Introduction
The hedgehog (Hh) signaling pathway is one of the
most significant molecular mechanisms for the regulation of the
embryonic developmental process, and its activation shows an
association with the emergence of a number of solid tumors
(1,2). Studying the function and regulatory
mechanism of the HH signaling pathway may be further elucidate the
mechanisms underlying the development of malignant tumors and aid
in their diagnosis and treatment. The Hh family mainly includes
SHH, HHIP, PTCH1, Smo and Gli, where PTCH1 is a negative regulatory
factor of the Hh signaling pathway. The Hh pathway could be
activated by inhibiting the expression of PTCH1 and could also be
involved in tumorigenesis. It has been reported that the PTCH1 gene
could be methylated and involved in the formation of certain tumors
(3). However, the association
between PTCH1 methylation and gastric cancer is rarely reported.
The purpose of the present study was to investigate the role of
PTCH1 hypermethylation on gastric carcinogenesis by observing PTCH1
gene methylation and expression in gastric cancer tissues and the
gastric cancer AGS cell line, as well as by investigating the
effect of the demethylating agent, 5-aza-2′-deoxycytidine
(5-Aza-dC), on PTCH1 gene methylation and expression in gastric
cancer cells.
Materials and methods
Specimens and cell culture
A total of 20 gastric cancer tissues and their
corresponding adjacent normal tissues were collected from 20
gastric patients who underwent curative resections. These cancer
tissue specimens and adjacent normal tissue specimens were
routinely confirmed by biopsy and stored in liquid nitrogen. The
study group consisted of 14 male and 6 female patients, with a
median age of 60.12 years. No primary tumors from other sites were
observed for these gastric patients. Prior to surgery, the gastric
patients received no other treatment. The human gastric cancer AGS
cell line was purchased from the cell center of Shanghai Life
Science Institutes of the Chinese Academy of Sciences (Shanghai,
China) and was cultured with 10% fetal bovine serum (Hyclone,
Shanghai, China) and Ham’s F12K medium Sigma-Aldrich (St. Louis,
MO, USA) at 37°C and 5% CO2. This study was performed at
the First Hospital of Zhangjiagang (Zhangjiagang, China) and also
approved by the Institutional Review Board of the First Hospital of
Zhangjiagang. Written informed consent was obtained from each
patient.
Instruments and reagents
5-Aza-2′-deoxycytidine (5-Aza-dc) was purchased from
Sigma-Aldrich, and TRIzol reagent was bought from Invitrogen Life
Technologies (Carlsbad, CA, USA). The RNA reverse transcription
kit, propidium iodide (PI) and Annexin-V/PI double-staining
streaming apoptosis detection kits were purchased from Shanghai
Jingmei Biological Engineering Co., Ltd. (Shanghai, China). The
methylation conversion kit, EZ DNA Methylation-Gold™, was purchased
from the Beijing Science and Technology Development Co., Ltd.
(Beijing, China). The ABI7500 Real-Time PCR instrument was
manufactured by Applied Biosystems (Life Technologies, Carlsbad,
CA, USA).
AGS cell treatment with 5-Aza-dC
AGS cells (3×105) were plated in 100-ml
flasks with Ham’s F12K medium containing 10% fetal bovine serum at
37°C with 5% CO2. Next, 24 h after the cells had reached
the logarithmic growth phase, they were treated with
5×10−6 mol/l 5-Aza-dc. The treatment medium was changed
every 24 h for 3 times, then the cells were collected. Control cell
groups without 5-Aza-dc treatment were also collected for
comparison.
Total RNA extraction, cDNA synthesis and
DNA extraction
Total RNA (dissolved with 50 μl Tris-EDTA) was
extracted by a conventional method once 100 mg tissue in liquid
nitrogen had been ground into a powder, or after 1×106
AGS cells had been washed with phosphate-buffered saline (PBS) and
collected by centrifugation at 12,000 rpm for 15 min. The
concentration and purity of the RNA were tested by electrophoresis.
To synthesize cDNA, 1 μl 0.5 μg/μl Oligo-(dT)18 and 10
μl DEPC-H2O were added to 1 μl total RNA and mixed, and
then bathed in 70°C water for 5 min and cooled rapidly on ice. The
following reagents were then added: 4 μl 5× reaction buffer, 1 μl
20 U/μl RNA enzyme inhibitors and 2 μl 10 mmol/l dNTP. The mixture
was held in a water bath at 37°C for 5 min, then 1 μl 200 U/μl
reverse transcriptase (M-MuLV) was added and the mixture was placed
in a 42°C water bath for 60 min. The process was completed with
immersion in a 70°C water bath for 10 min. The synthesis reaction
was terminated. Synthesized cDNA product were stored at −20°C. The
DNA of the tumor tissues and cells were obtained using the
phenol/chloroform extraction method. DNA quality was measured with
a NanoDrop-1000 full wavelength UV/VIS scanning spectrophotometer
(Thermo Fisher Scientific, Waltham, MA, USA).
PTCH1 gene quantitative (q)PCR
analysis
PTCH1 gene qPCR analysis was started with 2 μl cDNA
product as a template, adding 0.5 μl for each PTCH1 upstream and
downstream primer (Shanghai Sangon Biological Engineering
Technology and Services Co, Ltd., Shanghai, China; Table I), as well as 10 μl 2× SYBR Green
Real Time PCR Master Mix liquid (Shanghai GeneCore BioTechnologies
Co., Ltd., Shanghai, China) and 7 μl sterile water. Next, detection
of 35 amplification loops were conducted with the ABI7500 Real-Time
PCR instrument at conditions of 95°C for 5 sec, 55°C for 5 sec and
72°C for 30 sec. The melting curves of the amplified products were
analyzed, using β-actin as an internal reference. The relative
expression of the PTCH1 gene was calculated by 2−ΔΔCt
and analyzed by agarose gel electrophoresis.
 | Table IPrimer sequences and length of PCR
product. |
Table I
Primer sequences and length of PCR
product.
| PCR type | Primer name | Primer sequences | Product length,
bp |
|---|
| qPCR | PTCH1 |
5′-TGTGCGCTGTCTTCCTTCTG-3′
5′-ACGGCACTGAGCTTGATTC-3′ | 119 |
| β-actin |
5′-GCCATCCTGCGTCG-3′
5′-TGGGCACCGGAACCGCT-3′ | 260 |
| MSP | Methylation |
5′-GTTAATTCGTGATTTTTCGGA-3′
5′-ATAACAAACCTACGAACCGC-3′ | 197 |
| Unmethylation |
5′-AATGTTAATTTGTGATTTTTTGGA-3′
5′-TAAATAACAAACCTACAAACCAC-3′ | 197 |
Cell cycle and apoptosis detection by
flow cytometry
Treated and untreated AGS cells (~1×106
cells each) were collected and centrifuged at 1000 rpm for 5 min,
and then the culture medium was discarded. The cells were washed
once with 3 ml 0.01 mol/l PBS (pH 7.4), which was then removed by
centrifugation at 1,500 rpm for 10 min. The cells were fixed for 24
h at 4°C by adding 1 ml ice-cold 70% ethanol. The fixative was
centrifuged at 1,500 rpm for 10 min and discarded again. The cells
were resuspended with 3 ml PBS for 5 min. The cells were filtered
once with a 400-mesh screen and the PBS was removed by
centrifugation. The cells were stained with 1 ml PI dye (final
concentration at 1,500 rpm for 10 min of 100 μg/ml, 0.01 mol/l PBS,
pH 7.4) and stored at 4°C in the dark for 30 min. A flow cytometer
was used to detect the cell cycle and apoptosis (BD FACSCalibur, BD
Biosciences, Franklin Lakes, NJ, USA).
DNA bisulfite conversion
DNA bisulfite conversion was conducted using the EZ
DNA Methylation-Gold kit, according to the manufacturer’s
instructions. Briefly, 130 μl CT Conversion Reagent was added to 20
μl DNA (500 ng), then mixed and maintained at 98°C for 10 min. The
sample was then held at 64°C for 2.5 h. Storage was at 4°C. A total
of 600 μl M-Binding Buffer was added into an activated Zymo-spin
column. The converted DNA that was stored at 4°C was then added
into the spin column and the content was mixed by inversion.
Centrifugation at full-speed (12,000 rpm) was applied for 30 sec,
then the effluent was removed and 100 μl M-Wash Buffer was added
into the column. Centrifugation at 12,000 rpm was applied for 30
sec and then 200 μl M-Desulphonation Buffer was added into the
column prior to storage for 15–20 min and centrifugation again at
full speed (12,000 rpm) for 30 sec. A total of 200 μl M-Wash Buffer
was added into the column and centrifugation at 12,000 rpm was
applied for 30 sec. Another 30 μl M-Elution Buffer was added, and
then the transformed DNA was collected by centrifugation at 12,000
rpm and stored at −20°C for 1 week.
Methylation-specific PCR (MSP)
detection
CpG island analysis and primer design for PTCH1 mRNA
of the transcription start site (counted as 0:00) −3950 bp upstream
and +2050 bp downstream were conducted with Methyl Primer
Express® v1.0 software (Applied Biosystems; Life
Technologies). Table I lists the
MSP primers. PCR cooling amplification was performed in 8 μl
bisulfite-treated DNA with a conventionally configured reaction
system. Briefly, pre-degeneration was completed at 95°C for 5 min.
Prior to PCR, predegeneration was completed at 95°C for 5 min.
Next, cooling PCR was performed for 10 cycles (PCR degeneration at
94°C for 30 sec, with renaturation temperature decreases of 0.5°C
per cycle; temperature decreases from +3°C to −2°C for 30 sec; and
extension at 72°C for 30 sec). Next, the normal PCR was performed
for 40 cycles (PCR degeneration at 94°C for 30 sec; −2°C
renaturation for 30 sec; and extension at 72°C for 30 sec).
Amplification products were analyzed by 1.5% agarose gel
electrophoresis.
Statistical analysis
Data were analyzed with SPSS 13.0 statistical
software (SPSS, Inc., Chicago, IL, USA). A non-parametric
Mann-Whitney U test was applied to compare the differences in
relative PTCH1 mRNA expression between the gastric carcinoma and
adjacent normal tissues. Fisher’s exact test was applied to compare
the differences in PTCH1 gene promoter methylation rate between the
gastric cancer and adjacent normal tissues. Spearman’s test was
used to study the correlation between PTCH1 methylation and its
expression in gastric cancer tissues.
Results
Expression of PTCH1 mRNA in the gastric
cancer AGS cell line and gastric cancer and adjacent normal
tissues
The expression of the PTCH1 gene in the gastric
cancer tissues and gastric cancer AGS cell line were observed by
qPCR. Taking AGS as a reference sample, the relative expression
levels of PTCH1 in the gastric cancer and adjacent normal tissues
were 1.26±0.89 and 2.74±1.67, respectively. There was a significant
statistical difference between the two groups (n=20; P=0.023).
Methylation changes in the PTCH1 gene
promoter region of gastric cancer and adjacent normal tissues,
detected using MSP
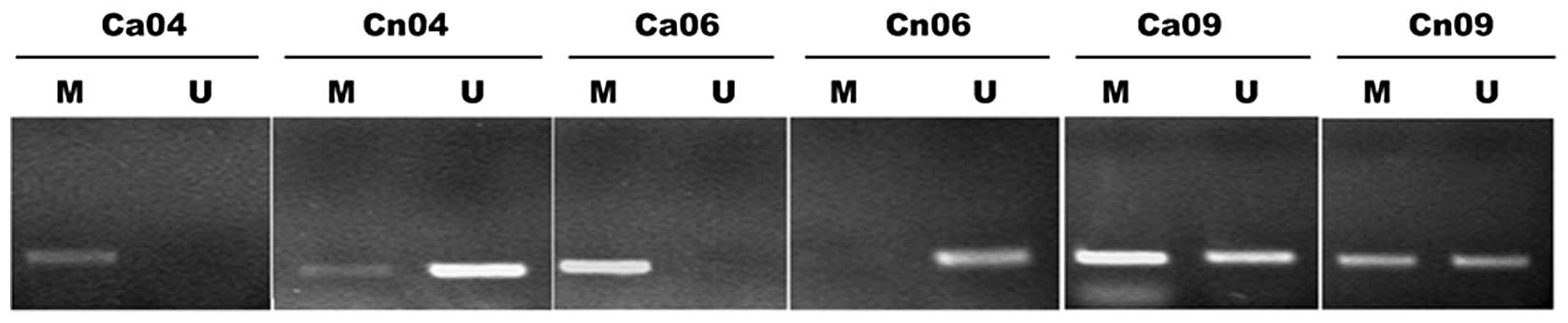
In order to observe the incidence of PTCH1 gene
methylation in the gastric cancer tissues, MSP detection was
conducted in the gastric cancer and adjacent normal tissues of 20
patients. Methylation amplified bands were observed in 12 cancer
tissues and 4 adjacent normal tissues, with an incident rate of 60%
and 20%, respectively (Fisher’s exact probability test; two-tailed
test P=0.057 and one-tailed test P=0.029). This indicated the
presence of high PTCH1 gene methylation in the gastric cancer
tissues. Fig. 1 shows typical
electrophoretograms.
Correlation between PTCH1 methylation and
expression in gastric cancer tissues
In order to further study the association between
PTCH1 expression and promoter methylation, the correlation between
PTCH1 gene methylation and its relative expression in gastric
cancer and adjacent normal tissues was analyzed in 20 gastric
cancer patients. The qPCR values of PTCH1 in methylated and
unmethylated tissues were 0.93±0.71 and 2.58±1.52, respectively.
The expression of PTCH1 was negatively correlated with the
methylation status, with a correlation coefficient of −0.591
(P=0.006).
Impact of 5-Aza-dC treatment on the cell
cycle, the apoptosis of AGS cells and the PTCH1 gene methylation
status
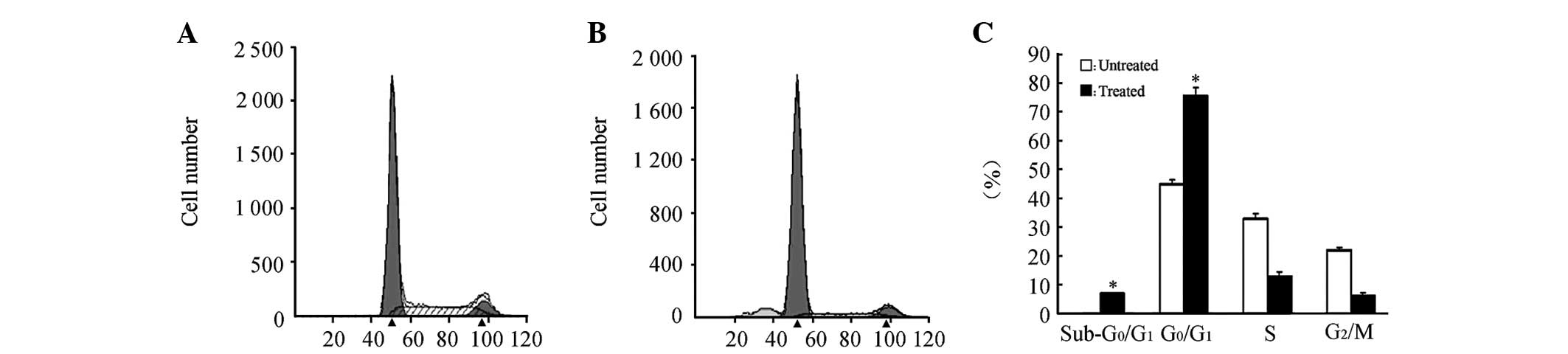
PI staining was conducted in the AGS cells at the
logarithmic phase at 72 h post-treatment with 5×10−6
mol/l 5-Aza-dC, then the cell cycle of the treated AGS cells was
analyzed (Fig. 2). A
G0/G1 block was observed in the treated AGS
cells. Meanwhile, Annexin V/PI double-staining was conducted to
detect the cell apoptosis of the treated AGS cells. Significant
levels of apoptosis were observed in the treated AGS cells. There
were significant differences between the treated group and the
control group according to the results of three independent
experiments (P<0.05; Fig. 2).
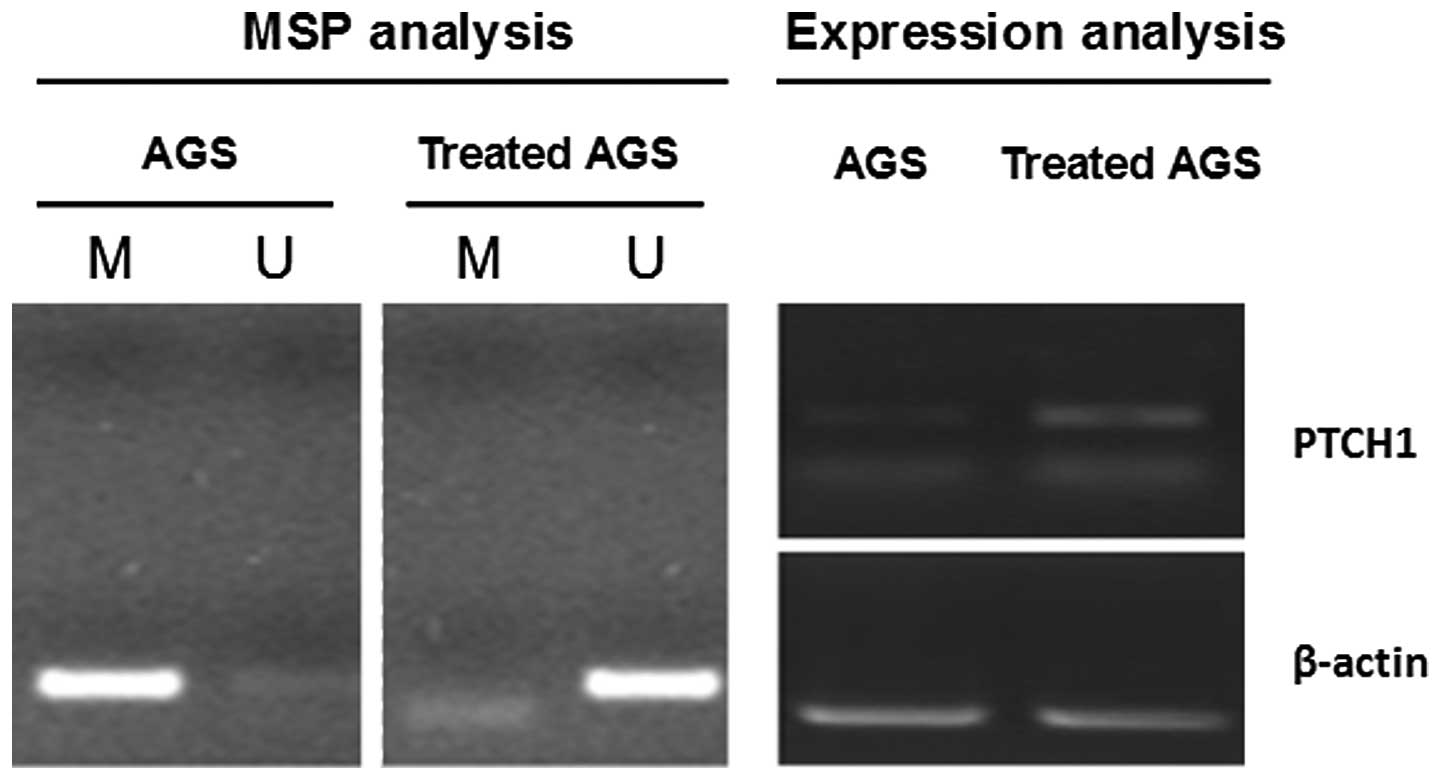
MSP and relative expression measurements were conducted on PTCH1
gene methylation and expression in the AGS cells prior to and
following the treatment with 5-Aza-dC AGS, as shown in Fig. 3 (4).
Amplification was obtained for the methylated sequence of the PTCH1
gene, but not for the demethylated sequence in the AGS cells prior
to the treatment. The relative PTCH1 mRNA expression was low in the
AGS cells prior to the treatment. Amplification was obtained for
the demethylated sequence of the PTCH1 gene, but not for the
methylated sequence in the AGS cells following the treatment. The
relative expression of the PTCH1 mRNA was increased in the AGS
cells following the treatment. This further indicates the negative
correlation between PTCH1 gene hypermethylation and expression.
Discussion
Cancer epigenetics studies have found that there is
widespread hypomethylation and partial regional hypermethylation of
CpG islands in the genomic DNA of cancer cells. Partial regional
hypermethylation of CpG islands may result in the inactivation of
certain tumor suppressor genes, which is an important mechanism for
causing the malignant transformation of cells (5,6).
Demethylation agents, such as 5-Aza-dC, could be used to obtain the
re-expression of these hypermethylation genes and to play a
significant role in tumor suppression (7).
The Hh signaling pathway is a crucial signal
transduction pathway in the regulation of embryonic development.
The Hh signal is most active in the embryonic formation period, and
has no expression or extremely low expression in normal mature
tissues. However, aberrant activation of the Hh signal transduction
pathway in cells of mature tissues and organs can result in various
diseases and tumorigenesis. The high expression of SHH in small
cell lung cancer tissues and cell lines has been reported in a
previous study (8).
In total, 81% of tumor cell lines from the digestive
tract (including the esophagus, stomach, bile duct and pancreas)
have been shown to express SHH and its receptor, PTCH1 (9). The hypomethylation of HHIP, the
inhibitor of SHH, has been observed in gastrointestinal tumors
(10). The missing or mutated PTCH1
gene has rarely been reported in gastric cancer in previous
studies. Few studies have also reported the correlation between the
methylation of the PTCH1 gene and gastric cancer. In the present
study, the expression of PTCH1 mRNA was detected in gastric cancer
tissues, adjacent normal tissues and a human gastric cancer cell
line, with a relatively high expression of PTCH1 mRNA in the
adjacent normal tissues compared with the cancer tissues. High
methylation levels of PTCH1 were observed in the gastric cancer
tissues and the cancer cell lines. This indicates that the high
methylation modification of PTCH1 as a tumor suppressor gene may be
one of the main mechanisms of gastric cancer.
In conclusion, the hypermethylation of the PTCH1
gene promoter region in gastric cancer was observed in the present
study. Following this preliminary result, further studies of
molecular mechanisms involved in regulating the PTCHI methylation
changes and the association between PTCH1 hypermethylation and the
biological features of gastric cancer are required. More scientific
experimental evidence regarding PTCH1 gene hypermethylation as a
gastric cancer marker and its function in guiding the treatment and
prognosis of gastric cancer are also required from these
studies.
Acknowledgements
This study was supported by a grant from the Open
Project Program of Key Discipline for Medicine of Jiangsu Province
(WKF2013-05).
References
|
1
|
Saqui-Salces M and Merchant JL: Hedgehog
signaling and gastrointestinal cancer. Biochim Biophys Acta.
1803:786–795. 2010.
|
|
2
|
Shahi MH, Lorente A and Castresana JS:
Hedgehog signaling in medulloblastoma, glioblastoma and
neuroblastoma. Oncol Rep. 19:681–688. 2008.
|
|
3
|
Cul’bová M, Lasabová Z, Stanclová A, et
al: Methylation of selected tumor-suppressor genes in benign and
malignant ovarian tumors. Ceska Gynekol. 76:274–279. 2011.(In
Slovak).
|
|
4
|
Zuo Y and Song Y: Detection and analysis
of the methylation status of PTCH1 gene involved in the hedgehog
signaling pathway in a human gastric cancer cell line. Exp Ther
Med. 6:1365–1368. 2013.
|
|
5
|
Tada M, Kanai F, Tanaka Y, et al:
Down-regulation of hedgehog-interacting protein through genetic and
epigenetic alterations in human hepatocellular carcinoma. Clin
Cancer Res. 14:3768–3776. 2008.
|
|
6
|
Caffarelli E and Filetici P: Epigenetic
regulation in cancer development. Front Biosci (Landmark Ed).
16:2682–2694. 2011.
|
|
7
|
To KF, Leung WK, Lee TL, et al: Promoter
hypermethylation of tumor-related genes in gastric intestinal
metaplasia of patients with and without gastric cancer. Int J
Cancer. 102:623–628. 2002.
|
|
8
|
Watkins DN, Berman DM, Burkholder SG, et
al: Hedgehog signalling within airway epithelial progenitors and in
small-cell lung cancer. Nature. 422:313–317. 2003.
|
|
9
|
Ma X, Sheng T, Zhang Y, et al: Hedgehog
signaling is activated in subsets of esophageal cancers. Int J
Cancer. 118:139–148. 2006.
|
|
10
|
Taniguchi H, Yamamoto H, Akutsu N, et al:
Transcriptional silencing of hedgehog-interacting protein by CpG
hypermethylation and chromatic structure in human gastrointestinal
cancer. J Pathol. 213:131–139. 2007.
|