Introduction
The extracellular matrix (ECM) triggers numerous
intercellular signaling pathways that regulate cellular growth,
division, migration and differentiation (1). The ECM consists of numerous large
macromolecules, including collagen, fibronectin and laminin
(2). In the breast, collagens and
other extracellular molecules may directly affect breast epithelial
proliferation and differentiation (3). Type I collagen, the major structural
component of the ECM, is a heterotrimer composed of three α-chains
encoded by the collagen, type I, α1 (COL1A1) and COL1A2 genes
(4). Type I collagen comprises ~84%
of all the collagen synthesized by fibroblasts and large
depositions of type I collagen lead to internal organ fibrosis
(5). In particular, type I collagen
acts as a physical barrier for cell migration and interferes with
the proliferative ability of both normal and cancer cells (6).
Matrix metalloproteinases (MMPs) are a family of 24
human zinc binding endopeptidases which degrade components of the
ECM and are associated with remodeling of the ECM and basement
membrane under physiological conditions (7). Excessive expression of MMPs has been
associated with numerous malignant processes, including cancer
proliferation, invasion and metastasis, as well as inflammatory
conditions, such as rheumatoid arthritis and osteoarthritis
(8,9). Elevated plasma levels of soluble
gelatinases, including MMP-2 and -9, have been positively
correlated with a higher incidence of metastases in different types
of cancer and have been considered as a valuable prognostic factor
in breast and colon cancer (10).
The degradation of type I collagen by MMP-1 and -9 has been
associated with rapid progression, poor overall survival and
secondary metastasis (11), and it
appears this process may have a pivotal role in the acquisition of
invasive characteristics in breast cancer (12,13).
However, despite the numerous studies that have been conducted to
further elucidate the cross-talking between malignant epithelial
cells and stromal cells (14–16),
the role of type I collagen, derived from fibroblasts, on the
changing characteristics of breast cancer cells, has not been fully
clarified. The present study examined the effect of type I collagen
and culture media of normal human dermal fibroblasts (HDFs) on
breast cancer cell lines.
Materials and methods
Cell lines and cell cultures
The human breast cancer cell lines, MCF-7 (luminal
type A) and MDA-MB231 (triple negative, basal type) were cultured
in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10%
fetal bovine serum (FBS), 100 IU/ml penicillin and 100 mg/ml
streptomycin. Primary HDF cultures were obtained from the foreskin
of healthy volunteers with their consent, aged 20–30 years old. The
HDF cultures was provided by Dr Chung Jin Ho of Seoul National
University (Seoul, Korea) (17).
The isolated cells were allowed to attach to plastic plates and
were cultured in DMEM supplemented with 10% FBS, 2 mM glutamine,
100 IU/ml penicillin and 100 μg/ml streptomycin. Following six or
eight passages, the fibroblasts were used for the experiments. Each
cell was maintained in a culture media supplemented without fetal
bovine serum (FBS) for 16–24 h. DMEM, antibiotics (penicillin and
streptomycin) and FBS were purchased from Life Technologies
(Rockville, MD, USA). MCF-7 and MDA-MB231 were obtained from
American Type Culture Collection (Manassas, VA, USA).
Cell morphology study
MDA-MB231 and MCF7 breast cancer cell lines were
seeded in the culture media of normal HDFs for 3 h and then
analyzed. Each cell line was also seeded in serum free-DMEM media
containing 50 μg/ml type I collagen (R&D Systems, Minneapolis,
MN, USA) and water, respectively. The morphology of each cell was
analyzed using the CK40 inverted microscope (Olympus, Tokyo,
Japan).
Dot-blotting
To verify the presence of secreted type I collagen
in cultured media, MDA-MB231 and MCF7 cells and normal HDFs were
incubated in serum-free media for 24 h. The indicated dose samples
were transferred onto nitrocellulose membranes (Sigma-Aldrich, St.
Louis, MO, USA) using the BIO-DOT™ apparatus (Bio-Rad, Hercules,
CA, USA) and the membranes were then blocked with 10% skimmed milk
in Tris-buffered saline with 0.01% Tween-20 (TBST; Sigma-Aldrich)
for 15 min. The blots were incubated with mouse anti-human type I
procollagen monoclonal antibody (SP1.D8; Developmental Studies
Hybridoma Bank, Iowa City, IA, USA; dilution, 1:10) in 1% TBST
buffer at 4°C overnight. Blots were washed three times in TBST and
subsequently incubated in rabbit anti-mouse peroxidase-conjugated
monoclonal antibody (Santa Cruz Biotechnology, Inc., Santa Cruz,
CA, USA; dilution, 1:2,000) in TBST buffer. Following 1 h of
incubation at room temperature, the blots were washed three times
in TBST and enhanced chemiluminescent reagents (Amersham
Biosciences, Buckinghamshire, UK) were used for development.
Quantitation of type I collagen and MMP-9
mRNA
Total RNA was extracted from cells using TRIzol
reagent (Invitrogen Life Technologies, Carlsbad, CA, USA),
according to the manufacturer’s instructions. Isolated RNA samples
were then used for reverse transcription polymerase chain reaction
(RT-PCR). The samples (total RNA, 1 μg) were reverse transcribed
into cDNA in 20 μl reaction volume using a first-strand cDNA
synthesis kit for RT-PCR, according to the manufacturer’s
instructions (MBI Fermentas, Hanover, MD, USA).
Gene expression was quantified by quantitative PCR
(qPCR) using SensiMix SYBR kit (Bioline Ltd., London, UK) and 100
ng of cDNA/reaction. The sequences of the primer sets used for this
analysis were as follows: human MMP-1: Forward, 5′-ATT CTA CTG ATA
TCG GGG CTT TGA-3′ and reverse, 5′-ATG TCC TTG GGG TAT CCG TGT AG
-3′; human MMP-9: Forward, 5′-CCC GGA CCA AGG ATA CAG-3′ and
reverse, 5′-GGC TTT CTC TCG GTA CTG-3′; and human GAPDH (as an
internal control): Forward, 5′-ATT GTT GCC ATC AAT GAC CC-3′ and
reverse, 5′-AGT AGA GGC AGG GAT GAT GT-3′. An annealing temperature
of 60°C was used for all of the primers. PCR was performed in a
standard 384-well plate format with an ABI 7900HT qPCR detection
system (Applied Biosystems, Foster City, CA, USA). For data
analysis, the raw threshold cycle (CT) value was first normalized
to the housekeeping gene for each sample to obtain the change in CT
(ΔCT). The normalized ΔCT was then calibrated to control cell
samples to obtain the ΔΔCT.
Cell adhesion assay
MDA-MB231 and MCF7 breast cancer cells were seeded
with serum-free media and 50 μg/ml type I collagen-containing media
for 3 h on a 96-well plate. MDA-MB231 and MCF7 breast cancer cells
were seeded with the culture media of breast cancer cells or normal
HDF for 3 h on a 96-well plate.
To analyze the adhesion capacities of each cell, the
cells were incubated with 5 mg/ml of
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
solution (Sigma-Aldrich) at 37°C for 1 h. The culture media was
removed and then the cells were dissolved with dimethylsulfoxide
(Sigma-Aldrich). The adhesion capacity of each cell was analyzed at
a wavelength of 595 nm on a spectrophotometer (Spectra Max 190;
Molecular Devices, Sunnyvale, CA, USA).
Statistical analysis
Student’s t-test was used to compare the cell
adhesion rates with the mRNA expression levels. Statistical
analyses were performed using PASW® Statistics 18.0
(SPSS, Inc., Chicago, IL, USA). The results are presented as the
mean ± standard error of mean. All P-values were two-tailed and
P<0.05 was considered to indicate a statistically significant
difference.
Results
Expression of type I collagen protein and
mRNA in breast cancer cells and normal HDFs
The level of type I collagen protein expression in
the conditioned culture media of MCF7 or MDA-MB231 breast cancer
cells and normal HDFs was observed in this study. As demonstrated
in Fig. 1A, type I collagen was not
detected in the culture media of MCF7 and MDA-MB231 breast cancer
cells, and was only detected in the culture media of normal HDFs.
To verify the non-specific binding of type I collagen antibodies,
type I collagen was loaded at the indicated doses in each well. It
was revealed that the level of type I collagen protein expression
increased in a dose-dependent manner (Fig. 1A). The expression of type I collagen
mRNA was high in the HDF-conditioned culture media, whereas it was
low or absent in the culture media of the breast cancer cell lines
(Fig. 1B).
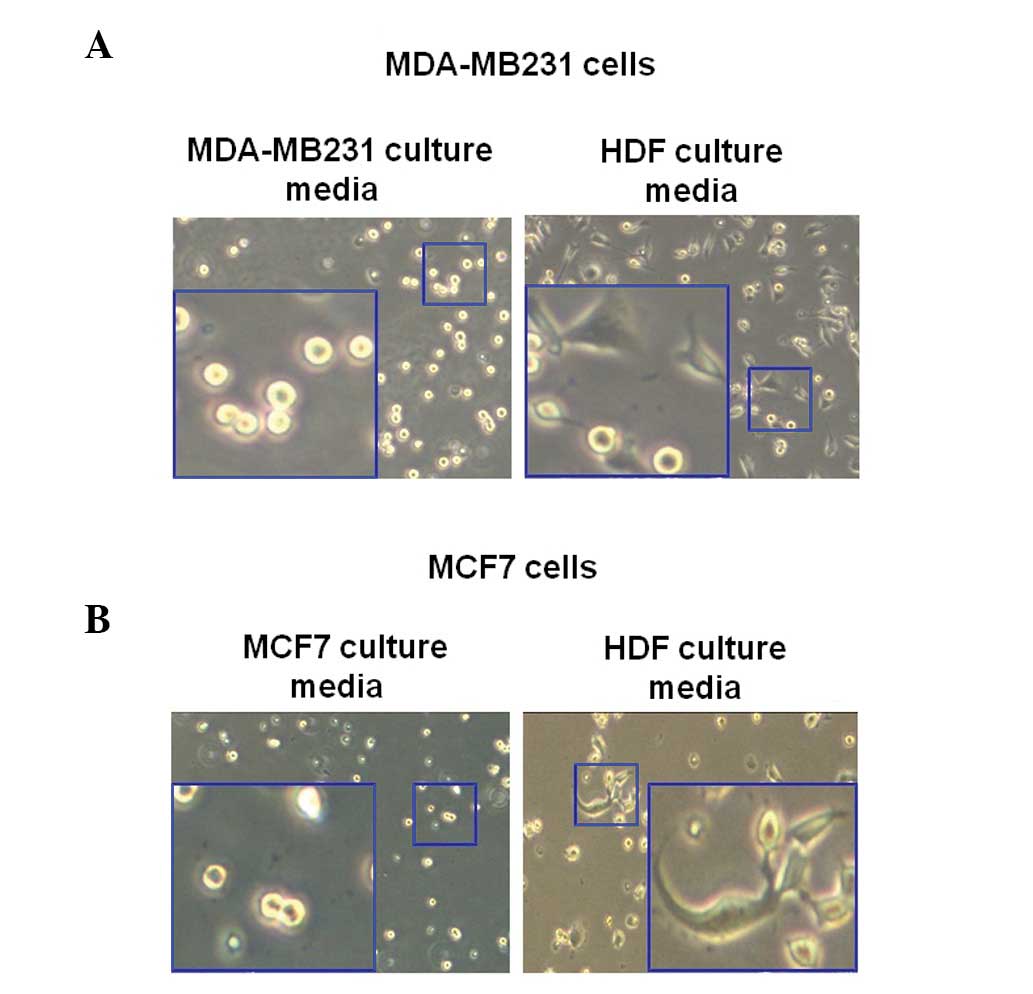
Morphological changes of human breast
cancer cells in HDF-conditioned cultured media
To observe the change of morphology of breast cancer
cells as induced by HDF-conditioned culture media, MDA-MB231 cells
were incubated with or without culture media of normal HDFs for 3
h. In the present study, MDA-MB231 and MCF7 cells were trypsinized
and then seeded with the culture media of each cell. Following 3 h,
the morphology of cells was observed using a CK40 inverted
microscope. As illustrated in Fig.
2A, the MDA-MB231 breast cancer cells exhibited enhanced
sprouting in the HDF-conditioned culture media (Fig. 2A). However, the morphology of the
MDA-MB231 breast cancer cells in the culture media of MDA-MB231
cells did not change (Fig. 2A). As
revealed in Fig. 2B, the morphology
of MCF7 cells markedly altered in the normal HDF culture media.
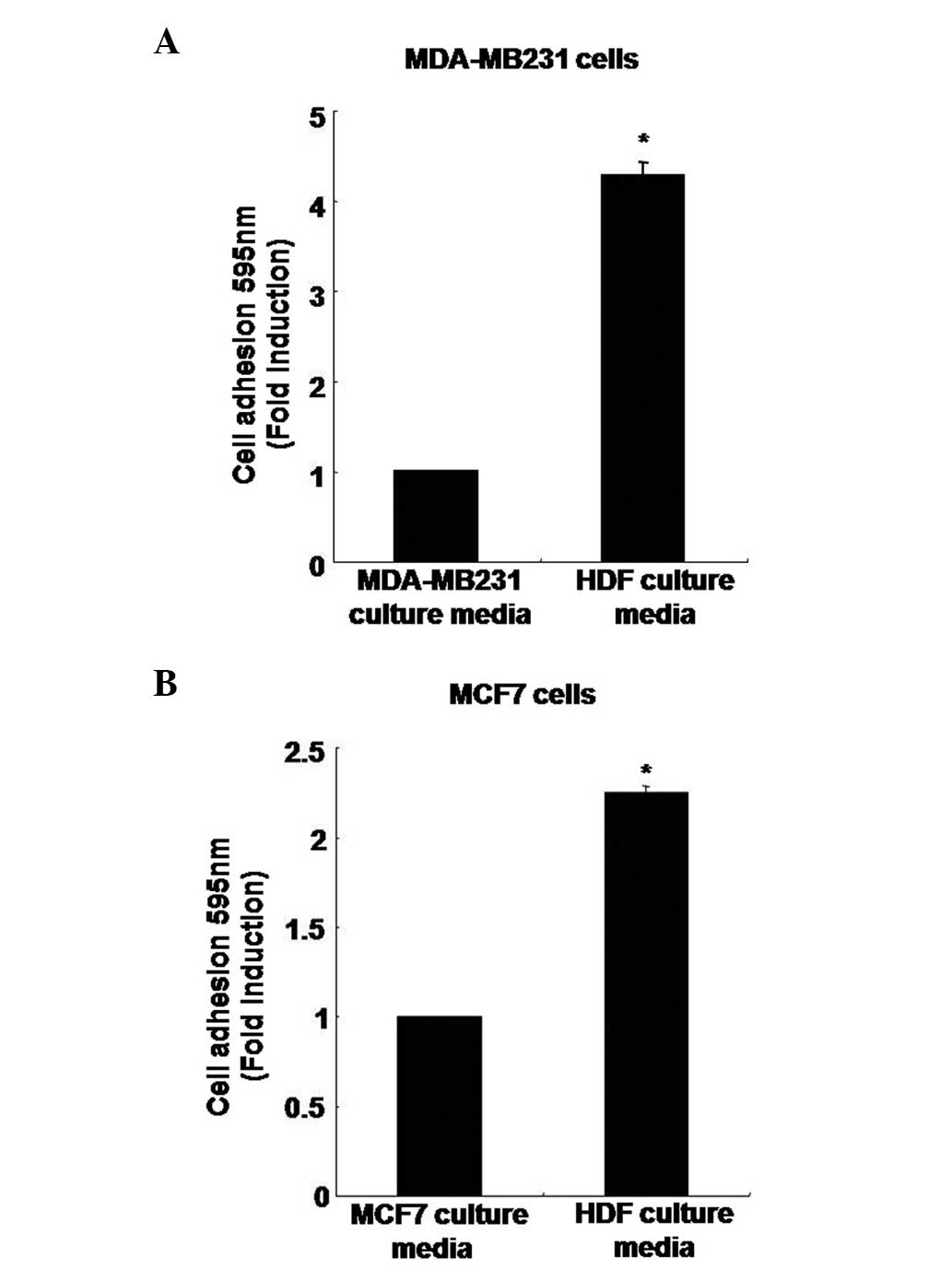
Adhesion capacity of human breast cancer
cells in HDF-conditioned culture media
To analyze the rate of adhesion capacity, MDA-MB231
cells were treated with the MDA-MB231 breast cancer cell culture
media or the culture media containing normal HDFs. After 3 h, the
adhesion capacity of MDA-MB231 breast cancer cells had increased by
4.27±0.15-fold when cultured in the HDF-conditioned culture media
compared with the MDA-MB231 culture media (Fig. 3A). In addition, the adhesion
capacity of the MCF7 breast cancer cells was also significantly
higher, with an increase of 2.25±0.03-fold in the HDF-conditioned
culture media compared with the MCF7 culture media (Fig. 3B).
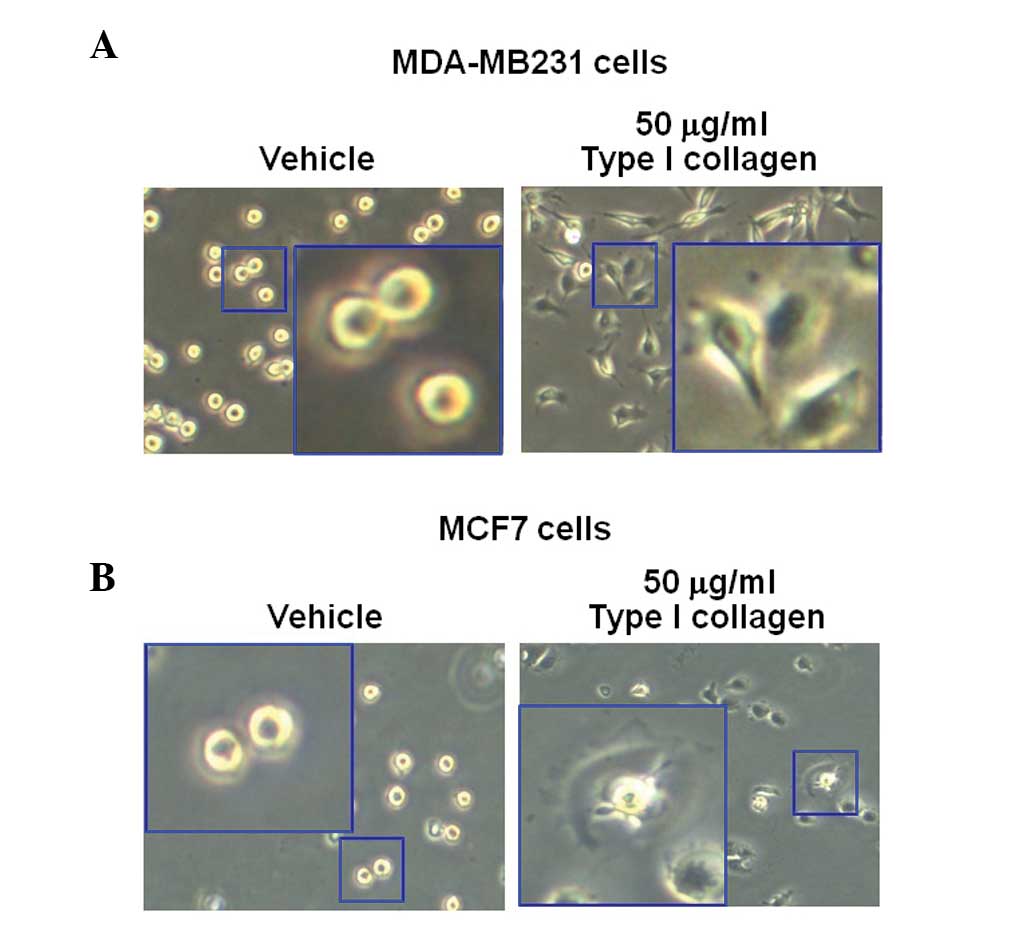
Type I collagen-induced morphological
changes in breast cancer cells
MDA-MB231 and MCF7 breast cancer cells were treated
with 50 mg/ml type I collagen. After 3 h, the morphology of the
cells was observed using an CK40 inverted microscope. The results
revealed that type I collagen augmented the sprouting of the cells
in the MDA-MB231 and MCF7 breast cancer cell lines, but not in the
vehicle-treated cells (Fig. 4A and
B). These results were similar to those in Fig. 2.
Type I collagen induces changes in the
adhesion capacity of human breast cancer cells
To verify the effect of type I collagen on the
adhesion capacity of MDA-MB231 cells by type I collagen, cells were
treated with type I collagen at the indicated concentration for 3
h. Treatment with type I collagen enhanced the adhesion capacity of
MDA-MB231 cells by 4.37±0.88-fold that of the vehicle-treated cells
(Fig. 5A). The adhesion capacity of
MCF7 breast cancer cells was also markedly increased by
3.72±0.52-fold (Fig. 5B).
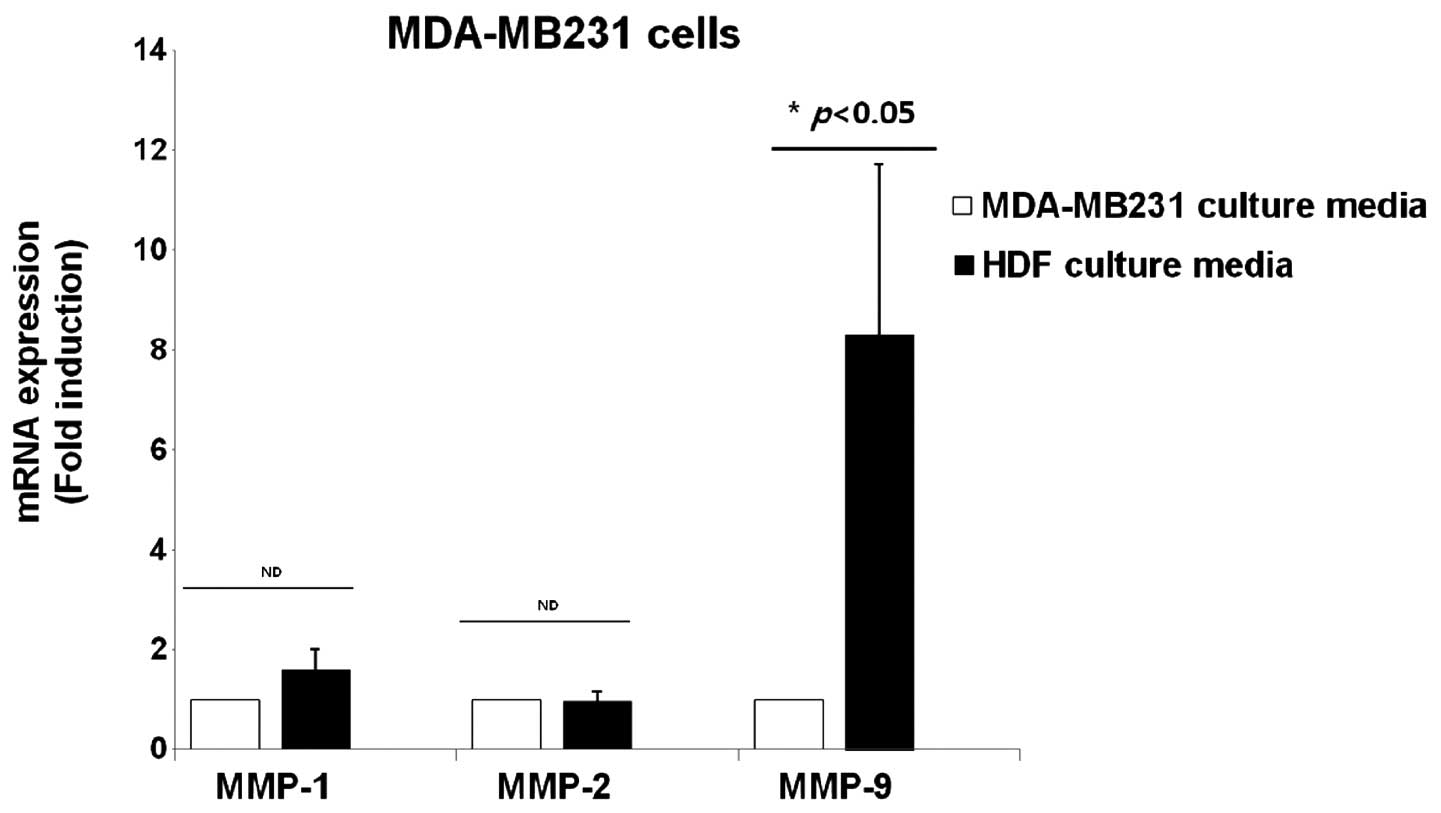
Expression of MMP-1, -2 and -9 mRNA in
the culture media of breast cancer cells and normal HDFs
The effect of HDF-conditioned culture media on the
expression of MMP-1, -2 and -9 was examined, which are all marker
proteins of cancer metastasis. MDA-MB231 breast cancer cells were
treated with culture media of MDA-MB231 and normal HDFs for 24 h,
and cell lysates were harvested for detecting the level of MMP-1,
-2 and -9 mRNA expression. It was demonstrated that the levels of
MMP-1 and -2 mRNA expression were not affected by the normal
HDF-conditioned culture media (Fig.
6). However, the level of MMP-9 mRNA expression was
significantly increased by 8.3±3.4-fold in the HDF-conditioned
culture media compared with the culture media of MDA-MB231 cells
(Fig. 6).
Discussion
ECM macromolecules are a major component of the
cellular microenvironment and are in immediate contact with tumor
cells (18). ECM molecules,
including type I collagen, inhibit the proliferation of tumor cells
by upregulating p27KIP1 in human M24met melanoma cells
(19). In addition, type I collagen
significantly augments the apoptotic cell death of MCF7 breast
cancer cells through the existence of membrane type-1 (MT1)-MMP
(8). In the present study, the
correlation between the secreted proteins of normal fibroblasts and
malignant breast cancer cells was investigated.
The interactions between malignant epithelial cells
and their microenvironment are well established in tumorigenesis
(20). The ECM is a key component
of the microenvironment and affects numerous characteristics of
tumor cells, including cell growth, survival and angiogenensis
(18). The ECM molecules, including
collagen, glycosaminoglycans and elastic fibers secreted from
fibroblasts (which are a component of stromal cells), are key to
its effects on tumorigenesis (5).
These fibroblasts have an important role in the synthesis and
remodeling of a variety of ECM molecules in the tumor stroma
(21). In a previous study, Maquoi
et al reported that collagen affects the fate of malignant
epithelial cells in breast cancer cells through MT1-MMP-dependent
mechanisms (8). In accordance with
these investigations, the present study identified that two breast
cancer cell lines, MDA-MB231 and MCF7, exhibited increased
sprouting and enhanced adhesion rates following treatment with
HDF-conditioned culture media. Therefore, it was demonstrated that
secreted proteins of fibroblasts may affect the characteristics of
breast cancer cells, which is consistent with the results of
several other studies (21–23).
It is well established that carcinoma-associated
fibroblasts stimulate cancer cell progression, through the
secretion of various cytokines, such as stromal cell-derived
factor-1 and transforming growth factor β (23,24).
In the present study, however, another stimulatory pathway of tumor
cells was investigated. MMPs contribute to a variety of malignant
processes, including tumor growth, invasion and metastasis
(9). In numerous types of cancer,
elevated plasma levels of soluble gelatinases, such as MMP-2 and
-9, have been positively correlated with a higher incidence of
MMP-9 expression, and appear to be regulated by the binding of
multiple factors, including nuclear factor κ-light-chain-enhancer
of activated B cells and the activator protein 1, to their response
elements (13,25). Kim et al reported that the
basal level of MMP-9 expression was significantly increased by
constitutively active mitogen-activated protein kinase kinase
overexpression in SKBR3 breast cancer cells (13). Therefore, MDA-MB231 cells were
treated with MDA-MB231 culture media and HDF-conditioned culture
media, and MMP-1, -2 and -9 expression was analyzed. As a result,
there was no difference in the expression of MMP-1 and -2 between
the two media; however MMP-9 was highly expressed in the
HDF-conditioned culture media with MDA-MB231 cells. Based on these
results, it was assumed that fibroblasts may stimulate breast
cancer cell metastasis and MMP-9 may effect this process. As
mentioned above, MMP-9 is known to degrade basement membranes in
breast cancer, and several studies have also reported that MMP-9
may be associated with breast cancer initiation and progression
through interactions between oncogenes and tumor suppressor genes
(12,26).
In a recent study, one of the microenvironment
components, type I collagen, induced apoptotic cell death in
luminal-like breast carcinoma cells but not in basal-like breast
carcinoma cells (8). Secreted
proteins of the microenvironment, including type I collagen and
laminin, are important in the invasiveness and progression of
breast cancer cells (27). The
results of the present study reveal that type I collagen may be one
of the ECM proteins which stimulates breast cancer cell metastasis,
which is a result that is consistent with other studies (28–30).
It is evident that type I collagen is not the only factor that
initiates and promotes cancer cells to become metastatic, among
other products of fibroblasts. However, it may be assumed that type
I collagen has an important role in the development and initiation
of metastasis in breast cancer cells. Further elucidating the
detailed mechanisms underlying the effect of type I collagen on
breast cancer cells requires further study. One investigation
demonstrated that females with highly dense breasts, which is
associated with a high density of type I collagen, also had a
higher risk of recurrence following a mastectomy or radiotherapy
(31). Therefore, it is possible
that type I collagen may be considered as a potential therapeutic
target or prognosis factor of the disease.
In the present study, the results revealed that
HDF-conditioned culture media augmented the aggressiveness of
breast cancer cells, through the induction of sprouting cells and
the enhancement of adhesion capacities. In addition, being one of
the key enzymes of metastasis, the expression of MMP-9 mRNA was
significantly enhanced by the HDF-conditioned culture media. Based
on this evidence, the present study demonstrates that
microenvironmental sources, including secreted cytokines and
proteins (i.e. type I collagen) of stromal fibroblasts cells, may
induce the development of aggressive characteristics in breast
cancer cells.
References
|
1
|
Nelson CM and Bissell MJ: Modeling dynamic
reciprocity: engineering three-dimensional culture models of breast
architecture, function, and neoplastic transformation. Semin Cancer
Biol. 15:342–352. 2005.
|
|
2
|
Ghajar CM and Bissell MJ: Extracellular
matrix control of mammary gland morphogenesis and tumorigenesis:
insights from imaging. Histochem Cell Biol. 130:1105–1118.
2008.
|
|
3
|
Lin CQ and Bissell MJ: Multi-faceted
regulation of cell differentiation by extracellular matrix. FASEB
J. 7:737–743. 1993.
|
|
4
|
Vuorio E and de Crombrugghe B: The family
of collagen genes. Annu Rev Biochem. 59:837–872. 1990.
|
|
5
|
Cutroneo KR: How is Type I procollagen
synthesis regulated at the gene level during tissue fibrosis. J
Cell Biochem. 90:1–5. 2003.
|
|
6
|
Sodek KL, Brown TJ and Ringuette MJ:
Collagen I but not Matrigel matrices provide an MMP-dependent
barrier to ovarian cancer cell penetration. BMC Cancer.
8:2232008.
|
|
7
|
Cauwe B, Van den Steen PE and Opdenakker
G: The biochemical, biological, and pathological kaleidoscope of
cell surface substrates processed by matrix metalloproteinases.
Crit Rev Biochem Mol Biol. 42:113–185. 2007.
|
|
8
|
Maquoi E, Assent D, Detilleux J, Pequeux
C, Foidart JM and Noël A: MT1-MMP protects breast carcinoma cells
against type I collagen-induced apoptosis. Oncogene. 31:480–493.
2012.
|
|
9
|
Chambers AF and Matrisian LM: Changing
views of the role of matrix metalloproteinases in metastasis. J
Natl Cancer Inst. 89:1260–1270. 1997.
|
|
10
|
Björklund M and Koivunen E:
Gelatinase-mediated migration and invasion of cancer cells. Biochim
Biophys Acta. 1755:37–69. 2005.
|
|
11
|
Nakajima M, Welch DR, Wynn DM, Tsuruo T
and Nicolson GL: Serum and plasma M(r) 92,000 progelatinase levels
correlate with spontaneous metastasis of rat 13762NF mammary
adenocarcinoma. Cancer Res. 53:5802–5807. 1993.
|
|
12
|
Duffy MJ, Maguire TM, Hill A, McDermott E
and O’Higgins N: Metalloproteinases: role in breast carcinogenesis,
invasion and metastasis. Breast Cancer Res. 2:252–257. 2000.
|
|
13
|
Kim S, Choi JH, Lim HI, et al: EGF-induced
MMP-9 expression is mediated by the JAK3/ERK pathway, but not by
the JAK3/STAT-3 pathway in a SKBR3 breast cancer cell line. Cell
Signal. 21:892–898. 2009.
|
|
14
|
Shekhar MP, Werdell J, Santner SJ, Pauley
RJ and Tait L: Breast stroma plays a dominant regulatory role in
breast epithelial growth and differentiation: implications for
tumor development and progression. Cancer Res. 61:1320–1326.
2001.
|
|
15
|
Mao Y, Keller ET, Garfield DH, Shen K and
Wang J: Stromal cells in tumor microenvironment and breast cancer.
Cancer Metastasis Rev. 32:303–315. 2013.
|
|
16
|
Angelucci C, Maulucci G, Lama G, et al:
Epithelial-stromal interactions in human breast cancer: effects on
adhesion, plasma membrane fluidity and migration speed and
directness. PLoS One. 7:e508042012.
|
|
17
|
Kim S, Kim Y, Lee Y and Chung JH: Ceramide
accelerates ultraviolet-induced MMP-1 expression through
JAK1/STAT-1 pathway in cultured human dermal fibroblasts. J Lipid
Res. 49:2571–2581. 2008.
|
|
18
|
Hynes RO: The extracellular matrix: not
just pretty fibrils. Science. 326:1216–1219. 2009.
|
|
19
|
Henriet P, Zhong ZD, Brooks PC, Weinberg
KI and DeClerck YA: Contact with fibrillar collagen inhibits
melanoma cell proliferation by up-regulating p27KIP1. Proc Natl
Acad Sci USA. 97:10026–10031. 2000.
|
|
20
|
Barkan D, El Touny LH, Michalowski AM, et
al: Metastatic growth from dormant cells induced by a
col-I-enriched fibrotic environment. Cancer Res. 70:5706–5716.
2010.
|
|
21
|
Bhowmick NA, Neilson EG and Moses HL:
Stromal fibroblasts in cancer initiation and progression. Nature.
432:332–337. 2004.
|
|
22
|
Proia DA and Kuperwasser C: Stroma: tumor
agonist or antagonist. Cell Cycle. 4:1022–1025. 2005.
|
|
23
|
Kojima Y, Acar A, Eaton EN, et al:
Autocrine TGF-beta and stromal cell-derived factor-1 (SDF-1)
signaling drives the evolution of tumor-promoting mammary stromal
myofibroblasts. Proc Natl Acad Sci USA. 107:20009–20014. 2010.
|
|
24
|
Orimo A, Gupta PB, Sgroi DC, et al:
Stromal fibroblasts present in invasive human breast carcinomas
promote tumor growth and angiogenesis through elevated SDF-1/CXCL12
secretion. Cell. 121:335–348. 2005.
|
|
25
|
Crowe DL, Tsang KJ and Shemirani B: Jun
N-terminal kinase 1 mediates transcriptional induction of matrix
metalloproteinase 9 expression. Neoplasia. 3:27–32. 2001.
|
|
26
|
Choi JY, Jang YS, Min SY and Song JY:
Overexpression of MMP-9 and HIF-1α in breast cancer cells under
hypoxic conditions. J Breast Cancer. 14:88–95. 2011.
|
|
27
|
Kim BG, An HJ, Kang S, et al:
Laminin-332-rich tumor microenvironment for tumor invasion in the
interface zone of breast cancer. Am J Pathol. 178:373–381.
2011.
|
|
28
|
Ma XJ, Dahiya S, Richardson E, Erlander M
and Sgroi DC: Gene expression profiling of the tumor
microenvironment during breast cancer progression. Breast Cancer
Res. 11:R72009.
|
|
29
|
Provenzano PP, Inman DR, Eliceiri KW, et
al: Collagen density promotes mammary tumor initiation and
progression. BMC Med. 6:112008.
|
|
30
|
Ramaswamy S, Ross KN, Lander ES and Golub
TR: A molecular signature of metastasis in primary solid tumors.
Nat Genet. 33:49–54. 2003.
|
|
31
|
Park CC, Rembert J, Chew K, Moore D and
Kerlikowske K: High mammographic breast density is independent
predictor of local but not distant recurrence after lumpectomy and
radiotherapy for invasive breast cancer. Int J Radiat Oncol Biol
Phys. 73:75–79. 2009.
|