Introduction
Non-Hodgkin lymphoma (NHL) is a diverse group of
blood cancer that includes any type of lymphoma with the exception
of Hodgkin’s lymphoma. The latest lymphoma classification, the 2008
WHO classification, largely abandoned the ‘Hodgkin’ versus
‘non-Hodgkin’ grouping and instead lists >80 different forms of
lymphoma in four broad groups (1).
The gastrointestinal (GI) tract is the most common extranodal site
of involvement in NHL, accounting for 5–10% of all NHL cases.
Intestinal lymphomas represent 15–20% of all GI lymphomas. The
stomach is the predominant location for GI lymphomas (50%), whereas
intestinal lymphomas are less frequently observed in the small
bowel (20–30%) (2,3).
Previous studies have shown that even though
surgical resection is necessary for local disease control and
preventing bleeding and/or perforation, it is rare for the
procedure to completely eliminate the lymphoma when used alone
(4–8). However, a surgical resection should
always be attempted for localized disease. Controversy remains with
regard to the management of extensive GI lymphoma. This lymphoma is
commonly diagnosed at an advanced stage, and surgical treatment is
only suitable for 30–40% of such patients. As a consequence,
radiotherapy and adjuvant chemotherapy are essential therapeutic
approaches.
The present study provides a retrospective analysis
of 11 cases of primary NHL of the small intestine, presenting the
clinical and pathological characteristics and analyzing the risk
factors contributing to perforation of the small bowel in this type
of lymphoma.
Patients and methods
Patients and data collection
A retrospective analysis was performed on the
clinical data from 313 cases of NHL that occurred between 1995 and
2008 in the Tri-Service General Hospital (National Defense Medical
Center, Taipei, Taiwan). From the 313 patients with NHL, 11
patients were confirmed to have primary NHL of the small
intestine.
The clinical data collected included: Age at
diagnosis, Ann Arbor stage, Eastern Cooperative Oncology Group
performance status, lactate dehydrogenase (LDH) level, extranodal
site and International Prognostic Index. The initial staging
involved a history and physical examination, standard blood tests
for LDH and other biochemical markers, chest X-rays, bone marrow
aspiration and biopsies, and computed tomography of the neck,
chest, abdomen and pelvis. This study was approved by the
Institutional Review Board of Tri-Service General Hospital,
National Defense Medical Center (Taipei, Taiwan). Patients provided
written informed consent.
Chemotherapy
A combination of cyclophosphamide,
hydroxydaunorubicin, vincristine and prednisone (CHOP) plus
rituximab (R-CHOP), or CHOP was administered for three or four
courses following radiotherapy for localized disease, and for 6–8
courses for advanced disease. A combination of cyclophosphamide,
etoposide, prednisolone and vincristine (CEOP) or CEOP minus
epirubicin was administered to patients who presented with cardiac
dysfunction or were older than 70 years. CHOP chemotherapy
consisted of 750 mg/m2 cyclophosphamide as an
intravenous (i.v.) infusion on day one, 50 mg/m2
doxorubicin i.v. on day one, 1.4 mg/m2 vincristine,
(maximum dose, 2 mg/body) i.v. on day one and 60 mg/m2
prednisone per orally (p.o.) on days 1–5. CEOP chemotherapy
consisted of 750 mg/m2 cyclophosphamide i.v. on day one,
80 mg/m2 epirubicin i.v. on day one, 1.4
mg/m2 vincristine (maximum dose, 2 mg/body) i.v. on day
one and 60 mg/m2 prednisone p.o. on days 1–5. The dosage
and schedule of rituximab included in the R-CHOP regimen was 375
mg/m2 every three weeks with chemotherapy.
The second-line chemotherapy regimens were a
combination of ifosfamide, carboplatin and etoposide or a
combination of etoposide, methylprednisolone, cytarabine and
cisplatin.
Endpoints and statistical analysis
Complete remission and partial remission were
assessed according to the Japanese 327123 International Working
Group criteria (9). Stable disease
was defined as less than partial remission, but not progressive
disease. Progressive disease was defined as the occurrence of new
lesions or a 25% increase in the sum of the products of the
cross-sectional diameters of all previously detected lesions.
The primary endpoint was overall survival (OS). The
final date for OS was defined as the day of mortality from any
cause or the last day the patient was known to be alive. OS was
assessed using the Kaplan-Meier method and compared between groups
using the log-rank test (10,11).
All survival analyses were performed using STATA statistical
software (StataCorp LP, College Station, TX, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Patient characteristics
Among the 11 patients with primary NHL of the small
intestine, seven of the patients were male (63.6%) and four were
female (36.3%). Nine of the patients (81.8%) were diagnosed with
B-cell lymphoma, of which five (45.4%) were also diagnosed with
diffuse large B-cell lymphoma (DLBL) (Table I). Abdominal pain and/or distention
were present in six (54.5%) of the patients and jejunum involvement
was also observed in six (54.5%) of the 11 patients. The clinical
characteristics of the patients with and without jejunum
involvement are shown in Table
II.
 | Table IClinical data of 11 patients with
primary NHL of the small intestine. |
Table I
Clinical data of 11 patients with
primary NHL of the small intestine.
| Case | Gender | Age, years | Involved site | Cx | Type | Stage | ECOG status | IPI | Surgery | C/T (cycles) | Outcome (months) |
|---|
| 1 | M | 47 | D, J, I | T | EATL | III | 1 | 3 | Y (Ru) | CHOP (1) | DOD (1.5) |
| 2 | M | 75 | D | B | Mantle | IV | 4 | 5 | | CHOP (2) | DOD (5) |
| 3 | M | 21 | D | T | Anaplastic | II | 1 | 2 | Y (AP) | CHOP (6)
ESHAP | AWD (57) |
| 4 | M | 63 | I | B | DLBL | III | 1 | 3 | Y (AP) | CHOP (6) | AWD (59) |
| 5 | F | 81 | J | B | Follicular | IV | 2 | 4 | | R-CHOP | AWD (58) |
| 6 | M | 47 | J | B | DLBL | IV | 2 | 4 | Y (AP) | R-CHOP (2)
R-ICE (2)+ ASCT | DOD (9) |
| 7 | F | 51 | D | B | DLBL | III | 1 | 3 | | R-CHOP (6) | AWD (75) |
| 8 | F | 80 | J | B | DLBL | III | 1 | 4 | | R-COP (6) | AWD (20) |
| 9 | M | 87 | J | B | Marginal zone | I | 3 | 4 | Y (AP) | Ex | AWD (9) |
| 10 | F | 50 | J | B | Follicular | II | 1 | 2 | Y (Ru) | R-CHOP
R-COP | AWD (8) |
| 11 | M | 17 | I | B | DLBL | II | 1 | 1 | | CEOP (2)
R-CHOP (4) | AWD (6) |
 | Table IIPatients with primary NHL of the small
intestine, with or without jejunum involvement. |
Table II
Patients with primary NHL of the small
intestine, with or without jejunum involvement.
| Jejunum
involvement |
|---|
|
|
|---|
| Parameter | Yes (n=6) | No (n=5) |
|---|
| Age, years |
| Mean | 65.3 | 45.4 |
| Range | 47–87 | 17–75 |
| Median | 65 | 51 |
| Gender, n |
| Male | 3 | 4 |
| Female | 3 | 1 |
| Cell type, n |
| B | 5 | 4 |
| T | 1 | 1 |
| ECOG, n |
| 0–1 | 3 | 4 |
| ≥2 | 3 | 1 |
| IPI, n |
| 1–2 | 1 | 2 |
| 3–5 | 5 | 3 |
| Surgery |
| Yes, n | 4 | 2 |
| No, n | 2 | 3 |
| Surgical rate,
% | 67 | 40 |
| Outcome |
| OS time, months | 16.9 | 39.6 |
| 4-year OS, n/total n
(%) | 1/6 (17) | 3/5 (60) |
Treatment
Surgical treatment was performed in four of the six
patients with jejunum involvement, due to an acute abdomen or
perforation-related peritonitis. The rate of surgical treatment in
patients with NHL and jejunum involvement was higher than in those
without jejunum involvement, although this difference was not
statistically significant (66.6 vs. 40%; P=0.763).
Outcome and survival
The mean OS time was 27.2 months and the four-year
survival rate was 36.3%. The mean OS time in the patients with
jejunum involvement was shorter than in those without jejunum
involvement (16.9 vs. 39.6 months), although this difference was
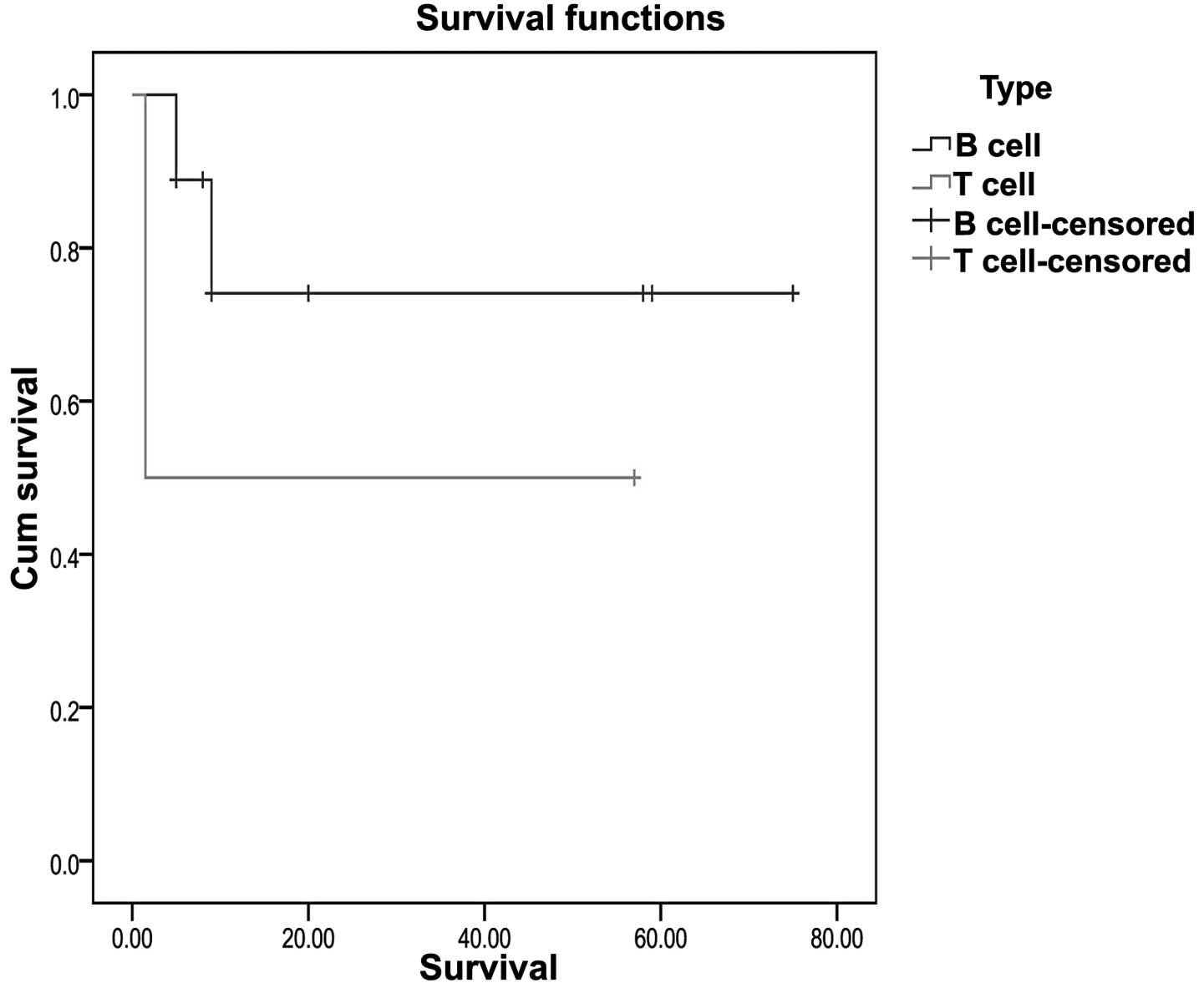
not found to be statistically significant (P=0.657; Fig. 1). Patients with T-cell lymphoma
(n=2) were observed to have a poorer OS time compared with those
with B-cell lymphoma (P=0.338; Fig.
2), but this difference was not statistically significant. The
patients with primary lymphoma of the small intestine with
disseminated disease were found to have a poorer OS time compared
with those with localized disease, although this difference was
also not statistically significant (P=0.202; Fig. 3).
Discussion
The treatment strategies for intestinal NHL have yet
to be established, although surgical resection is accepted as a
primary treatment modality based on the results of small series of
patients with intestinal NHL. Primary surgical resection has been
proposed as a rational treatment choice, as it simultaneously
establishes the diagnosis and reduces the tumor burden (12).
Previous studies have shown that ileocecal lymphomas
frequently require surgical intervention on an emergency basis due
to the anatomical site. However, even if 54% of ileocecal lymphomas
require immediate surgery, 30% of non-ileocecal lymphomas also
present with complications (3,13–16).
Thus, lymphomas in other anatomical locations also present with
complications that require emergency surgical intervention.
Previous studies investigating the rate of emergency surgery in
primary lymphomas of the small intestine are summarized in Table III (3,16–20).
In previous studies, the rate of complications that required
primary surgery was found to be between 9.3 and 23.8%, however, the
rate in the present study was 54.5% among all the patients with
primary NHL of the small intestine and 66.6% in the patients with
NHL with jejunum involvement. In the present study, two cases had
emergency surgery for intestinal perforations during chemotherapy.
Thus, patients with NHL of the small intestine with jejunum
involvement may benefit from primary surgical intervention followed
by chemotherapy.
 | Table IIIComparison of the rates of emergency
surgery in primary non-Hodgkin’s lymphoma of the small intestine
reported in previous studies. |
Table III
Comparison of the rates of emergency
surgery in primary non-Hodgkin’s lymphoma of the small intestine
reported in previous studies.
| First author/s, year
(ref.) | Small intestine | Jejunum | Complications
requiring primary surgery [jejunum] | Emergency surgery
rate, n/total n (%) [jejunum] |
|---|
| Turowski and Basson,
2004 (15) | 21 | NA | 5 | 5/21 (23.8) |
| Koch et al,
2001 (2) | 32 | NA | 3 | 3/32 (9.4) |
| Daum et al,
2003 (16) | 83 | 30 | 19 | 19/83 (22.9) |
| Ibrahim et al,
2001 (17) | 37 | - | - | - |
| Kako et al,
2009 (18) | 23 | 3 | 3 | 3/23 (13.0) |
| Kim et al,
2011 (19) | 92 | 17 | 12 | 12/92 (13.0) |
| Present study | 11 | 6 | 6 | 6/11 (54.5) |
| | | [4] | [4/6 (66.7)] |
B-cell lymphoma is the most common type of NHL of
the small intestine, and DLBL accounts for the majority of cases of
B-cell NHL. Previous studies have shown that T-cell lymphomas and
NHL with disseminated disease have poorer OS times, however, in the
present study this difference was not statistically significant
(P>0.05) (3,16–19).
In the present study, the OS time was shorter in the patients with
jejunum involvement compared with those without jejunum
involvement.
The majority of previous studies on primary GI NHL,
which has an incidence rate of one case per 100,000
individuals/year, are small, retrospective studies reporting small
patient sample sizes or studies which have been performed over long
periods. Such studies are often heterogeneous, combining a variety
GI NHL types and using differing histological classifications,
staging systems and forms of treatment.
The present study had certain significant
limitations. While the study was not a randomized trial, the
patient characteristics in the two groups were essentially
different. Furthermore, the numbers of patients and the follow-up
times were limited. A longer follow-up may stabilize the trends and
allow conclusions to be made.
The results of the present indicate that DLBL is the
most common subtype of NHL in primary lymphoma of the small
intestine and also show that the involvement site in NHL may be an
independent factor affecting surgical potential in patients with
intestinal lymphoma. The outcome for patients with primary lymphoma
of the small intestine is poor compared with that for patients with
lymphoma in other locations in the GI tract. The present study
identified that NHL with jejunum involvement may require primary
surgical intervention following chemotherapy, however, the sizes of
the subgroups of primary lymphoma of the small intestine were too
small for individual analysis.
Acknowledgements
The present study was supported by a grant from the
Tri-Service General Hospital (no. TSGH-C100-65).
References
|
1
|
Campo E, Swerdlow SH, Harris NL, Pileri S,
Stein H and Jaffe ES: The 2008 WHO classification of lymphoid
neoplasms and beyond: evolving concepts and practical applications.
Blood. 117:5019–5032
|
|
2
|
Zinzani PL, Magagnoli M, Pagliani G, et
al: Primary intestinal lymphoma: clinical and therapeutic features
of 32 patients. Haematologica. 82:305–308. 1997.
|
|
3
|
Koch P, del Valle F, Berdel WE, et al;
German Multicenter Study Group. Primary gastrointestinal
non-Hodgkin’s lymphoma: I. Anatomic and histologic distribution,
clinical features, and survival data of 371 patients registered in
the German Multicenter Study GIT NHL 01/92. J Clin Oncol.
19:3861–3873. 2001.
|
|
4
|
DiSario JA, Burt RW, Vargas H and
McWhorter WP: Small bowel cancer: epidemiological and clinical
characteristics from a population-based registry. Am J
Gastroenterol. 89:699–701. 1994.
|
|
5
|
Al-Mondhiry H: Primary lymphomas of the
small intestine: east-west contrast. Am J Hematol. 22:89–105.
1986.
|
|
6
|
Salles G, Herbrecht R, Tilly H, et al:
Aggressive primary gastrointestinal lymphomas: review of 91
patients treated with the LNH-84 regimen. A study of the Groupe
d‘Etude des Lymphomes Agressifs. Am J Med. 90:77–84. 1991.
|
|
7
|
Rackner VL, Thirlby RC and Ryan JA Jr:
Role of surgery in multimodality therapy for gastrointestinal
lymphoma. Am J Surg. 161:570–575. 1991.
|
|
8
|
Shepherd FA, Evans WK, Kutas G, et al:
Chemotherapy following surgery for stages IE and IIE non-Hodgkin’s
lymphoma of the gastrointestinal tract. J Clin Oncol. 6:253–260.
1988.
|
|
9
|
Cheson BD, Horning SJ, Coiffier B, Shipp
MA, Fisher RI, Connors JM, et al: Report of an international
workshop to standardize response criteria for non-Hodgkin’s
lymphomas. NCI Sponsored International Working Group. J Clin Oncol.
17:12441999.
|
|
10
|
Kaplan EL and Meier P: Nonparametric
estimation from incomplete observations. J Am Stat Assoc.
53:457–481. 1958.
|
|
11
|
Mantel N: Evaluation of survival data and
two new rank order statistics arising in its consideration. Cancer
Chemother Rep. 50:163–170. 1966.
|
|
12
|
Lee J, Kim WS, Kim K, et al: Intestinal
lymphoma: exploration of the prognostic factors and the optimal
treatment. Leuk Lymphoma. 45:339–344. 2004.
|
|
13
|
Gurney KA, Cartwright RA and Gilman EA:
Descriptive epidemiology of gastrointestinal non-Hodgkin’s lymphoma
in a population-based registry. Br J Cancer. 79:1929–1934.
1999.
|
|
14
|
Liang R, Todd D, Chan TK, Chiu E, Lie A,
Kwong YL, et al: Prognostic factors for primary gastrointestinal
lymphoma. Hematol Oncol. 13:153–163. 1995.
|
|
15
|
Radaszkiewicz T, Dragosics B and Bauer P:
Gastrointestinal malignant lymphomas of the mucosa-associated
lymphoid tissue: factors relevant to prognosis. Gastroenterology.
102:1628–1638. 1992.
|
|
16
|
Turowski GA and Basson MD: Primary
malignant lymphoma of the intestine. Am J Surg. 169:433–441.
1995.
|
|
17
|
Daum S, Ullrich R, Heise W, et al:
Intestinal non-Hodgkin’s lymphoma: a multicenter prospective
clinical study from the German Study Group on Intestinal
non-Hodgkin’s Lymphoma. J Clin Oncol. 21:2740–2746. 2003.
|
|
18
|
Ibrahim EM, Ezzat AA, El-Weshi AN, et al:
Primary intestinal diffuse large B-cell non-Hodgkin’s lymphoma:
clinical features, management, and prognosis of 66 patients. Ann
Oncol. 12:53–58. 2001.
|
|
19
|
Kako S, Oshima K, Sato M, et al: Clinical
outcome in patients with small-intestinal non-Hodgkin lymphoma.
Leuk Lymphoma. 50:1618–1624. 2009.
|
|
20
|
Kim SJ, Kang HJ, Kim JS, et al: Comparison
of treatment strategies for patients with intestinal diffuse large
B-cell lymphoma: surgical resection followed by chemotherapy versus
chemotherapy alone. Blood. 117:1958–1965. 2011.
|