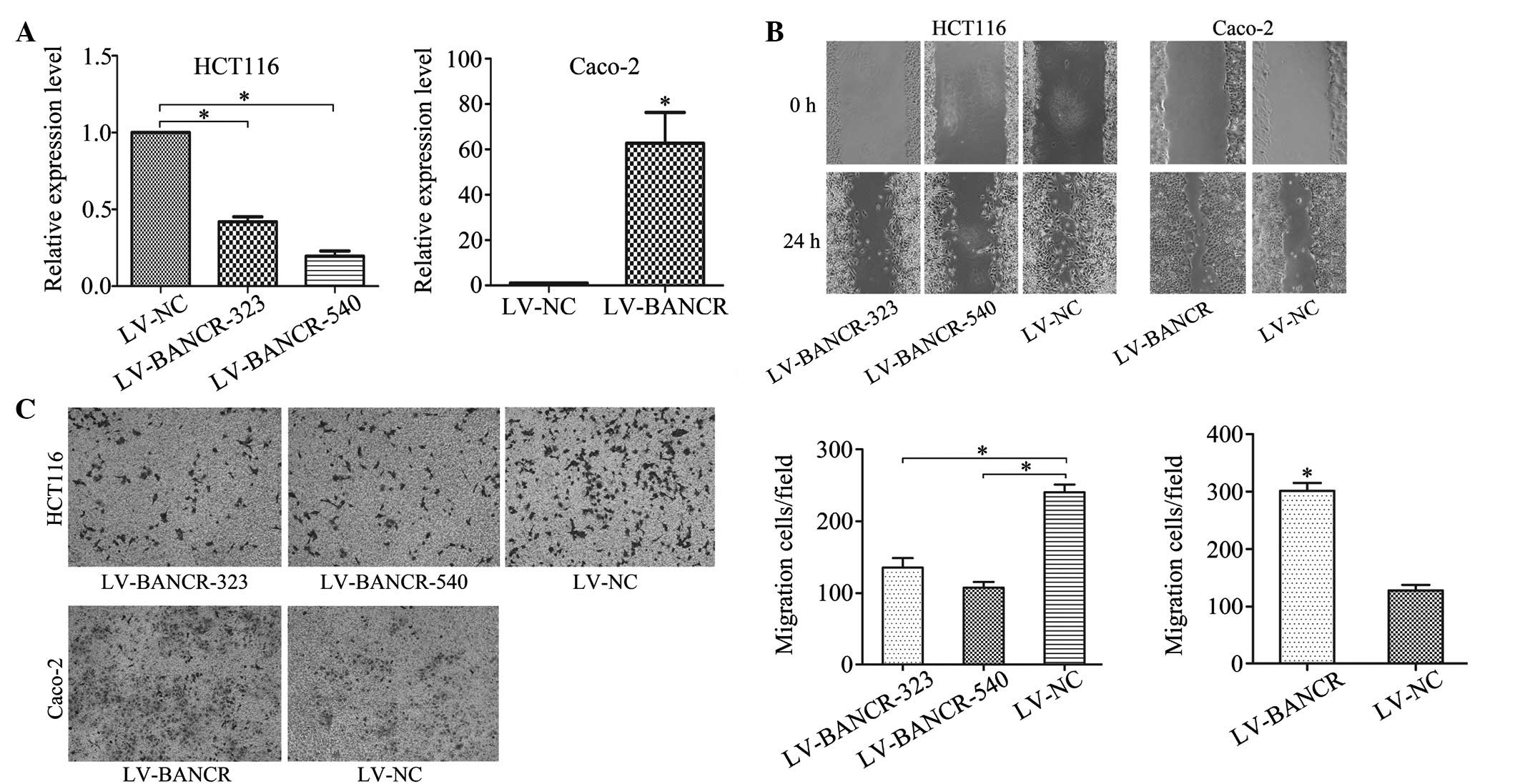 | Figure 2Modulation of BANCR expression affects
cell migration. (A) Following treatment with LV-BANCR-323 and
LV-BANCR-540, BANCR expression in HCT116 cell lines was
downregulated compared with those treated with LV-NC, and BANCR
expression in Caco-2 cell lines infected with LV-BANCR was
significantly upregulated. (B) Wound healing experiments and (C)
Transwell migration assays showed that BANCR overexpression
promoted cell migration in Caco-2 cells, however, cell migration
reduced in BANCR-downregulated HCT116 cells (crystal violet stain;
magnification, ×100). The bar graph represent at least three
independent experiments and the bars indicate the number of
migrated cells per field. *P<0.05. LV-BANCR-323,
recombinant lentiviruses containing shRNA-323; LV-BANCR-540,
recombinant lentiviruses containing shRNA-540; LV-BANCR,
recombinant lentiviruses containing human full-length BANCR cDNA;
NC, negative control; BANCR, BRAF-activated long non-coding RNA;
shRNA, short hairpin RNA. |
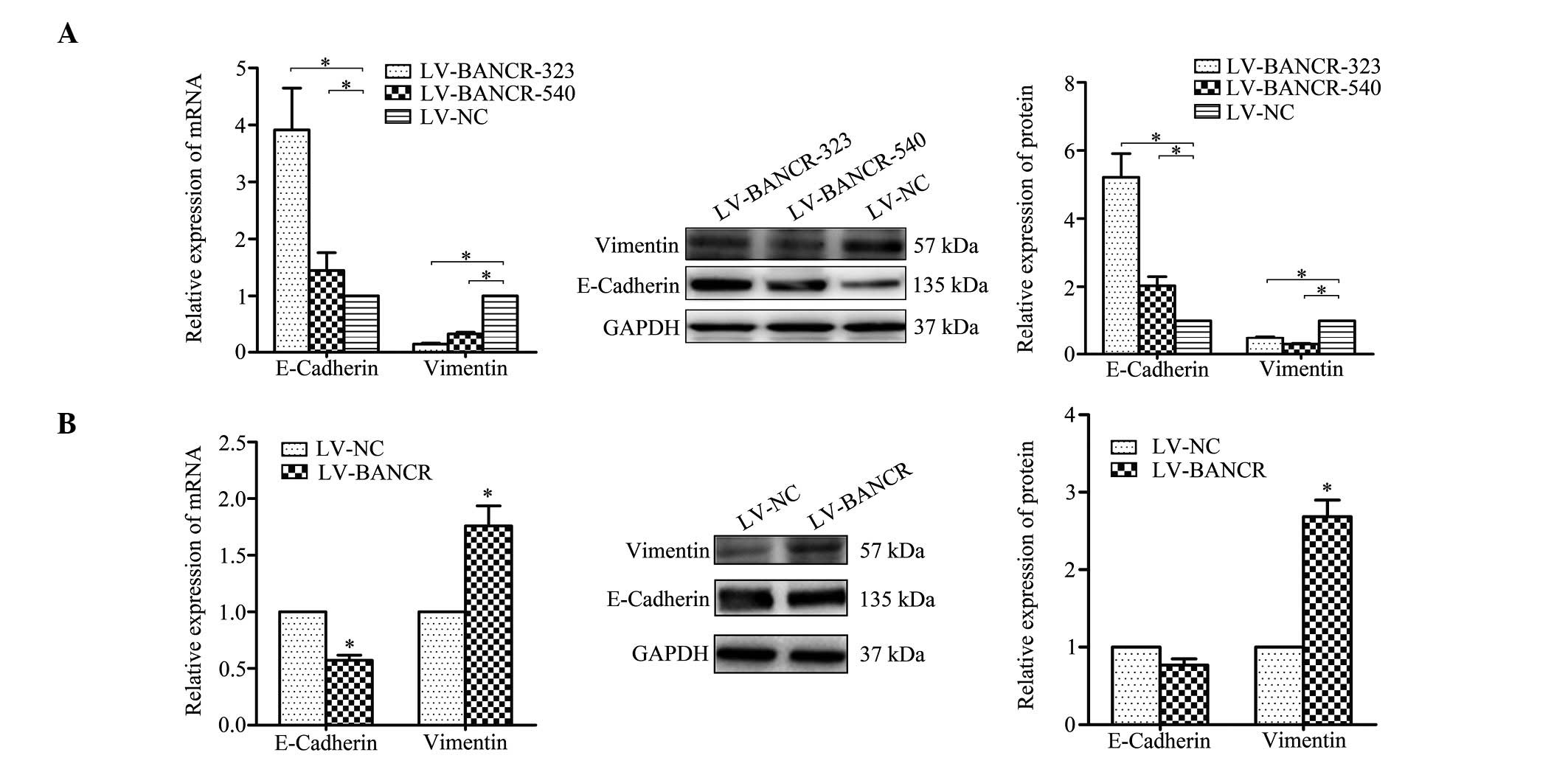
BANCR induces EMT phenotypes
EMT is one of the key processes for primary tumour
cells to acquire a migratory capacity (16). To define the role of BANCR in the
progression of cell migration within CRC cells, the changes in
expression of epithelial and mesenchymal markers were detected
following modulation of the BANCR expression level. The
downregulation of the BANCR levels within the HCT116 cells was
found to be associated with the upregulated E-cadherin and
downregulated vimentin expression at the mRNA and protein levels
(Fig. 3A). Vimentin expression was
upregulated and E-cadherin was downregulated in the
BANCR-overexpressed Caco-2 cells (Fig.
3B).
BANCR contributes to HCT116 cell
migration through an ERK-dependent mechanism
Considering that the MEK signalling pathway has a
specific link with the BRAF gene (19), the correlation between BANCR and the
MEK signalling pathway was investigated and treatment with the
specific MEK inhibitor, U0126 (30 μM) appeared to reduce HCT116
migration. Next, stable cell lines were generated that expressed
BANCR cDNA in the HCT116 cells by lentiviral transfection with
LV-BANCR. As predicted, LV-BANCR significantly increased the cell
migration, however, the cell migration was decreased as a result of
treatment with U0126 (Fig. 4A). In
addition, BANCR overexpression was found to upregulate vimentin
expression and downregulate E-cadherin expression (mRNA and
protein). By contrast, treatment with LV-BANCR+U0126 was found to
downregulate vimentin expression and upregulate E-cadherin
expression (mRNA and protein; Fig.
4B). These results showed that BANCR contributes moderately to
HCT116 cell migration via an ERK-dependent mechanism.
 | Figure 4BANCR contributes to HCT116 cell
migration via an extracellular signal-regulated kinase-dependent
mechanism. (A) Transwell migration assay showed that treatment with
MEK inhibitor, U0126, reduced HCT116 migration and that treatment
with LV-BANCR induced HCT116 migration, however, this was also
inhibited by U0126 (crystal violet stain; magnification, ×100). (B)
Following treatment with U0126, LV-BANCR and LV-BANCR + U0126, the
expression of E-cadherin and vimentin was impaired.
*P<0.05. LV-BANCR, recombinant lentiviruses
containing human full-length BANCR cDNA; NC, negative control;
BANCR, BRAF-activated long non-coding RNA; shRNA, short hairpin
RNA. |
Discussion
Advances in molecular techniques have led to the
discovery of a novel type of regulatory gene. Although initially
argued to be spurious transcriptional noise, lncRNAs are implicated
to have oncogenic and tumour-suppressing roles (20,21).
In CRC, one example of such oncogenic lncRNA is the Hox transcript
antisense intergenic RNA (HOTAIR). HOTAIR expression levels are
higher in cancerous tissues than in corresponding non-cancerous
tissues of stage IV CRC patients. Furthermore, patients with high
HOTAIR expression exhibit a relatively poorer prognosis (22). An additional classic oncogenic
lncRNA is termed the highly upregulated in liver cancer (HULC).
Colorectal carcinomas, which metastasise to the liver and not to
the LNs, upregulate HULC ncRNA (23). MALAT-1, H19 and CRNDE RNAs are also
associated with CRC (24–26).
BANCR is recurrently overexpressed in melanoma. The
shRNA-mediated knockdown of BANCR in melanoma cells changes the
expression levels of 88 genes, a number of which are involved in
cell migration and chemotaxis. In addition, BANCR depletion impairs
the migration of melanoma cells in vitro (12,13).
In the present study, the BANCR expression levels in CRC patients
were examined and the associated clinical implications were
investigated. The qPCR showed that BANCR was upregulated in CRC
tumour tissues compared with adjacent normal tissues. Furthermore,
upregulated BANCR expression was found to correlate with the TNM
stage and LN metastasis. In addition, the potential role of BANCR
in CRC cells in vitro was examined. Altered BANCR expression
in the HCT116 and Caco-2 cells was found to affect migration and
thus, the current results are consistent with those of a previous
study (13), indicating that BANCR
expression enhances the aggressive biological behaviour of cancer
cells of various origins. However, considering that the tissues
were resected in the recent two years, the long-term overall
survival rates were not analysed, however, will be investigated in
future studies.
EMT processes, which are characterised by diminished
epithelial characteristics and increased mesenchymal attributes,
provide epithelial cells with enhanced migratory potential and are
implicated in numerous physiological and pathological processes
that require cell migration. LncRNA is important in regulating
cancer progression (6,7) and therefore, recent studies are
beginning to elucidate the association between EMT and lncRNAs.
Ying et al (27)
demonstrated that upregulated MALAT-1 contributes to bladder cancer
cell migration by activating Wnt signalling and subsequently
inducing EMT. Luo et al (28) observed that lncRNA H19 increases
bladder cancer metastasis by associating with EZH2 and inhibiting
E-cadherin expression. In addition, Xu et al (29) showed that the knockdown of lncRNA
HOTAIR suppresses tumour invasion and reverses EMT in gastric
cancer. Although direct supporting evidence is insufficient, there
is a suspected role of BANCR in the regulation of EMT. The present
in vitro study identified that altered BANCR expression
affects the expression of epithelial and mesenchymal markers,
implying that BANCR may regulate EMT. Furthermore, the effect of
BANCR on cell migration and EMT regulation may depend on the
MEK/ERK signalling pathway as the MEK/ERK signalling pathway
inhibitor, U0126, inhibits the BANCR overexpression-induced
promotion of cell migration, vimentin upregulation and E-cadherin
downregulation. These observations indicate that BANCR contributes
to CRC migration by inducing EMT via an MEK/ERK-dependent
mechanism.
In conclusion, to the best of our knowledge, the
present study is the first to report that BANCR is highly expressed
in CRC and that BANCR is likely to present as a useful biomarker of
CRC. Furthermore, identifying that BANCR induces CRC migration by
inducing EMT via an MEK/ERK signalling pathway expands on the
current understanding of the molecular mechanisms of BANCR. Most
importantly, BANCR may be used as a potential molecular target
during the treatment of human CRC.
Acknowledgements
The present study was supported by the Department of
Health of the Jiangsu Province Fund (grant no. BK20131448).
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013.
|
|
2
|
Ponting CP, Oliver PL and Reik W:
Evolution and functions of long noncoding RNAs. Cell. 136:629–641.
2009.
|
|
3
|
Mercer TR, Dinger ME and Mattick JS: Long
non-coding RNAs: insights into functions. Nat Rev Genet.
10:155–159. 2009.
|
|
4
|
Loewer S, Cabili MN, Guttman M, et al:
Large intergenic non-coding RNA-RoR modulates reprogramming of
human induced pluripotent stem cells. Nat Genet. 42:1113–1117.
2010.
|
|
5
|
Sigova AA, Mullen AC, Molinie B, et al:
Divergent transcription of long noncoding RNA/mRNA gene pairs in
embryonic stem cells. Proc Natl Acad Sci USA. 110:2876–2881.
2013.
|
|
6
|
Gupta RA, Shah N, Wang KC, et al: Long
non-coding RNA HOTAIR reprograms chromatin state to promote cancer
metastasis. Nature. 464:1071–1076. 2010.
|
|
7
|
Tsai MC, Spitale RC and Chang HY: Long
intergenic noncoding RNAs: new links in cancer progression. Cancer
Res. 71:3–7. 2011.
|
|
8
|
Gutschner T, Hämmerle M, Eissmann M, et
al: The noncoding RNA MALAT1 is a critical regulator of the
metastasis phenotype of lung cancer cells. Cancer Res.
73:1180–1189. 2013.
|
|
9
|
Meola N, Pizzo M, Alfano G, Surace EM and
Banfi S: The long noncoding RNA Vax2os1 controls the cell cycle
progression of photoreceptor progenitors in the mouse retina. RNA.
18:111–123. 2012.
|
|
10
|
Chisholm KM, Wan Y, Li R, Montgomery KD,
Chang HY and West RB: Detection of long non-coding RNA in archival
tissue: correlation with polycomb protein expression in primary and
metastatic breast carcinoma. PLoS One. 7:e479982012.
|
|
11
|
Qi P and Du X: The long non-coding RNAs, a
new cancer diagnostic and therapeutic gold mine. Mod Pathol.
26:155–165. 2013.
|
|
12
|
McCarthy N: Epigenetics. Going places with
BANCR. Nat Rev Cancer. 12:4512012.
|
|
13
|
Flockhart RJ, Webster DE, Qu K, et al:
BRAFV600E remodels the melanocyte transcriptome and induces BANCR
to regulate melanoma cell migration. Genome Res. 22:1006–1014.
2012.
|
|
14
|
Davies H, Bignell GR, Cox C, et al:
Mutations of the BRAF gene in human cancer. Nature. 417:949–954.
2002.
|
|
15
|
Wish TA, Hyde AJ, Parfrey PS, et al:
Increased cancer predisposition in family members of colorectal
cancer patients harboring the p. V600E BRAF mutation: a
population-based study. Cancer Epidemiol Biomarkers Prev.
19:1831–1839. 2010.
|
|
16
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009.
|
|
17
|
Knauf JA, Sartor MA, Medvedovic M, et al:
Progression of BRAF-induced thyroid cancer is associated with
epithelial-mesenchymal transition requiring concomitant MAP kinase
and TGFβ signaling. Oncogene. 30:3153–3162. 2011.
|
|
18
|
Levine RA, Chawla B, Bergeron S and
Wasvary H: Multidisciplinary management of colorectal cancer
enhances access to multimodal therapy and compliance with National
Comprehensive Cancer Network (NCCN) guidelines. Int J Colorectal
Dis. 27:1531–1538. 2012.
|
|
19
|
Bhatt KV, Spofford LS, Aram G, McMullen M,
Pumiglia K and Aplin AE: Adhesion control of cyclin D1 and p27Kip1
levels is deregulated in melanoma cells through BRAF-MEK-ERK
signaling. Oncogene. 24:3459–3471. 2005.
|
|
20
|
Wilusz JE, Sunwoo H and Spector DL: Long
noncoding RNAs: functional surprises from the RNA world. Genes Dev.
23:1494–1504. 2009.
|
|
21
|
Ishibashi M, Kogo R, Shibata K, et al:
Clinical significance of the expression of long non-coding RNA
HOTAIR in primary hepatocellular carcinoma. Oncol Rep. 29:946–950.
2013.
|
|
22
|
Kogo R, Shimamura T, Mimori K, et al: Long
noncoding RNA HOTAIR regulates polycomb-dependent chromatin
modification and is associated with poor prognosis in colorectal
cancers. Cancer Res. 71:6320–6326. 2011.
|
|
23
|
Matouk IJ, Abbasi I, Hochberg A, Galun E,
Dweik H and Akkawi M: Highly upregulated in liver cancer noncoding
RNA is overexpressed in hepatic colorectal metastasis. Eur J
Gastroenterol Hepatol. 21:688–692. 2009.
|
|
24
|
Tsang WP, Ng EK, Ng SS, et al: Oncofetal
H19-derived miR-675 regulates tumor suppressor RB in human
colorectal cancer. Carcinogenesis. 31:350–358. 2010.
|
|
25
|
Xu C, Yang M, Tian J, Wang X and Li Z:
MALAT-1: a long non-coding RNA and its important 3′ end functional
motif in colorectal cancer metastasis. Int J Oncol. 39:169–175.
2011.
|
|
26
|
Graham LD, Pedersen SK, Brown GS, et al:
Colorectal neoplasia differentially expressed (CRNDE), a novel gene
with elevated expression in colorectal adenomas and
adenocarcinomas. Genes Cancer. 2:829–840. 2011.
|
|
27
|
Ying L, Chen Q, Wang Y, Zhou Z, Huang Y
and Qiu F: Upregulated MALAT-1 contributes to bladder cancer cell
migration by inducing epithelial-to-mesenchymal transition. Mol
Biosyst. 8:2289–2294. 2012.
|
|
28
|
Luo M, Li Z, Wang W, Zeng Y, Liu Z and Qiu
J: Long non-coding RNA H19 increases bladder cancer metastasis by
associating with EZH2 and inhibiting E-cadherin expression. Cancer
Lett. 333:213–221. 2013.
|
|
29
|
Xu ZY, Yu QM, Du YA, et al: Knockdown of
long non-coding RNA HOTAIR suppresses tumor invasion and reverses
epithelial-mesenchymal transition in gastric cancer. Int J Biol
Sci. 9:587–597. 2013.
|