Introduction
Cervical cancer is the third most common type of
cancer in the female population worldwide. Globally, cervical
cancer is considered to be the seventh most common type of cancer,
with 530,232 cases reported in 2008. More than 85% of cases occur
in developing countries (1). Latin
America and the Caribbean have a very high incidence of cervical
cancer. The World Health Organization reports 33,000 new cases per
year (2,3). At present, in Mexico, cervical cancer
is the second most common cause of cancer-associated mortality in
females, and there is a population of 40.06 million 15-year old
females who are at risk of developing cervical cancer. It is
estimated that each year, 13,960 females are diagnosed with
cervical cancer and that 4,476 succumb due to the disease (4). Cervical cancer develops due to
persistent infection with the oncogenic Human Papilloma Virus
(HPV), which progresses into an intraepithelial lesion, then into
invasive cervical cancer.
The majority of patients are diagnosed at an
advanced stage of cervical cancer, losing the most important window
for treatment. This cancer is preceded by precursor lesions which
have been classified into three progressive degrees, termed
cervical intraepithelial neoplasia (CIN) I–III by Richart (5,6), while
the Bethesda classification system divides the precursor lesions
into low-grade squamous intraepithelial lesions (LSILs) and
high-grade squamous intraepithelial lesions (HSILs) (7). HSILs have been proposed to be the true
precursors to squamous cell cervical carcinoma. A number of studies
have reported that persistent HSILs develop into carcinomas in
40–100% of cases, while LSILs show spontaneous regression (8,9).
The most common diagnostic method that is used to
detect these lesions is based on the cytology and histopathology of
the cervical tissues and cells. The Papanicolaou (PAP) smear, also
known as the PAP test, is a low-cost method that is easily
accessible, with 50% sensitivity and high susceptibility to intra-
and inter-individual variability (10,11).
This technique has many limitations, with false negative results
often reported (20–30%) due to sample manipulation and
contamination (12). The
introduction of liquid-based cytology has contributed to a
reduction in the efficiency problem associated with sample
processing; however, validation in terms of sensitivity and
specificity still presents deficiencies (10,11,13).
Liquid-based cytology enables the detection of low- and high-grade
dysplasia. Despite not being a definitive diagnostic method,
liquid-based cytology does determine the presence of a lesion, as
well as its topography, extension and severity. It also allows
direct biopsies to be taken for further histopathological analysis
(14).
In the last few years, there has been a trend toward
identifying novel molecular biomarkers using proteomic tools that
enable the identification of early lesions that have the greatest
risk of malignant transformation. These types of tools allow the
screening of proteins on a larger scale and from different
biological samples, including serum, plasma, cells and tissues.
They also allow the identification of molecules expressed at very
low concentrations (fentomoles) with high sensitivity and
specificity (15,16). Biofluids, including serum and
plasma, are the main source of biomarkers due to their low cost,
ease of collection, non-invasive collection and their easy
processing (17,18).
A number of studies have investigated novel
biomarkers in the serum (19–22),
plasma (23–25) and tissue (26–31)
from patients with early cervical lesions and/or cervical cancer,
using proteomic tools. A large number of proteins have been
identified that show differential expression between samples from
healthy females and those from females with different types of
intraepithelial lesions or cervical cancer. However, none of the
proteins comply with the characteristics required for a specific
marker, according to international requirements, which would be
useful for the detection of lesions that have a greater probability
of being transformed into cancer. At present, the markers under
validation are Ki67, pINK4A, MIB-1 and ProExC (32–34).
The present study aimed to investigate a marker in the serum of
females infected with HPV and the histopathological diagnosis of
advanced CIN (CIN III).
Materials and methods
Patients and biological samples
The present study is a descriptive and transversal
pilot study on serum samples from a population of Mexican females.
A total of 10 samples (five controls and five cases) of blood were
obtained from females aged between 28 and 65 years who were
recruited from the Hospital General Regional del Estado de Morelos
(IMSS; Cuernavaca, Mexico) who fulfilled the inclusion criteria and
accepted to donate a blood sample under informed consent. The
present study was reviewed and approved by the Ethics Committee of
the IMSS and the IRB from the Instituto Nacional de Salud Pública
(Cuernavaca, Mexico). The inclusion criteria for the cases were:
(i) a positive Hybrid Capture test; (ii) an abnormal PAP test; and
(iii) CIN III lesion diagnosis confirmed via colposcopy and
histopathology, using the Bethesda criteria. Colposcopic and
histopathologic analyses were performed by two specialists from the
Colposcopy Unit at the IMSS and two pathologists form the Pathology
Unit at the IMSS. In addition, positivity for HPV 16 was confirmed
using polymerase chain reaction (PCR) analysis (Table I). Only the serum samples from the
females who were diagnosed with CIN III were included in the
present study. The inclusion criteria for the control group were:
(i) a negative hybrid capture test; (ii) a normal PAP test; and
(iii) no apparent injury through gynecological physical
examination. For the control and case groups, the inclusion
criteria also included not having had a HPV vaccine, not having
taken oral contraceptives in the previous six months and not having
received chemotherapy.
 | Table ICharacteristics of the individuals in
the case and control groups who were included for 2D-gel
analysis. |
Table I
Characteristics of the individuals in
the case and control groups who were included for 2D-gel
analysis.
| Sample | Number | Age (years) | PAP test | Hybrid capture
test | Colposcopy | Histopathology | HPV type |
|---|
| Control group |
| 1 | C1-IMSS | 38 | Normal cytology | (−) | Nda | Nda | Nd |
| 2 | C2-IMSS | 41 | Normal cytology | (−) | Nda | Nda | Nd |
| 3 | C3-IMSS | 47 | Normal cytology | (−) | Nda | Nda | Nd |
| 4 | C4-IMSS | 47 | Normal cytology | (−) | Nda | Nda | Nd |
| 5 | C7-IMSS | 40 | Normal
cytology | (−) | Nda | Nda | Nd |
| Case group |
| 1 | T1-IMSS | 65 | Abnormal
cytology | (+) | HSIL | CIN III | 16 |
| 2 | T3-IMSS | 60 | Abnormal
cytology | (+) | HSIL | CIN III | 16 |
| 3 | T7-IMSS | 42 | Abnormal
cytology | (+) | HSIL | CIN III | 16 |
| 4 | T8-IMSS | 32 | Abnormal
cytology | (+) | HSIL | CIN III | 16 |
| 5 | T10-IMSS | 28 | Abnormal
cytology | (+) | HSIL | CIN III | 16 |
Blood sample processing
Blood samples (3 ml) were obtained using
venipuncture in Vacutainer® SSTTM tubes
(Becton-Dickinson, Mexico City, Mexico) with separator gel and were
left at room temperature for 1 h. Samples were then centrifuged at
1,300 × g for 15 min. The serum was stored at −80°C in 100 μl
aliquots until processed. Serum aliquots (10 μl) in triplicate from
each patient were passed through a high-affinity column to remove
albumin and immunoglobulin G (IgG) using a commercial kit
(ProteoSeek™ 162 Albumin/IgG removal kit; Pierce Biotechnology
Inc., Rockford, IL, USA). Other interfering substances, including
detergents, salts, lipids, nucleic acids and phenolic acids, were
removed from the samples using the Two-dimensional (2D) Clean Up
kit (GE Healthcare; Little Chalfont, UK). Serum protein
quantification was performed using the 2D Quant kit (GE
Healthcare).
2D gel electrophoresis (2D-GE)
A total of 200 μg protein (in triplicate) was mixed
with rehydration buffer [8 M urea, 2% CHAPS (Roche Diagnostics
GmbH, Mannheim, Germany), 0.5% IPG2 buffer (pH 3–10; GE
Healthcare), 0.002% bromophenol blue and 0.56 M dithiothreitol
(DTT; Sigma-Aldrich, Munich, Germany)] in a final volume of 250 μl.
To each tube, 1 μl IPG2 buffer and 0.00056 g DTT was added.
Isoelectric focusing was performed using an Ettan™ IPGphor™ unit
using 13-cm strips (GE Healthcare). The rehydration time was 14 h
at a constant current of 50 mA per strip. The voltages used for
isoelectric focusing were 500, 1,000 and 8,000 V/h. The strips were
then equilibrated in buffer [50 mM Tris-HCl (pH 8.8;
Sigma-Aldrich), 6 M urea, 30% Glycerol, 2% SDS (Sigma-Aldrich),
0.002% bromophenol blue (Sigma-Aldrich) and Milli-Q™ water
(Millipore Milli-Q lab water system; Millipore, Billerica, MA,
USA)] supplemented with DTT (50 mg/5 ml) for 15 min. The strips
were then equilibrated with iodoacetamide (125 mg/5 ml; GE
Healthcare) for the same duration of time. Protein separation was
performed using SDS-PAGE in two dimensions on gradient gels of
7.5–20% at a constant voltage of 150 V for 5.5 h. Gels were stained
with Colloidal Coomassie Blue G-250 (Bio-Rad, Hercules, CA, USA),
as described previously (34).
Subsequent to staining, the gels were scanned using LabScan™ 5.0
(GE Healthcare). Digital images were used for detection and
analysis using ImageMaster 2D Platinum 6.0 software (GE
Healthcare). The selected spots were identified using electrospray
ionization time-of-flight mass spectrometry (ESI-TOF-MS). In brief,
protein spots were excised from the Coomassie-stained 2D gels and
destained using 50% (v/v) methanol and 5% (v/v) acetic acid
(Mallinckrodt, Baker Inc., Paris, KY, USA) for 12 h. The destained
gels were then washed with deionized water, soaked for 10 min in
100 mM ammonium bicarbonate (Sigma-Aldrich), cut into small pieces,
completely dehydrated using 100% acetonitrile (Mallinckrodt, Baker
Inc.) and vacuum-dried. In-gel digestion was performed through
adding 30 μl modified porcine trypsin solution (20 ng/ml; Promega
Corporation, Madison, WI, USA) to 50 mM ammonium bicarbonate
followed by overnight incubation at room temperature. Peptides were
extracted using 50% (v/v) acetonitrile and 5% (v/v) formic acid
(Mallinckrodt, Baker Inc.) twice for 30 min with sonication. The
extract volumes were reduced using evaporation in a vacuum
centrifuge and were adjusted to 20 μl using 1% (v/v) formic
acid.
Liquid chromatography tandem mass spectrometry
(LC/MS/MS). Mass spectrometric analysis of the
tryptic peptides was performed using an integrated nano-LC ESI
MS/MS system (Synapt G2 High Definition mass spectrometer; Waters
Corporation, Milford, MA, USA) equipped with a NanoLockSpray™ ion
source. The instrument was coupled online to a NanoAcquity Ultra
Performance liquid chromatograph (UPLC; Waters Corporation). The
binary solvent system used was 2% acetonitrile in Milli-Q water
with 0.1% formic acid (mobile phase A) and 98% acetonitrile in
Milli-Q water with 0.1% formic acid (mobile phase B). Samples were
concentrated and desalted through injection into a Symmetry C18
UPLC trapping column (5 mm, 180×20 mm; Waters Corporation) and
washed with 100% mobile phase A at a flow rate of 15 μl/min for 3
min. Next, the trap column was switched in-line (coupled) with the
analytical BEH C18 UPLC column (1.7 μm, 75 μmx100 mm; Waters
Corporation) for peptide seperation, using a linear gradient to 40%
B over a 30 min period, at a flow rate of 0.3 μl/min. The column
was then washed for 10 min with 98% mobile phase B.
The mass spectrometer was calibrated using an NaCl
solution and operated in ESI positive V-mode at a resolution of
10,000 full width at half height. Spectra were acquired in the
automated mode using data-dependent acquisition (DDA). Fibrin
peptide B solution (100 fmol/μl) was infused through the reference
sprayer of the NanoLockSpray source at a flow rate of 500 nl/min
and was sampled at 30 sec intervals during the acquisition. MS
survey scans of 1 sec over the m/z range 300–1,600 were used for
the peptide detection followed by two MS/MS scans of 2 sec each
(m/z, 50–2,000) of detected precursors. Collision energies were
automatically adjusted based on the ion charge state and the mass.
The five most intensive precursor ions were interrogated per MS/MS
switching event. Dynamic exclusion for 60 sec was used in order to
minimize multiple MS/MS events for the same precursor.
Data processing and protein
identification
DDA raw data files were processed and converted to
pkl files using ProteinLynx Global Server software, version 2.4
(Waters Corporation). Pkl files were subsequently database-searched
using the Mascot search algorithm (version 1.6b9; Matrix Science,
London, UK). The specific genome was not available; thus, searches
were performed using the human subset of the National Center for
Biotechnology Information non-redundant database (NCBInr;
http://www.ncbi.nih.gov). Trypsin was used as a
specific protease and one missed cleavage was allowed with mass
tolerances of 50 ppm and 0.05 Da for the precursor and fragment
ion, respectively. Variable modifications included methionine
oxidation and glutamine-asparagine deamination. Peptide matches
with Mascot scores exceeding the 95% level of confidence were
accepted as correct matches. The threshold score was 48 for
P<0.05.
Western blot analysis for α-1-B
glycoprotein (A1BG) and complement component 3 (C3)
The serum samples used for western blot analysis
were obtained from a group of 55 females participating in the HPV
Detection Service at the IMSS. This group of females underwent a
Pap smear and a hybrid capture test. If the Pap smear showed any
alterations, the participants also underwent colposcopic and
histopathologic analysis. For the western blot analysis, the
control group (n=30) included females with a normal Pap smear and a
negative hybrid capture test, while the case group (n=25) included
females with an abnormal Pap smear, positive hybrid capture test
and a diagnosis of CIN III using colposcopy and histopathology. In
addition, in the case group, the HPV type was identified using PCR
(HPV 16, 56%; HPV 58, 8%; HPV 33, 8%; HPV 18, 4%; and unidentified,
24%). A total of 20 μg total protein from the serum was separated
using unidimentional 10% SDS-PAGE and transferred to polyvinylidene
fluoride membranes (GE Healthcare). The membranes were blocked for
1 h in 0.1% Tween 20 and 5% non-fat dry milk in Tris-buffered
saline (TBS) at room temperature. Primary antibodies against mouse
monoclonal Ig-G anti-human A1BG (clone 51A6) and mouse monoclonal
Ig-G anti-human complement C3 (clone 2898) (Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA) were diluted 1:2,000 and
1:1,000, respectively in 0.1% Tween 20 and 5% non-fat dry milk in
TBS. The membranes were then incubated with the primary antibodies
overnight at 4°C. Membranes were subsequently washed with 0.1%
Tween 20 in TBS and incubated with polyclonal goat anti-mouse
IgG-horseradish peroxidase secondary antibodies (Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA) for 1 h at a dilution of
1:2,000. Peroxidase activity was visualized using colorimetry with
3,3′,5,5′-tetramethylbenzidine (Invitrogen Life Technologies,
Carlsbad, CA, USA). Subsequent to immunodetection, membranes were
washed twice with TBS and stained with 0.1% Coommassie R-250 (GE
Healthcare), which was used as a loading control, according to the
method described by Welinder and Ekblad (36). Densitometric quantification of the
western blots was determined using Image J software (National
Institutes of Health, Bethesda, MA, USA).
Even though the haptoglobins and apolipoproteins
identified by spectrometry showed higher scores than the complement
C3 and A1BG, only the latter was analyzed. This was due to the
evidence that, in 2008, Barba de la Rosa et al (20) had shown increases in serum
haptoglobins in females with different degrees of cervical cancer
lesions. There is also little information with regard to the
possible role of C3 and A1BG in this type of cancer, particularly
in precursor lesions.
Detection and typification of viral
DNA
Cervical cells were obtained using endocervical
curettage and collected in transport medium (HC2 DNA Collection
Device; Digene, Gaithersburg, MD, USA). Samples were transported
and stored at −20°C until use. The presence of infection with
oncogenic types was determined through hybrid capture using the
Hybrid Capture II kit (Digene), according to the manufacturer’s
instructions, which detects the following HPV oncogenic types: 16,
18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59 and 68 (37). Positive samples were used for viral
typification using PCR analysis. In brief, extraction and
purification of DNA was performed using the Genomic DNA
Purification kit (Fermentas, Burlington, ON, Canada) according to
the manufacturer’s instructions. DNA integrity was determined
through amplifying a 450-bp fragment of the constitutive GAPDH gene
using the following oligonucleotide sequences: Forward, 5′-ACC ACA
GTC CAT GCC ATC AC-3′ and reverse, 5′-TCC ACC ACC CTG TTG CTG
TA-3′. Verification of the presence of the HPV L1 gene was
performed using PCR amplification of a 450-bp fragment using the
MY11 (5′-GCM CAG GGW CAT AAY AAT GG-3′) and MY09 (5′-CGT CCM ARR
GGA WAC TGA TC-3′) oligonucleotide sequences. DNA samples which
amplified the HPV L1 fragment were analyzed using restriction
fragment length polymorphism to typify the HPV (38). The samples were digested with four
restriction endonucleases (Rsa1, Acc I, Dde I
and XbaI), and incubated at 30°C for 2 h. The band profile
was visualized on 6% polyacrylamide gels. SiHa and CasKi cells were
used for the DNA positive control, while water was used as a
negative control. Viral type confirmation was determined through
sequencing using specific oligonucleotides for each viral type.
Results
Sample characteristics
Serum protein profiles were analyzed in samples from
females in the control group (n=5) and those diagnosed with CIN III
through histopathological analysis (n=5). The individuals in each
group fulfilled their respective inclusion criteria. The range of
ages, diagnosed oncogenic HPV infection, viral type and
histological and cytological diagnoses are shown in Table I, which demonstrates the homogeneity
of the analyzed samples. All of the females in the control group
were found to have a normal PAP result and were negative for
oncogenic viruses, while those in the case group had an abnormal
PAP result, were positive for oncogenic virus HPV 16 and had a
confirmed diagnosis of CIN III. The average age of the individuals
in in the control and case groups was 42.6 and 45.4 years,
respectively, with the two groups having a similar median age (41
and 42 years, respectively).
2D-GE
The serum from the individuals in the case and
control groups was analyzed in triplicate using 2D SDS-PAGE and the
differential expression was assessed using ImageMaster 2D Platinum
6.0 software (GE Healthcare). The program detected 337 spots that
belonged to the healthy group and 516 spots that belonged to the
cases diagnosed with CIN III. Matching between the groups resulted
in 189 matches common to the two groups. Eight spots were selected
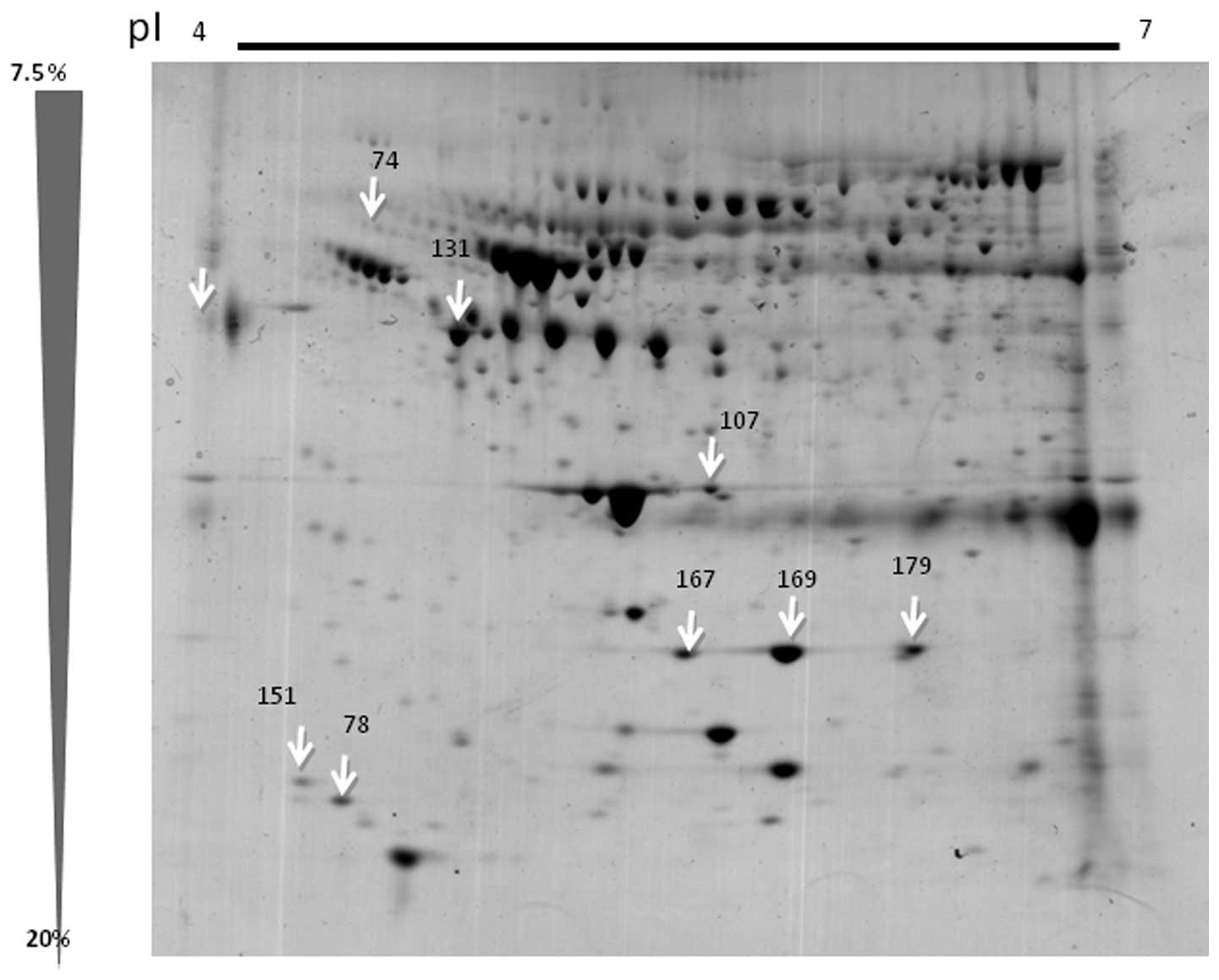
for further identification (Fig.
1). The parameters used for the selection of these spots were
that the spots were present in 8–11 gels, and that they were only
present in the case group and not in the control group, indicating
a constant presence of the spot. These spots were excised from the
gels, digested and identified using ESI-TOF-MS analysis.
LC-ESI-MS-MS analysis
The eight spots selected from the 2D gels were
identified using ESI-TOF-MS analysis. The peak of the mass of the
peptide was compared to that in the NCBInr database. Table II shows the proteins that were
sequenced from the eight spots, which correspond to the A1B319
glycoprotein, C3, one pro-apolipoprotein, two apolipoproteins and
three haptoglobins.
 | Table IIProteins analyzed using ESI-TOF-MS
and identified using the Mascot search algorithm. |
Table II
Proteins analyzed using ESI-TOF-MS
and identified using the Mascot search algorithm.
| Spot number | Access
numbera | Description | Function | Score | Peptide
matchedb | Coverage (%) | MW (kDa) | pI |
|---|
| 74 | gi69990 |
α-1-B-glycoprotein | Unknown | 96 | 6 (2) | 5 (2) | 51,908 | 6.23 |
| 78 | gi78101271 | Complement
component 3 | Immune system | 128 | 18 (3) | 13 (5) | 39,463 | 6.23 |
| 107 | gi178775 | Pro
apolipoprotein | Trygliceride
components and high lipoproteins | 474 | 36 (12) | 13 (9) | 28,944 | 6.23 |
| 131 | gi52120 | Apolipoprotein | Trygliceride
components and high lipoproteins | 474 | 36 (12) | 13 (9) | 28,944 | 5.23 |
| 151 | gi521205 | Apolipoprotein C
III | Trygliceride
components and high lipoproteins | 57 | 5 (1) | 3 (1) | 10,815 | 5.65 |
| 167 | gi22397 | Haptoglobin H2 | Antioxidant,
angiogenesis inductor, antiinflamatory effect | 142 | 13 (3) | 8 (4) | 41,717 | 5.45 |
| 169 | gi223976 | Haptoglobin H2 | Antioxidant,
angiogenesis inductor, antiinflamatory effect | 204 | 21 (4) | 100 | 41,717 | 5.45 |
| 179 | gi296653 | Haptoglobin H2 | Antioxidant,
angiogenesis inductor, antiinflamatory effect | 142 | 13 (3) | 100 | 41,499 | 5.00 |
Western blot analysis
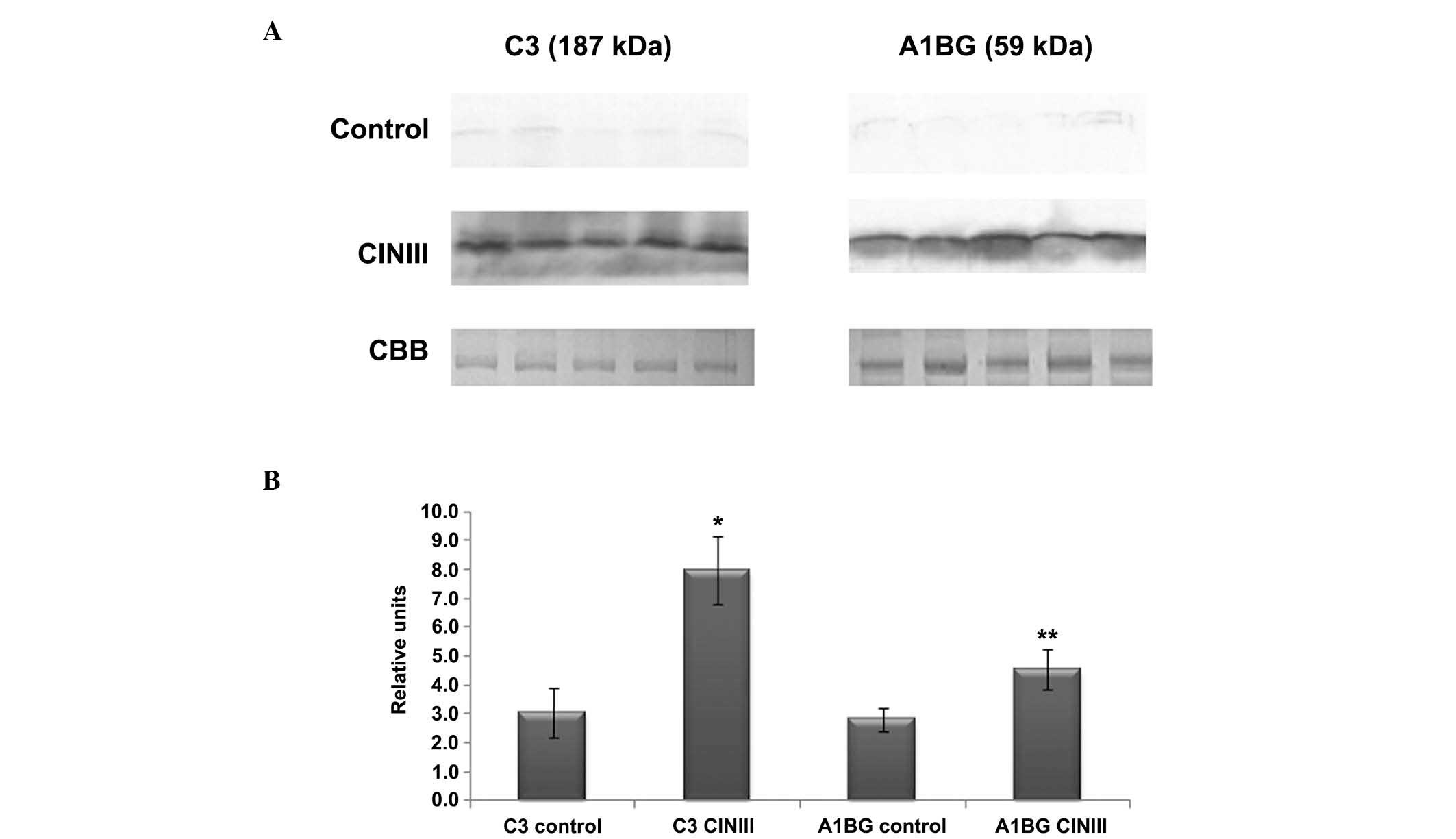
Fig. 2A shows a
representative western blot of the expression of A1BG and C3 in the
serum of the females diagnosed with CIN III (n=25) and those in the
healthy control group (n=30). Fig.
2B shows densitometry analysis of the corresponding bands from
the two groups. The relative density of the A1BG and C3 protein
bands was observed to be greater in the serum samples of the
females with CIN III, compared with those in the control group. For
C3, statistical analysis revealed that the expression values for
the individuals in the control and CIN III groups were 3.1±0.85 and
8.0±1.17 (t=5.7; P≤0.05), respectively. For A1BG, the expression
values were 2.8±0.40 and 4.5±0.68 in the control and CIN III groups
(t=4.3; P≤0.05), respectively.
Discussion
Proteins are encoded by the genes that constitute
the human genome, and they are directly responsible for regulating
cellular function through the activation or inactivation of
different signaling pathways associated with cell proliferation,
death and metabolism. It is well established that the number of
proteins expressed in a cell at a particular time does not
correspond with the total number of activated genes. Thus, it is
necessary to detect the profile of the proteins expressed at
specific times and under particular conditions in healthy subjects
and those with particular pathologies. Furthermore, the protein
profiles of the serum, plasma, tissue, urine and other human fluids
may be modified as a result of acute or chronic diseases, either
due to proteins produced by infectious agents, including viruses,
bacteria and fungi, or due to alterations at the genetic level. The
identification if these protein profiles has enabled the
identification of biomarkers for certain pathologies, which may be
used for disease prevention, diagnosis and follow-up (39). Certain biomarkers have been approved
by the Food and Drug Administration for certain types of cancer
(40).
Cervical cancer is a pathology whose causal agent is
HPV, which modifies the intracellular biology of the host during
infection and through its integration into the genome. In addition,
HPV induces an immune response on local and systemic levels. A
number of these changes are reflected in the expression profiles of
certain proteins in the tissue, plasma, serum and cervical mucus of
the infected patients. Our group has been interested in
investigating these profiles in the serum, plasma and cervical
mucus of females with different pre-malignant lesions and cervical
cancer. The present study utilized proteomic tools to investigate
serum proteins in females diagnosed with CIN III compared with
healthy females.
The present study identified eight proteins with an
increased expression in the serum of patients with CIN III lesions
compared with healthy females. These eight proteins included A1BG,
C3, a pro-apolipoprotein, two apolipoproteins and three
haptoglobins. The serum expression of A1BG and C3 was confirmed
using western blot analysis in 25 females with CIN III and 30
control individuals. The remaining six proteins were not analyzed
in this manner, as previous studies have demonstrated their
overexpression in the serum (20)
and plasma (22) of females with
different grades of intraepithelial lesions and cervical cancer. In
the present study, western blot analysis revealed that A1BG and C3
were overexpressed in all of the females in the CIN III group
compared with those in the control group, suggesting that these two
proteins may participate in the regulation of a pre-malignant
lesion-induced immune response or may be involved in
carcinogenesis. However, further studies using other types of
methodologies are required to investigate this hypothesis. Similar
findings were reported by Jeong et al (24) who analyzed plasma samples from six
healthy females compared with six females with squamous cell
carcinoma (SCC) and identified that A1BG and C3 were overexpressed
using 2D-GE analysis.
This increased expression of C3 was also confirmed
and validated in this study in a group of females diagnosed with
cancer in situ. However, the present study identified that
C3 overexpression was present in earlier disease stages. Thus, it
is important to elucidate the point at which C3 expression is
initiated in order to determine whether this increase is associated
with the presence of HPV during the infection stage and whether it
may be useful as a biological marker.
C3 is a key protein in the complement cascade and
its expression is essential for the activation of all three
complement pathways (41). It is
involved in the immune system and has a key role in destroying
invasive microorganisms and cleaning up dead and apoptotic cells.
The complement system is one of the most highly conserved cellular
systems (42). Complement proteins
act as zymogens, which are transformed into enzymes and activate
complement proteins or different receptors. Elevated concentrations
of complement proteins have been found in the serum of patients
with ovarian cancer (43),
hepatitis C-associated hepatocellular cancer (44), pancreatic cancer (45), small cell renal carcinoma (46) and SCC (24). In 1980, through simple
immunodiffusion assays, Pulay et al (47) demonstrated that the average level of
C3 increases with the progression of cervical cancer lesions up to
stage III, but diminishes by stage IV (48). In contrast to these previous
findings, fragments of C3 and C4 A/B have been reported to be
reduced in the plasma of patients with SCC of the penis, and this
reduction was found to become more evident as the disease
progressed, suggesting that these fragments may be good candidates
for prognostic tools (48). The
cause of this reduction is unknown; however, it may be the result
of infection with HPV or the Epstein Barr virus, which are
prevalent in this type of cancer in males (49), and whose proteins may be attacking
the immune system (50,51). However, this hypothesis has yet to
be elucidated.
These contradictory findings suggest that complement
proteins may be differentially regulated, depending on the type and
origin of the cancer, thus further research is required. A previous
study suggested that not only are there certain proteins with a
high variability under normal conditions, including haptoglobin
(0–40 mg/ml), lysozyme (0.01–0.1 mg/ml) and C-reactive protein
(0,01–0,3 mg/ml), but also those that have a low variability,
including albumin, which has a coefficient of variation (CV) of 9%,
as well as transferrin 410 (CV, 14%), C3, (CV, 17%), α-1 acid
glycoprotein (CV, 21%), α 2-macroglobulin (CV, 20%), transthyretin
fragment (CV, 28.3%) and β-chain α 2-HS-glycoprotein (CV, 29,7%)
(52), which may be important for
determining the state of health of an individual. It is important
to determine whether the changes in the expression of C3 and A1BG
identified in the present study, are capable of providing insight
into the changes that occur throughout the progression of cervical
lesions, prior to their transformation into cervical cancer.
A1BG is a protein found in the serum and plasma
whose function has yet to be elucidated. A1BG shows homology to the
immunoglobulin family, through its duplication and its nucleic acid
sequence (53). A1BG is present in
normal adult plasma at a concentration of 22 mg/dl (54). However, the biological function of
A1BG has yet to be elucidated and it has been found to be elevated
in certain types of cancer (19).
A1BG has been reported to be elevated in the serum of patients with
endometrial cancer and cervical cancer (19), as well as patients with cervical
squamous cell carcinoma (24,54).
These findings suggest that A1BG may be involved in cervical
carcinogenesis; thus. elucidating its function is important.
Haptoglobins are glycoproteins which are capable of
binding hemoglobin and are secreted by hepatic cells in response to
different stimuli and function as iron transporters and recyclers.
Haptoglobin concentration depends on the level of hemoglobin and
the greater the hemoglobin concentration, the lower the
concentration of haptoglobins (55). It has been reported that iron favors
the growth of cancer cells and that it preferentially accumulates
in cancer cells compared with normal cells (56).
Thus, it has been suggested that haptoglobins may be
potential markers for patients with ovarian, lung (57) and colon cancer (58). However, the role of haptoglobins in
cervical cancer has yet to be elucidated, particularly as the
difference in the expression of these proteins in females with
different lesion grades (low grade, high grade and cancer) is very
discreet as the lesions progress (20), suggesting that haptoglobins may not
be good candidate biomarkers for this pathology. The present study
found that three isoforms of haptoglobins were expressed in the
individuals in the healthy control group, as well as those with CIN
III, and that there was no significant difference in their
expression between the two groups. Apolipoprotein (APO) C-III is a
transporter molecule for high density lipoproteins which regulates
different cellular proteins involved in oxidation, apoptosis,
cellular recognition and transport (59). Apolipoproteins have been associated
with different types of cancer, including hepatocellular cancer
(60) and breast cancer (55). For example, ApoC-I has been found to
be increased in colon, prostate and liver cancer, while ApoC-III
has been reported to be associated with pancreatic, breast and
colon cancer (61). However, the
expression of apolipoproteins in patients with cervical cancer has
yet to be elucidated.
In conclusion, serum may be alternative source for
investigating differential protein profiles between healthy females
and those with HSILs, for example CIN III, secondary to HPV
infection. A number of studies, reviewed by Rutkowski et al
(51) and Pio et al
(62), suggest that modifications
in the complement system may contribute to tumor development due to
the influence of these proteins on processes involving
proliferation, angiogenesis, invasion, migration and survival.
Thus, it is possible that in the case of cervical cancer,
complement system proteins, including C3, may act as coadjuvants in
the development of lesions into the disease. However, further
investigations are required to elucidate the mechanisms involved
and to determine whether other proteins from the same family are
associated with this type of cancer.
Acknowledgements
The present study is dedicated to Dr Guillermo
Mendoza-Hernández from the Laboratory of Peptides and Proteins,
Department of Biochemistry, Faculty of Medicine, National
Autonomous University of Mexico (Mexico City. Mexico) who passed
away in 2011 and who performed the mass spectrometry analysis for
the identification of the proteins. The present study was supported
by the National Institute of Public Health and the Biology Science
Graduate Program (National Autonomous University of Mexico).
References
|
1
|
Weiderpass E and Labrèche F: Malignant
tumors of the female reproductive system. Saf Health Work.
3:166–180. 2012.
|
|
2
|
Ferlay J, Shin HR, Bray F, et al:
Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int
J Cancer. 127:2893–2917. 2010.
|
|
3
|
Jemal A, Bray F, Center MM, et al: Global
cancer statistics. CA Cancer J Clin. 61:69–90. 2011.
|
|
4
|
Bruni L, Barrionuevo-Rosas L, Serrano B,
et al: Human Papillomavirus and Related Diseases Report. Mexico:
http://www.hpvcentre.net/statistics/reports/MEX.pdf.
Accessed March 17, 2014
|
|
5
|
Richart RM: Cervical intraepithelial
neoplasia. Pathol Annu. 8:30–28. 1973.
|
|
6
|
Richart RM: A modified terminology for
cervical intraepithelial neoplasia. Obstet Gynecol. 75:131–133.
1990.
|
|
7
|
Solomon D, Davey D, Kurman R, Moriarty A,
O‘Connor D, Prey M, et al: The 2001 Bethesda System: terminology
for reporting results of cervical cytology. JAMA. 287:2114–2119.
2002.
|
|
8
|
Syrjänen K: Histology, classification and
natural history of cervical intraepithelial neoplasia (CIN). CME J
Gynecol Oncol. 14:4–21. 2009.
|
|
9
|
Correa P: A human model of gastric
carcinogenesis. Cancer Res. 48:3554–3560. 1988.
|
|
10
|
Lie AK, Risberg B, Borge B, Sandstad B,
Delabie J, Rimala R, et al: DNA- versus RNA-based methods for human
papillomavirus detection in cervical neoplasia. Gynecol Oncol.
97:908–915. 2005.
|
|
11
|
Wright TC Jr: Cervical cancer screening in
the 21st century: is it time to retire the Pap smear? Clin Obstet
Gynecol. 50:313–323. 2007.
|
|
12
|
Burd E: Human papillomavirus and cervical
cancer. Clin Microbiol Rev. 16:1–17. 2003.
|
|
13
|
Snijders PJ, Heideman DA and Meijer CJ:
Methods for HPV detection in exfoliated cell and tissue specimens.
APMIS. 118:520–528. 2010.
|
|
14
|
Grubisić G, Klarić P, Jokanović L, et al:
Diagnostic approach for precancerous and early invasive cancerous
lesions of the uterine cervix. Coll Antropol. 33:1431–1436.
2009.
|
|
15
|
Cho W: Proteomics technologies and
challenges. Genomics Proteomics Bioinformatics. 5:77–85. 2007.
|
|
16
|
Breuer EK and Murph MM: The role of
proteomics in the diagnosis and treatment of women’s cancers:
current trends in technology and future opportunities. Int J
Proteomics. 2011:1–17. 2011.
|
|
17
|
Veenstra TD, Conrads TP, Hood BL, et al:
Biomarkers: mining the biofluid proteome. Mol Cell Proteomics.
4:409–418. 2005.
|
|
18
|
Xu X and Veenstra TD: Analysis of
biofluids for biomarker research. Proteomics Clin. 2:1403–1412.
2008.
|
|
19
|
Abdul-Rahman PS, Lim BK and Hashim OH:
Expression of high-abundance proteins in sera of patients with
endometrial and cervical cancers: analysis using 2-DE with silver
staining and lectin detection methods. Electrophoresis.
28:1989–1996. 2007.
|
|
20
|
Barba de la Rosa AP, Lugo-Melchor OY,
Briones-Cerecero EP, Chagolla-López A, de León-Rodríguez A, Santos
L, et al: Analysis of human serum from women affected by cervical
lesions. J Exp Ther Oncol. 7:65–72. 2008.
|
|
21
|
Matthews R, Azuero A, Asmellash S, et al:
Usefulness of serum mass spectrometry to identify women diagnosed
with higher grades of cervical intraepitelial neoplasia may differ
by race. Inter J Womens Health. 3:185–192. 2011.
|
|
22
|
Guo X, Abliz G, Reyimu H, Zhao F, Kadeer
N, Matsidik R, et al: The association of a distinct plasma
proteomic profile with the cervical high-grade squamous
intraepithelial lesion of Uyghur women: a 2D liquid-phase
chromatography/mass spectrometry study. Biomarkers. 17:352–361.
2012.
|
|
23
|
Piyathilake CJ, Oelschlager DK, Meleth S,
Partridge EE and Grizzle WE: Plasma protein profiles differ between
women diagnosed with cervical intraepithelial neoplasia (cin) 1 and
3. Cancer Inform. 2:345–349. 2007.
|
|
24
|
Jeong DH, Kim HK, Prince AE, Lee DS, Kim
YN, Han J and Kim KT: Plasma proteomic analysis of patients with
squamous cell carcinoma of the uterine cervix. J Gynecol Oncol.
19:173–180. 2008.
|
|
25
|
Looj ML, Karsani SA, Rahman MA, et al:
Plasma proteome analysis of cervical intraepithelial neoplasia and
cervical squamous cell carcinoma. J Biosci. 34:917–925. 2009.
|
|
26
|
Ono A, Kumai T, Koizumi H, et al:
Overexpression of heat shock protein 27 in squamous cell carcinoma
of the uterine cervix: a proteomic analysis using archival
formalin-fixed, paraffin-embedded tissues. Hum Pathol. 40:41–49.
2009.
|
|
27
|
Zhu X, Lv J, Yu L, et al: Proteomic
identification of differentially-expressed proteins in squamous
cervical cancer. Gynecol Oncol. 112:248–256. 2009.
|
|
28
|
Liu C, Pan C, Shen J, et al:
Discrimination analysis of mass spectrometry proteomics for
cervical detection. Med Oncol. 28(Suppl 1): S553–S559. 2011.
|
|
29
|
Fukushima C, Murakami A, Yoshitomi K,
Sueoka K, Nawata S, Nakamura K and Sugino N: Comparative proteomic
profiling in squamous cell carcinoma of the uterine cervix.
Proteomics Clin Appl. 5:133–140. 2011.
|
|
30
|
Uleberg KE, Munk AC, Skaland I, Furlan C,
van Diermen B, Gudlaugsson E, et al: A protein profile study to
discriminate CIN lesions from normal cervical epithelium. Cell
Oncol (Dordr). 34:443–450. 2011.
|
|
31
|
Uleberg KE, Munk AC, Brede C, Gudlaugsson
E, van Diermen B, Skaland I, et al: Discrimination of grade 2 and 3
cervical intraepithelial neoplasia by means of analysis of water
soluble proteins recovered from cervical biopsies. Proteome Sci.
9:362011.
|
|
32
|
Pinto A, Crum C and Hirsch M: Molecular
markers of early cervical neoplasia. Diagn Histopathology (Oxf).
16:445–454. 2010.
|
|
33
|
Galgano MT, Castle PE, Atkins KA, et al:
Using biomarkers as objective standards in the diagnosis of
cervical biopsies. Am J Surg Pathol. 34:1077–1087. 2010.
|
|
34
|
Martin C and O‘Leary JJ: Histology of
cervical intraepithelial neoplasia and the role of biomarkers. Best
Pract Res Clin Obstet Gynaecol. 25:605–615. 2011.
|
|
35
|
Anderson NL, Esquer-Blasco R, Hofmann JP
and Anderson NG: A two-dimensional gel database of rat liver
proteins useful in gene regulation and drug effects studies.
Electrophoresis. 12:907–930. 1991.
|
|
36
|
Welinder C and Ekblad L: Coomassie
staining as loading control in Western blot analysis. J Proteome
Res. 10:1416–1419. 2011.
|
|
37
|
Lörincz A: Hybrid Capture method for
detection of human papillomavirus DNA in clinical specimens: a tool
management of equivocal Pap smears and for population screening. J
Obstet Gynaecol Res. 22:629–636. 1996.
|
|
38
|
Yoshikawa H, Kawana K, Kitagawa K, et al:
Detection and typing of multiple genital human papillomaviruses by
DNA amplification with consensus primers. Jpn J Cancer Res.
82:524–531. 1991.
|
|
39
|
Mishra A and Verma M: Cancer biomarkers:
are we ready for the prime time? Cancers (Basel). 2:190–208.
2010.
|
|
40
|
Gutman S and Kessler LG: The US Food and
Drug Administration perspective on cancer biomarker development.
Nat Rev Cancer. 6:565–571. 2006.
|
|
41
|
de Visser KE, Korets LV and Coussens LM:
Early neoplastic progression is complement independent. Neoplasia.
6:768–776. 2004.
|
|
42
|
Pio R: Control of complement activation by
cancer cells and its implications in antibody-mediated cancer
immunotherapy. Revisión Inmunología. 25:173–87. 2006.(In
Spanish).
|
|
43
|
Bjørge L, Hakulinen J, Vintermyr OK, Jarva
H, Jensen TS, Iversen OE and Meri S: Ascitic complement system in
ovarian cancer. Br J Cancer. 92:895–905. 2005.
|
|
44
|
Lee IN, Chen CH, Sheu JC, Lee HS, Huang
GT, Chen DS, et al: Identification of complement C3a as a candidate
biomarker in human chronic hepatitis C and HCV-related
hepatocellular carcinoma using a proteomics approach. Proteomics.
6:2865–2873. 2006.
|
|
45
|
Hanas JS, Hocker JR, Cheung JY, Larabee
JL, Lerner MR, Lightfoot SA, et al: Biomarker identification in
human pancreatic cancer sera. Pancreas. 36:61–69. 2008.
|
|
46
|
Xu G, Hou CR, Jiang HW, Xiang CQ, et al:
Serum protein profiling to identify biomarkers for small renal cell
carcinoma. Indian J Biochem Biophys. 47:211–218. 2010.
|
|
47
|
Pulay A, Füst G and Csömör A: Serum
complement levels in patients with cancer of the uterine cervix
before and after radiation therapy. Neoplasma. 27:211–216.
1980.
|
|
48
|
Ornellas P, Ornellas AA, Chinello C,
Gianazza E, Mainini V, Cazzaniga M, et al: Down regulation of C3
and C4A/B complement factor fragments in plasma from patients with
squamous cell carcinoma of the penis. Int Braz J Urol. 38:739–749.
2012.
|
|
49
|
Alfonso L, Moyses N, Alves G, Ornellas AA,
Passos MR, do Oliveira LH and Cavalcanti SM: Prevalence of human
papillomavirus and Epstein-Barr virus DNA in penile cancer cases
from Brazil. Mem Inst Oswaldo Cruz. 107:18–23. 2012.
|
|
50
|
Campo MS, Graham SV, Cortese MS, Ashrafi
GH, Araibi EH, Dornan ES, et al: HPV-16 E5 down-regulates
expression of surface HLA class I and reduces recognition by CD8 T
cells. Virology. 407:137–142. 2010.
|
|
51
|
Rutkowski MJ, Sughurue ME, Kane AJ, Mills
SA and Parsa AT: Cancer and the complement cascade. Mol Cancer Res.
8:1453–1465. 2010.
|
|
52
|
Pakharukova NA, Pastushkova LKh,
Moshkovskiǐ SA and Larina IM: Variability of healthy human
proteome. Biomed Khim. 58:514–529. 2012.(In Russian).
|
|
53
|
Ishioka N, Takahashi N and Putnam FW:
Amino acid sequence of human plasma alpha 1B-glycoprotein: homology
to the immunoglobulin supergene family. Proc Natl Acad Sci USA.
83:2363–2367. 1986.
|
|
54
|
Piyaphanee N, Ma Q, Kremen O, Czech K,
Greis K, Mitsnefes M, et al: Discovery and initial validation of α
1-B glycoprotein fragmentation as a differential urinary biomarker
in pediatric steroid-resistant nephrotic syndrome. Proteomics Clin
Appl. 5:334–342. 2011.
|
|
55
|
Huang HL, Stasyk T, Morandell S,
Dieplinger H, Falkensammer G, Griesmacher A, et al: Biomarker
discovery in breast cancer serum using 2-D differential gel
electrophoresis/MALDI-TOF/TOF and date validation by routine
clinical assays. Electrophoresis. 27:1641–1650. 2006.
|
|
56
|
Weinberg ED: The role of iron in cancer.
Eur J Cancer Prev. 5:19–36. 1996.
|
|
57
|
Thadikkaran L, Siegenthaler MA, Crettaz D,
et al: Recent advances in blood-related proteomics. Proteomics.
5:3019–3034. 2005.
|
|
58
|
Dowling P, Clarke C, Hennessy K,
Torralbo-Lopez B, Ballot J, Crown J, et al: Analysis of acute-phase
proteins, AHSG, C3, CLI, HP and SAA, reveals distinctive expression
patterns associated with breast, colorectal and lung cancer. Int J
Cancer. 131:911–923. 2012.
|
|
59
|
Jiang JT, Xu N, Zhang XY and Wu CP: Lipids
changes in liver cancer. J Zhejiang Univ Sci B. 8:398–409. 2007.(In
Chinese).
|
|
60
|
Steel LF, Shumpert D, Trotter M, Seeholzer
SH, Evans AA, London WT, et al: A strategy for the comparative
analysis of serum proteomes for the discovery of biomarkers for
hepatocellular carcinoma. Proteomics. 3:601–609. 2003.
|
|
61
|
Cohen M, Yossef R, Erez T, Kugel A, Welt
M, Karpasas MM, et al: Serum apolipoproteins C-I and C-III are
reduced in stomach cancer patients: results from MALDI-based
peptidome and immuno-based clinical assays. Plos One.
6:e145402011.
|
|
62
|
Pio R, Ajona D and Lambris JD: Complement
inhibition in cancer therapy. Semin Immunol. 25:54–64. 2013.
|