Introduction
The phosphatidylinositol 3-kinase (PI3K)/Akt pathway
is a cell survival pathway that is important in cell growth and
proliferation (1). In addition,
this pathway is known to be activated by radiation. Mammalian
target of rapamycin (Rap) (mTOR) is a 289-kDa serine/threonine
kinase and a downstream target of Akt (2). The normal activation of mTOR may lead
to an increase in protein translation, as mTOR phosphorylates and
activates the translation regulators, eukaryotic initiation factor
4E-binding protein 1 and ribosomal p70S6 kinase (3,4). In
addition, it has been shown that mTOR is important for the
oncogenic transformation induced specifically by PI3K and Akt,
components of a pathway that has also been indicated to be involved
in tumorigenesis (5), which is
becoming an important target for cancer treatment (6,7).
The PI3K/Akt pathway has also been demonstrated to
be associated with the occurrence, development and prognosis of
esophageal carcinoma. Hou et al (8) reported that the overexpression of mTOR
signaling in esophageal carcinoma Eca109 and EC9706 cells was found
to positively correlate with the malignancy of cancer cells. In
addition, Hirashima et al (9) reported that mTOR signaling was
abnormally activated in 116/167 (69.5%) cases of esophageal
squamous cell carcinoma (ESCC) in five ESCC cell lines. Clinically,
Hirashima et al (10) also
reported that the overexpression of phosphorylated (p)-mTOR was an
independent factor associated with a poor prognosis in esophageal
carcinoma. Furthermore, Hildebrandt et al (11) reported that gene mutations in the
PI3K/Akt/mTOR signaling pathway (Akt1, Akt2 and FRAP1) are
associated with the clinical prognosis of chemoradiotherapy.
mTOR has also been investigated as a target for
cancer therapy (6,12). Nishikawa et al (13) reported that temsirolimus (a
rapamycin derivative) treatment reduced the ability of ESCC cells
to proliferate, and thus inhibited subcutaneous tumors in nude mice
and effectively prolonged the survival of orthotopic esophageal
cancer-bearing mice. The mTOR inhibitor was also demonstrated to
decrease the phosphorylation of its downstream effectors, and
decrease gene expression and protein synthesis, thus, effectively
obstructing the pro-growth, pro-proliferation and pro-survival
effects of mTOR (14).
It has been reported that the combination of Rap and
the DNA-damaging chemotherapeutic agent, cisplatin, may present an
effective means of improving cancer treatment (15,16).
However, whether mTOR inhibition enhances radiation-induced DNA
damage in esophageal carcinoma cells remains unclear. The aim of
the present study was to investigate the effects of radiation on
mTOR signaling and to determine whether the inhibition of mTOR by
Rap enhances the radiosensitivity of Eca109 cells.
Materials and methods
Cell culture
The Eca109 cell lines were obtained from Chongqing
Medical University (Chongqing, China) and were cultured in
Dulbecco’s modified Eagle’s medium (Gibco-BRL, Carlsbad, CA, USA)
supplemented with 10% fetal bovine serum (Gibco-BRL), 100 U/ml
penicillin and 100 μg/ml streptomycin. All cells were incubated at
37°C in an atmosphere of 5% CO2.
Western blotting
All cells were homogenized in protein lysis buffer
(Beyotime Institute of Biotechnology, Nanjing, China) and
centrifuged at 15,000 × g for 15 min, and the supernatant was
harvested to obtain the total cellular protein extracts. The
protein concentrations were determined using the bicinchoninic acid
method. The total cellular protein extracts were separated by 6%
SDS-PAGE for p-mTOR and DNA-dependent protein kinase catalytic
subunit (DNA-PKcs), on 10% SDS-PAGE for p-p70S6K, Ku70, Ku80 and
β-actin, and on 12% SDS-PAGE for cleaved caspase-3, bax and bcl-2.
The proteins were electrotransferred to nitrocellulose membranes
(Amersham Pharmacia Biotech, Stockholm, Sweden) by a wet or
semi-dry transfer. The membranes were then blocked with 0.5%
skimmed milk and Tris-buffered saline with Tween 20 (TBST) for 2 h
at room temperature (RT) and rinsed three times with TBST for 30
min. Next, the cells were incubated with primary polyclonal rabbit
anti-human antibodies against p-mTOR, DNA-PKcs, p-p70S6K, p-p70S6K,
Ku70, Ku80 and β-actin purchased from Bioworld (Dublin, OH, USA)
and cleaved polyclonal rabbit anti-human caspase-3, polyclonal
rabbit anti-human bax and polyclonal mouse anti-human bcl-2
purchased from Santa Cruz Biotechnology, Inc., (Santa Cruz, CA,
USA) and diluted with 0.5% skimmed milk in TBST at 4°C overnight,
followed by rinsing three times with TBST for 30 min. The cells
were then incubated with the appropriate monoclonal goat anti-mouse
immunofluorescence-conjugated secondary antibodies (Odyssey,
Lincoln, NE, USA). Finally, the bands of specific proteins on the
nitrocellulose membranes (Amersham Pharmacia Biotech) were
visualized with an Odyssey infrared imaging system (Odyssey).
MTT assay
The cell suspension (200 μl) was seeded in 96-well
plates (3,000 cells/well), into five repeat wells and cultured for
24 h. Next, the cells were treated with 0, 100, 200 and 400 nmol/l
Rap or the same volume of dimethyl sulfoxide (DMSO) treatment for 1
h, in addition to treatment with different radiation doses of 0, 1,
2, 4 and 6 Gy, followed by a five day incubation period. Next, 20
μl/well of MTT solution (5 g/l; Sigma-Aldrich, St. Louis, MO, USA)
was added and the cells were incubated at 37°C for 4 h. The medium
was then aspirated and 150 μl DMSO was added and oscillated for 10
min for formazan solubilization. The absorbance was determined at a
wavelength of 470 nm using a microplate reader (BioTek Instruments,
Inc., Winooski, VT, USA).
Clonogenic assay
The cell suspensions (2 ml) were seeded in six-well
plates (1,000 cells/well), into three repeat wells, and cultured
for 24 h, following treatment with 200 nmol/l Rap or the same
volume of DMSO for 1 h, and radiation with various doses of 0, 1,
2, 4 or 6 Gy. The cells were then incubated for 10–14 days and
fixed with 4% paraformaldehyde (Beijing Solarbio Sciences and
Technology Co., Ltd., Beijing, China) and stained with crystal
violet (Sigma-Aldrich). The clone formations (≥50 cells) were
counted using an Olympus microscope (Olympus, Tokoyo, Japan).
Fluorescence-activated cell sorting
(FACS)
The Eca109 cells treated with a combination of Rap
and radiation, and Rap or radiation alone, were trypsinized, washed
with cold phosphate-buffered saline (PBS) and resuspended in PBS. A
total of 500 μl binding buffer, 5 μl Annexin V-fluorescein
isothiocyanate (final concentration of 1 μg/ml) and 5 μl propidium
iodide (final concentration of 250 ng/ml) (BD Biosciences, Franklin
Lakes, NJ, USA) was added to the mixture. The cells were then
vortexed and incubated for 10 min at RT in the dark for flow
cytometric analysis using a FACScan Flow Cytometer (BD
Biosciences).
Comet assay
The cell suspension was added to PBS and mixed with
low-melting point agarose (200 cells/100 μl) to prepare the slides
for the comet assay. The cells were lysed for 2 h in 4°C precooling
PBS (pH 8.0–8.4) and the DNA was uncoiled for 20 min in
Tris-borate-EDTA buffer. Electrophoresis was conducted at 20 V and
200 mA for 20 min, followed by staining with ethidium bromide (2.5
μg/ml) for 10 sec. The cells were then examined at ×200
magnification using a fluorescence microscope (Nikon Inc.,
Melville, NY, USA). The tail moment of 50 randomly selected cells
per group was measured using comet assay analysis CASP1.2 software
(Krzysztof Konca, Wroclaw, Poland).
Statistical analysis
The experimental data were analyzed using SPSS
version 16.0 (SPSS, Inc., Chicago, IL, USA) and quantitative data
was presented as χ2 ± standard deviation. Two groups
were compared using the t-test and multiple groups were compared
using one-way analysis of variance. P<0.05 was considered to
indicate a statistically significant difference and all P-values
were two-sided.
Results
Radiation induces mTOR signaling of
esophageal carcinoma Eca109 cells, and mTOR inhibitor Rap inhibits
this effect
Western blotting revealed that following irradiation
with 4 Gy radiation for 1 or 3 h, the expression levels of p-mTOR
and p-P70S6K were increased, and were higher than those of the
control groups. These results showed that radiation induced mTOR
signaling in the Eca109 cells (Fig.
1A). In addition, the combination of Rap and 4 Gy radiation for
1 h was found to decrease the expression of p-mTOR and p-P70S6K in
the Eca109 cells of the Rap and 4 Gy + Rap groups, which indicated
that the mTOR inhibitor, Rap, inhibits the mTOR signaling pathway,
and may also inhibit the radiation-induced mTOR signaling pathway
(Fig. 1B).
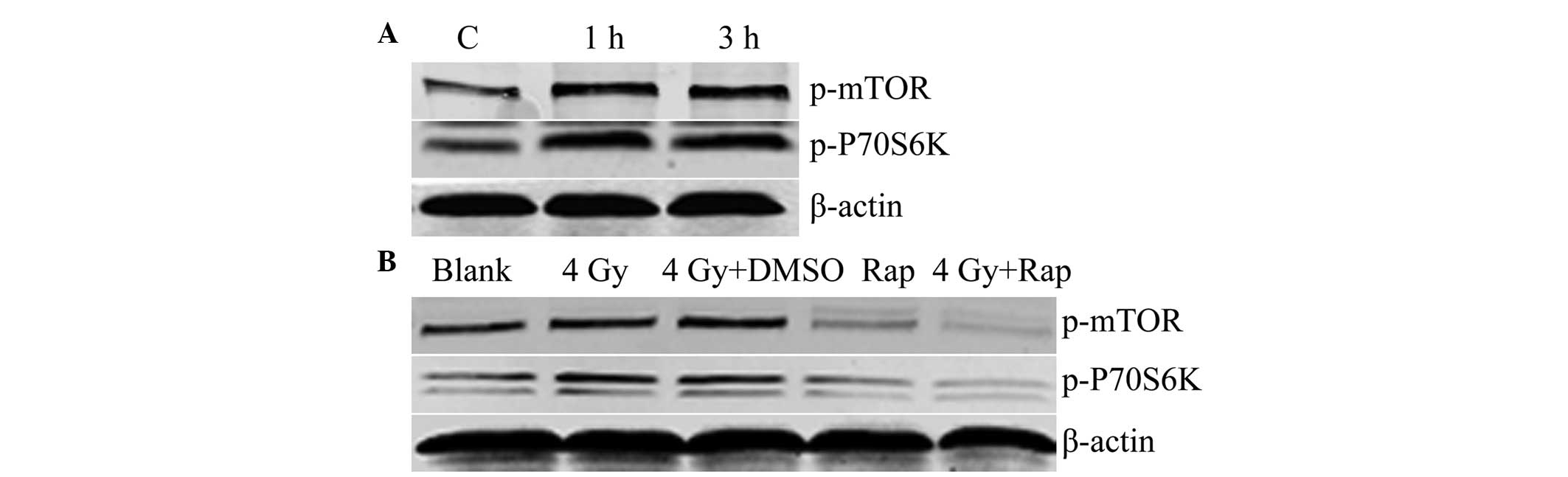 | Figure 1(A) Radiation-induced mTOR signaling
(C, control with no radiation; 1 h, 1 h post-irradiation with 4 Gy;
and 3 h, 3 h post-irradiation with 4 Gy). (B) Rap inhibited mTOR
signaling (blank group, PBS only; 4 Gy group; 4 Gy + DMSO group;
Rap group, 200 nmol/l Rap; 4 Gy + Rap group, 4 Gy + 200 nmol/l
Rap). mTOR, mammalian target of rapamycin; p-mTOR, phosphorylated
mammalian target of rapamycin; DMSO, dimethyl sulfoxide; Rap,
rapamycin; PBS, phosphate-buffered saline. |
mTOR inhibition by Rap improves the
proliferation inhibition rate of Eca109 cells treated with
radiation
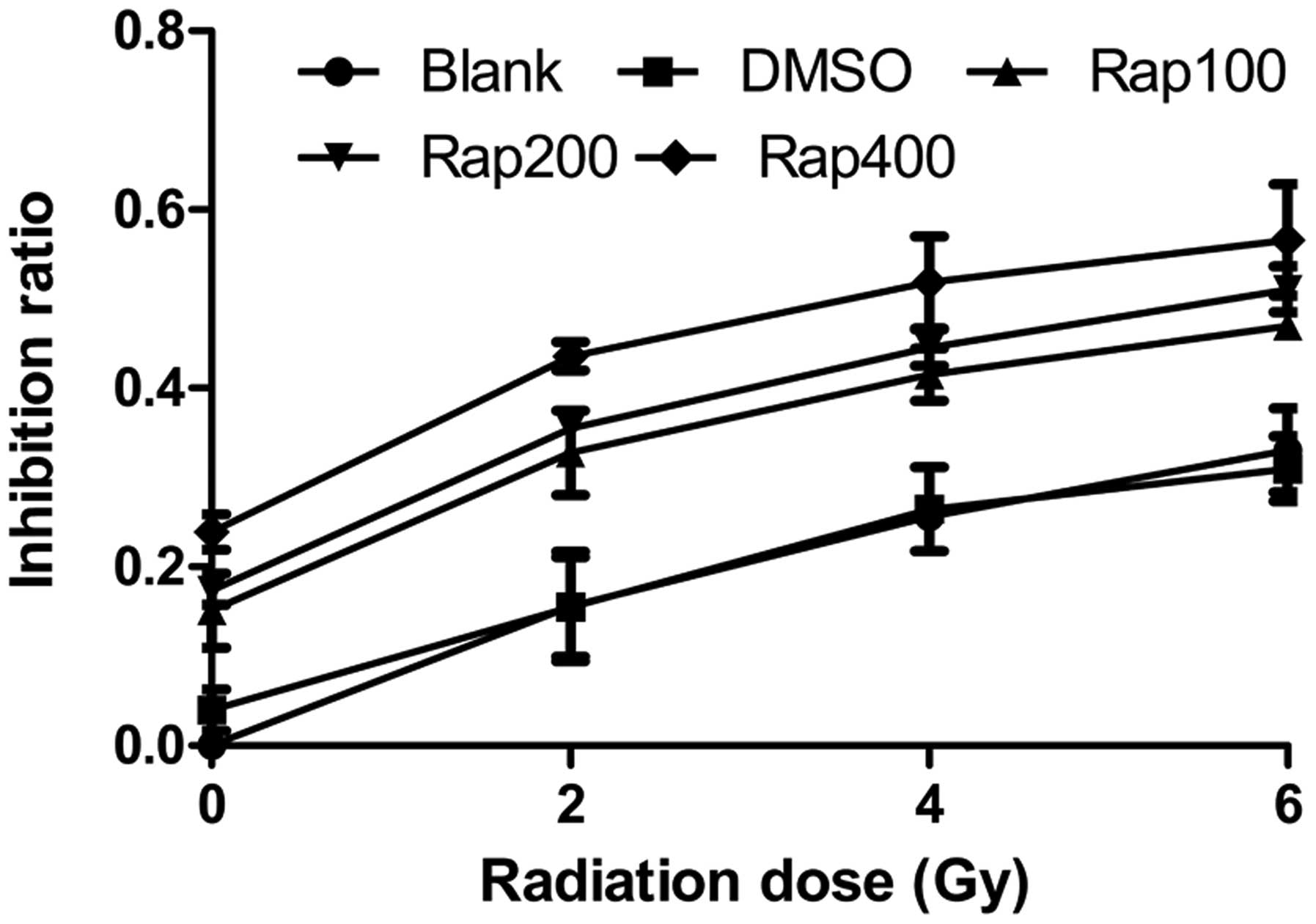
The MTT assay showed that following irradiation with
0, 2, 4, and 6 Gy, the proliferation inhibition rates of the Eca109
cells treated with 100, 200 (Rap200) and 400 nmol/l Rap were higher
than that of the blank group (P=0.005 for 100 nmol; P=0.001 for 200
nmol; and P<0.001 for 400 nmol) and the DMSO group (P=0.007 for
100 nmol; P=0.001 for 200 nmol; and P<0.001 for 400 nmol)
(Fig. 2), while no significant
difference was identified between the blank and DMSO groups
(P=0.899). Within the same radiation dose with increasing Rap
concentrations, the proliferation inhibition rate of the Eca109
cells improved. In addition, within the same Rap concentration with
increasing radiation doses, the proliferation inhibition rate also
improved. These results showed that the mTOR inhibitor, Rap,
inhibits the proliferation of Eca109 cells, and may also inhibit
the proliferation of Eca109 cells treated with irradiation.
mTOR inhibition by Rap decreases the
survival fraction of Eca109 cells treated with radiation
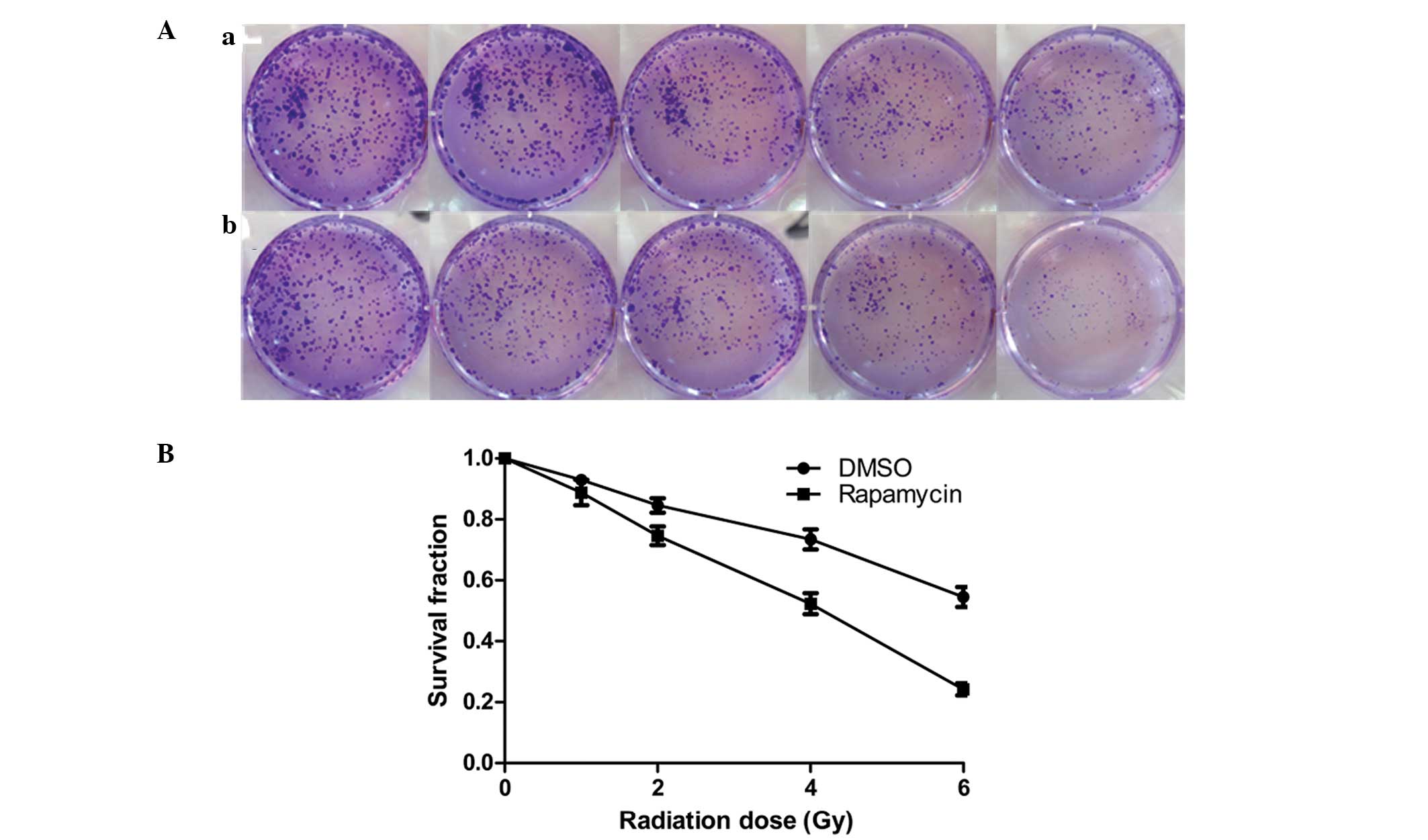
The clonogenic assay revealed that with increasing
radiation doses (0, 1, 2, 4 and 6 Gy) cell clone formation was
reduced, and at each radiation dose the clone formation of the
Eca109 cells in the radiation + Rap200 groups was less than that of
the radiation + DMSO groups (Fig.
3A). In addition, the survival curve for the Eca109 cells in
the radiation + Rap200 groups was found to be significantly lower
compared with that of the radiation + DMSO groups (P=0.015;
Fig. 3B).
mTOR inhibition by Rap promotes the
apoptosis of Eca109 cells treated with radiation
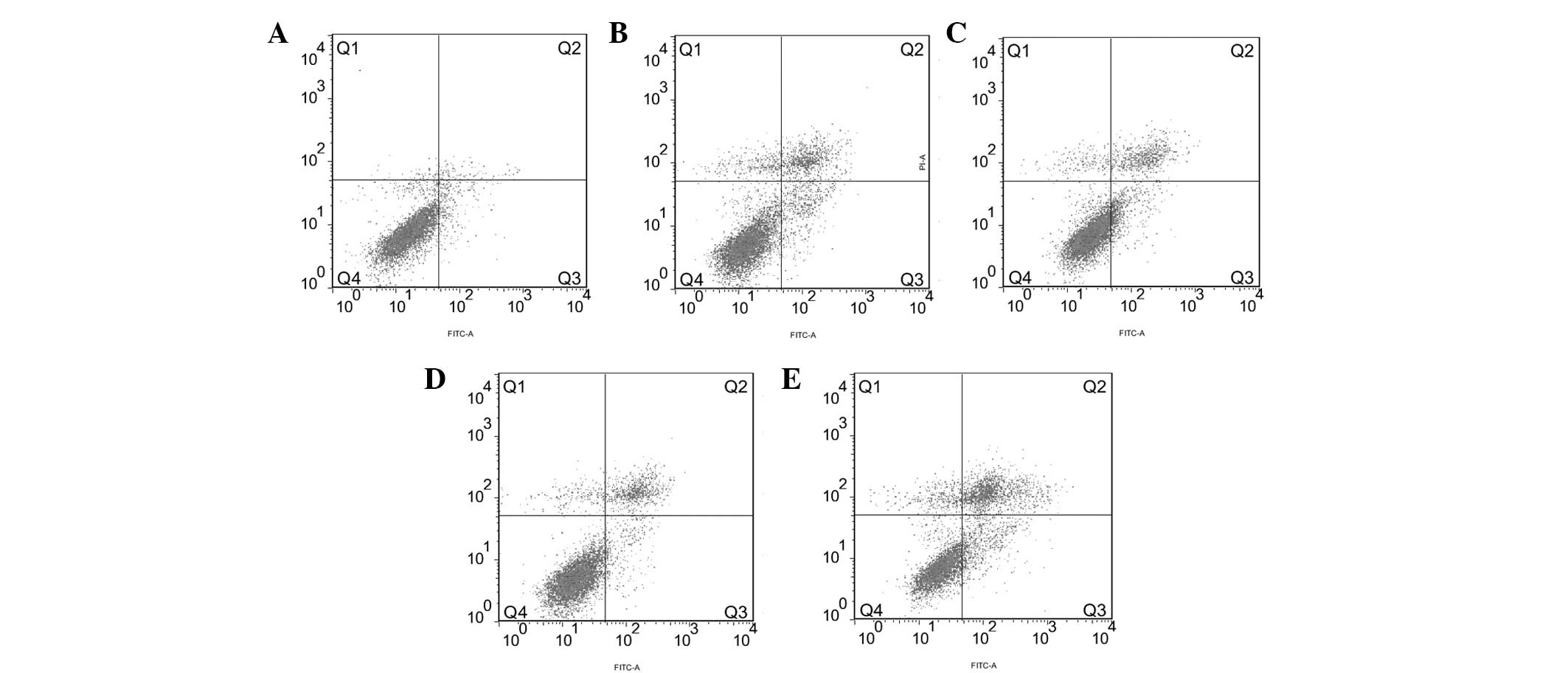
FACS analysis showed that the apoptosis rate of the
Eca109 cells in the 4 Gy + Rap200 group (31.63±0.90%) was higher
than that of the Rap200 (14.04±0.15%), 4 Gy + DMSO (17.96±0.31%), 4
Gy only (17.35±0.61%) and blank (4.19±0.48%) groups (all
P<0.001; Fig. 4). The apoptosis
rate of the Rap200 group was lower than that of the 4 Gy + DMSO
(P=0.002) and 4 Gy (P=0.001) groups, while no significant
difference was identified between the 4 Gy + DMSO and 4 Gy
groups.
mTOR inhibition by Rap promotes the
apoptotic protein expression of Eca109 cells treated with
radiation
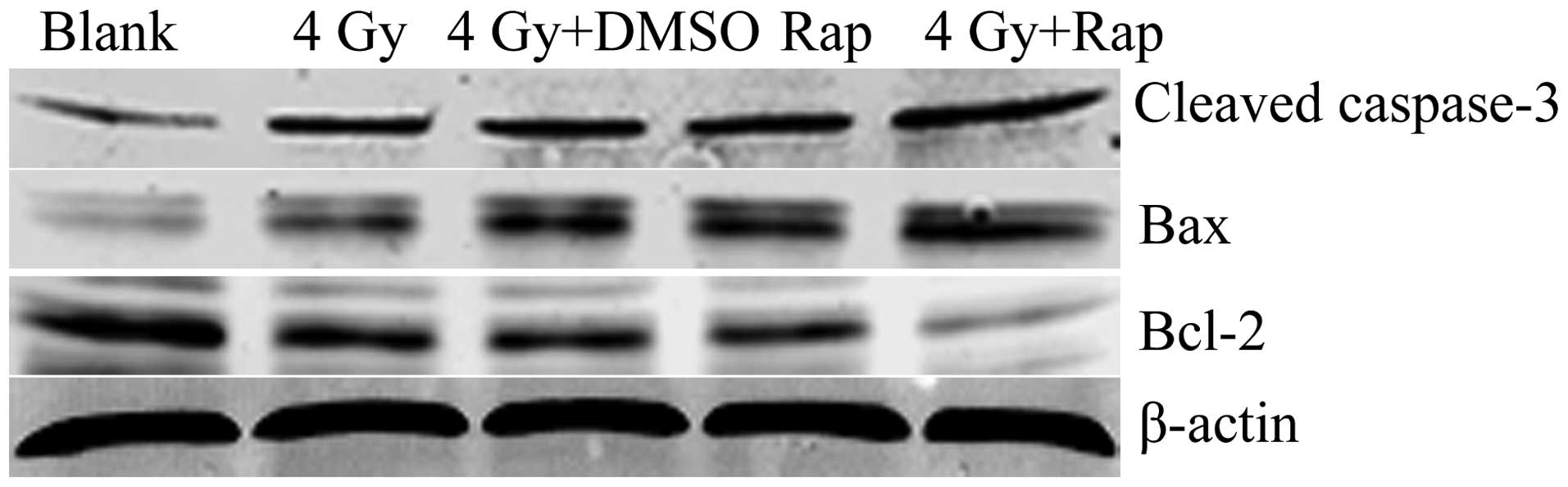
Western blotting revealed that the expression of the
apoptotic proteins, cleaved caspase-3 and bax, in the 4 Gy + Rap200
group was significantly higher than that of the Rap only, 4 Gy +
DMSO, 4 Gy only and blank groups. In addition, the expression of
the apoptosis-inhibiting protein, bcl-2, was less than that of the
control groups. These results indicated that Rap may promote
radiation-induced apoptotic protein expression and inhibit the
apoptosis-inhibiting protein expression in Eca109 cells (Fig. 5).
Radiation induces the expression of DNA
damage repair proteins of esophageal carcinoma Eca109 cells, and
mTOR inhibitor Rap inhibits this effect
The opportunity for DNA damage-repair in cells comes
between 4 and 6 h post-radiation treatment. Thus, 1 and 3 h
following 4 Gy radiation were selected for analysis. Western
blotting showed that the expression of Ku70, Ku80 and DNA-PKcs was
increased and higher than that of the control group. These results
indicated that radiation induces the expression of the DNA
damage-repair proteins in Eca109 cells (Fig. 6A). The combination of Rap and the
irradiation of Eca109 cells at 4 Gy for 1 h was found to result in
a decrease in the expression of Ku70, Ku80 and DNA-PKcs in the Rap
and 4 Gy + Rap groups, which indicated that the mTOR inhibitor Rap
inhibits expression of DNA damage-repair proteins, and may also
inhibit radiation-induced DNA damage-repair protein expression
(Fig. 6B).
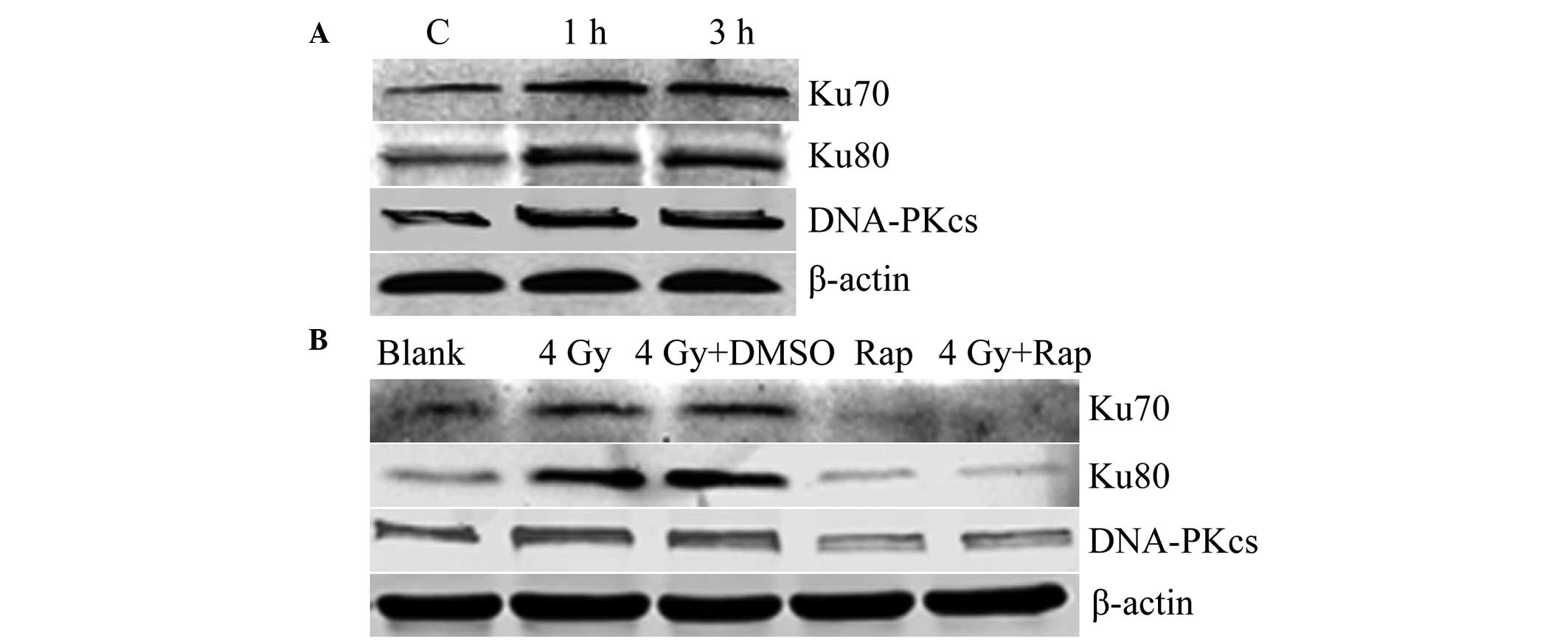 | Figure 6Expression of the DNA damage-repair
protein (A) increased following radiation (C, control with no
radiation; 1 h, 1 h post-irradiation with 4 Gy; and 3 h, 3 h
post-irradiation with 4 Gy) and (B) decreased following mTOR
inhibition by Rap (blank group, PBS only; 4 Gy group; 4 Gy + DMSO
group; Rap group, 200 nmol/l Rap only; and 4 Gy + Rap group, 4 Gy +
200 nmol/l Rap). DNA-PKcs, DNA-dependent protein kinase catalytic
subunit; DMSO, dimethyl sulfoxide; Rap, rapamycin; PBS,
phosphate-buffered saline. |
mTOR inhibition by Rap enhances
radiation-induced DNA damage in Eca109 cells
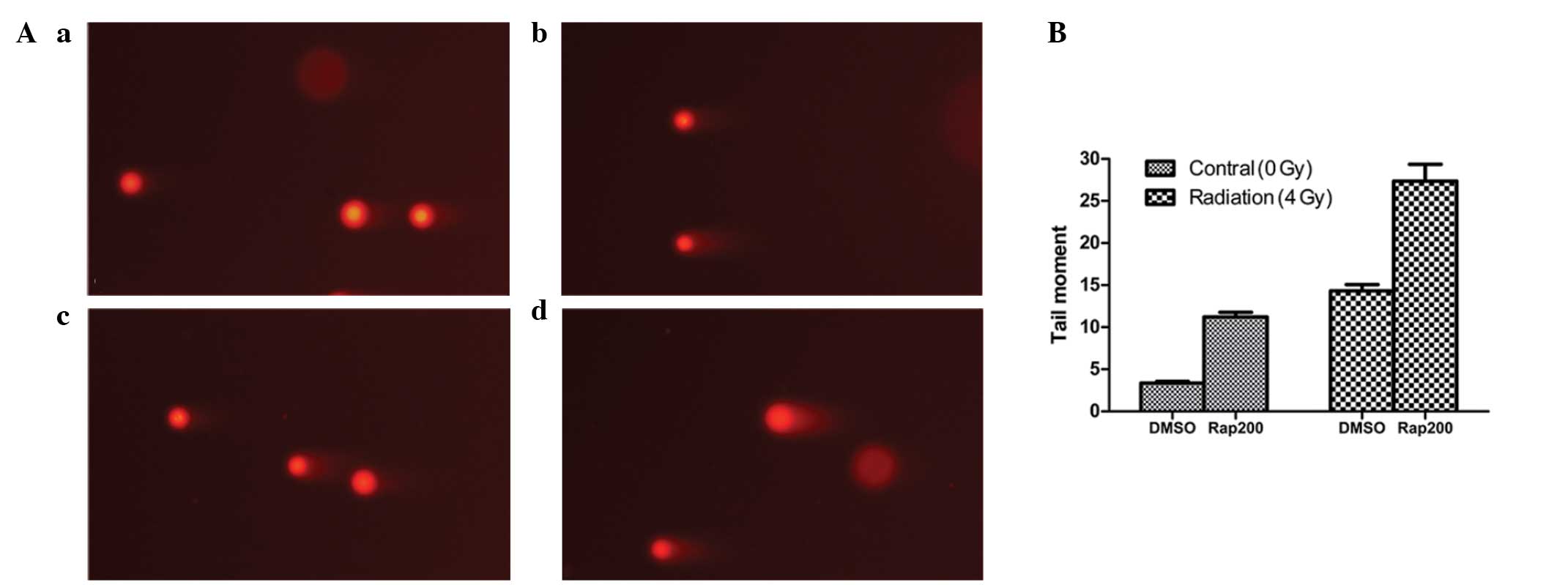
The comet assay revealed that following irradiation
with 4 Gy radiation for 30 min, the DNA damage in the Eca109 cells
in the 4 Gy + Rap group was greater than that in the control groups
(4 Gy + DMSO, Rap200 and DMSO groups; Fig. 7A). Furthermore, the tail moment of
the 4 Gy+Rap group (27.35±2.02) was longer than that of the control
groups (all P<0.001), whereas the tail moment of the DMSO + 4 Gy
(14.32±0.74) and Rap200 (11.21±0.55) groups was longer than that of
the DMSO group (3.37±0.20) (both P<0.001; Fig. 7B). These results indicated that the
mTOR inhibitor, Rap, may enhance the radiation-induced DNA damage
of Eca109 cells.
Discussion
mTOR, a downstream kinase of the PI3K/Akt pathway,
is crucial for cell growth and survival (1). Therefore, the use of mTOR as a
therapeutic target is becoming increasingly significant in cancer
research. In the present study, radiation was found to increase
mTOR signaling in the Eca109 cell line, and the mTOR inhibitor was
found to block this signaling activation, enhancing the
radiosensitivity of the Eca109 cells.
As radiotherapy is an important adjuvant therapy for
esophageal carcinoma, it is undesirable that radiation may
contribute to cancer cell survival. In the present study, mTOR
signaling was increased by ionizing radiation in the esophageal
carcinoma Eca109 cells. Therefore, blocking the activation of
radiation-induced mTOR signaling presents a method of enhancing the
cytotoxic effects of radiation. To demonstrate this, the Eca109
cells were treated with the mTOR inhibitor, Rap, to block the
increase in phosphorylation of the downstream marker p70S6K
protein. The results showed that the proliferation inhibition rate
of the Eca109 cells treated with a combination of Rap and radiation
was increased (Fig. 2), while the
survival fraction was decreased (Fig.
3). This indicated that the inhibition of mTOR by Rap may
effectively block the pro-survival response of esophageal carcinoma
cells to radiation, and that mTOR inhibition may present a method
for enhancing the efficacy of radiotherapy.
Hou et al (15) used a combination of Rap and the
DNA-damaging agent, cisplatin, to treat the subcutaneous tumors of
ectopic esophageal carcinoma in nude mice, and revealed that it
resulted in greater inhibition of tumor growth, while Rap
sensitized the cancer cells to DNA damage-induced apoptosis, which
was similar to the effect reported by Beuvink et al
(16). We hypothesized that
combining radiation with the mTOR inhibitor Rap would also
effectively sensitize cancer cells to apoptosis. Therefore, the
apoptosis rate of the Eca109 cells treated with Rap and radiation
was determined. The FACS results revealed that the apoptosis rate
of the 4 Gy + Rap200 group was 31.63±0.90, which was found to be
significantly higher than that of the Rap200 (14.04±0.15), 4 Gy +
DMSO (17.96±0.31), 4 Gy only (17.35±0.61) and blank (4.19±0.48)
groups (Fig. 4). In addition, the
western blotting results showed that the expression of cleaved
caspase-3 and bax were significantly higher than that of the
control groups, whereas the expression of bcl-2 was less than that
of the control groups (Fig. 5).
These results showed that the apoptosis of the cells treated with
Rap and radiation was greatly increased, indicating that
caspase-dependent and -independent apoptosis contribute to the
observed increase in cell apoptosis.
Radiation predominantly results in the death of
cancer cells by reducing the number of DNA double-strand breaks
(DSBs), however, there are various degrees of DSB repair potency in
cells, which affects the radiosensitivity of cancer cells. Two
repair pathways have been identified (17): DNA non-homologous end-joining (NHEJ)
involving DNA-protein kinases, including Ku70, Ku80 and DNA-PKcs;
and homologous recombination involving the ataxia telangiectasia
mutated protein. The NHEJ repair pathway is the predominant
radiation-induced DNA damage repair pathway in humans (18). In the present study, radiation was
found to induce mTOR signaling (Fig.
1A) and simultaneously increase the expression of Ku70, Ku80
and DNA-PKcs following irradiation (Fig. 6A). Therefore, we hypothesized a
correlation among the NHEJ repair pathway, radiation and mTOR
signaling. The mTOR inhibitor, Rap, was used to inhibit mTOR
signaling, and western blotting revealed that when mTOR signaling
was obstructed (Fig. 1B), the Ku70,
Ku80 and DNA-PKcs expression in the Rap200 and 4 Gy + Rap200 groups
was decreased (Fig. 6B). In
addition, the comet assay showed that the radiation-induced DNA
damage of the 4 Gy + Rap200 group was greater than that of the
control groups (Fig. 7A).
Furthermore, the tail moment of the 4 Gy + Rap200 group
(27.35±2.02) was longer than that of the 4 Gy only (14.32±0.74),
Rap200 only (11.21±0.55) and blank (3.37±0.20) groups (Fig. 7B). These results showed that the
inhibition of mTOR by Rap also inhibits the NHEJ repair pathway
prior to and following radiation, indicating that mTOR inhibition
may be a mechanism for decreasing the potency of radiation-induced
DNA damage repair, which may contribute to the increased
radiosensitivity observed in the present study.
It is known that cell cycle arrest may affect
radiosensitivity (19), as cells
exhibit varying radiosensitivities in different cell cycle phases,
and thus, cell cycle regulation is important for radiosensitivity.
The cells are most sensitive to radiation during the
G2-M phase, less sensitive during the G1
phase and least sensitive during the end of the S phase (20). Hou et al (15) reported that Rap inhibiting mTOR may
result in the cell cycle G0/G1 arrest of
esophageal carcinoma cells. However, this was not investigated in
the present study. Whether cell cycle G0/G1
arrest affects the radiosensitivity of Eca109 cells requires
further study.
Additionally, it has been shown that Rap may inhibit
angiogenesis (21), and that the
mTOR inhibitors, Rap and RAD001, may significantly enhance the
radiosensitivity of the tumor vasculature in vitro and in
vivo (22). We hypothesize that
mTOR inhibition with Rap may exhibit a greater increase in the
radiosensitivity of esophageal carcinoma in vivo. It is
possible that the combination of mTOR inhibition and radiation may
improve the efficacy of tumor radiotherapy via a dual mechanism
that promotes radiation-induced tumor cell cytotoxicity and
inhibits tumor angiogenesis. Therefore, whether mTOR inhibition
enhances the radiosensitivity of esophageal carcinoma in
vivo also requires further study.
References
|
1
|
Cantley LC: The phosphoinositide 3-kinase
pathway. Science. 296:1655–1657. 2002.
|
|
2
|
Navé BT, Ouwens M, Withers DJ, Alessi DR
and Shepherd PR: Mammalian target of rapamycin is a direct target
for protein kinase B: identification of a convergence point for
opposing effects of insulin and amino-acid deficiency on protein
translation. Biochem J. 344:427–431. 1999.
|
|
3
|
Beugnet A, Wang X and Proud CG: Target of
rapamycin (TOR)-signaling and RAIP motifs play distinct roles in
the mammalian TOR-dependent phosphorylation of initiation factor
4E-binding protein 1. J Biol Chem. 278:40717–40722. 2003.
|
|
4
|
Burnett PE, Barrow RK, Cohen NA, Snyder SH
and Sabatini DM: RAFT1 phosphorylation of the translational
regulators p70 S6 kinase and 4E-BP1. Proc Natl Acad Sci USA.
95:1432–1437. 1998.
|
|
5
|
Nicholson KM and Anderson NG: The protein
kinase B/Akt signalling pathway in human malignancy. Cell Signal.
14:381–395. 2002.
|
|
6
|
Wong KK, Engelman JA and Cantley LC:
Targeting the PI3K signaling pathway in cancer. Curr Opin Genet
Dev. 20:87–90. 2010.
|
|
7
|
Morgensztern D and McLeod HL:
PI3K/Akt/mTOR pathway as a target for cancer therapy. Anticancer
Drugs. 16:797–803. 2005.
|
|
8
|
Hou G, Xue L, Lu Z, Fan T, Tian F and Xue
Y: An activated mTOR/p70S6K signaling pathway in esophageal
squamous cell carcinoma cell lines and inhibition of the pathway by
rapamycin and siRNA against mTOR. Cancer Lett. 253:236–248.
2007.
|
|
9
|
Hirashima K, Baba Y, Watanabe M, et al:
Aberrant activation of the mTOR pathway and anti-tumour effect of
everolimus on oesophageal squamous cell carcinoma. Br J Cancer.
106:876–882. 2012.
|
|
10
|
Hirashima K, Baba Y, Watanabe M, et al:
Phosphorylated mTOR expression is associated with poor prognosis
for patients with esophageal squamous cell carcinoma. Ann Surg
Oncol. 17:2486–2493. 2010.
|
|
11
|
Hildebrandt MA, Yang H, Hung MC, et al:
Genetic variations in the PI3K/PTEN/AKT/mTOR pathway are associated
with clinical outcomes in esophageal cancer patients treated with
chemoradiotherapy. J Clin Oncol. 27:857–871. 2009.
|
|
12
|
Gomez-Pinillos A and Ferrari AC: mTOR
signaling pathway and mTOR inhibitors in cancer therapy. Hematol
Oncol Clin North Am. 26:483–505. 2012.
|
|
13
|
Nishikawa T, Takaoka M, Ohara T, et al:
Antiproliferative effect of a novel mTOR inhibitor temsirolimus
contributes to the prolonged survival of orthotopic esophageal
cancer-bearing mice. Cancer Biol Ther. 14:230–236. 2013.
|
|
14
|
Emerling BM and Akcakanat A: Targeting
PI3K/mTOR signaling in cancer. Cancer Res. 71:7351–7359. 2011.
|
|
15
|
Hou G, Zhang Q, Wang L, Liu M, Wang J and
Xue L: mTOR inhibitor rapamycin alone or combined with cisplatin
inhibits growth of esophageal squamous cell carcinoma in nude mice.
Cancer Lett. 290:248–254. 2010.
|
|
16
|
Beuvink I, Boulay A, Fumagalli S, et al:
The mTOR inhibitor RAD001 sensitizes tumor cells to DNA-damaged
induced apoptosis through inhibition of p21 translation. Cell.
120:747–759. 2005.
|
|
17
|
van Gent DC, Hoeijmakers JH and Kanaar R:
Chromosomal stability and the DNA double-stranded break connection.
Nat Rev Genet. 2:196–206. 2001.
|
|
18
|
Lieber MR, Ma Y, Pannicke U and Schwarz K:
Mechanism and regulation of human non-homologous DNA end-joining.
Nat Rev Mol Cell Biol. 4:712–720. 2003.
|
|
19
|
Hwang HS, Davis TW, Houghton JA and
Kinsella TJ: Radiosensitivity of thymidylate synthase-deficient
human tumor cells is affected by progression through the G1
restriction point into S-phase: implications for fluoropyrimidine
radiosensitization. Cancer Res. 60:92–100. 2000.
|
|
20
|
Pawlik TM and Keyomarsi K: Role of cell
cycle in mediating sensitivity to radiotherapy. Int J Radiat Oncol
Biol Phys. 59:928–942. 2004.
|
|
21
|
Guba M, von Breitenbuch P, Steinbauer M,
et al: Rapamycin inhibits primary and metastatic tumor growth by
antiangiogenesis: involvement of vascular endothelial growth
factor. Nat Med. 8:128–135. 2002.
|
|
22
|
Shinohara ET, Cao C, Niermann K, Mu Y,
Zeng F, Hallahan DE and Lu B: Enhanced radiation damage of tumor
vasculature by mTOR inhibitors. Oncogene. 24:5414–5422. 2005.
|