Introduction
JSI-124 is a potent inhibitor of the signal
transducer and activator of transcription 3 (STAT3) signaling
pathway (1). Previous studies have
reported that JSI124 has anti-tumor activities in human breast
cancer (2), lung cancer (3), neuroblastoma (4,5),
murine melanoma cell lines (6) and
B-cell leukemia (7). The mechanisms
underlying this anti-tumor activity include the activation of the
nuclear factor κ-light-chain-enhancer of activated B cells pathway
in human glioblastoma cells (8),
Rac 1 inhibition in breast cancer cells by a reactive oxygen
species-mediated function (9),
diminishing self-renewing and radiochemoresistant abilities in
thyroid cancer-derived cluster of differentiation
(CD)133+ cells (10) and
the suppression of cell motility through indirectly interfering
with actin dynamics in B16-F1 mouse melanoma cells (11). Su et al (12) also reported that the
G2/M-phase cell cycle arrest and apoptosis augmentation
caused by JSI124 inhibits glioblastoma multiforme cell
proliferation.
B cells are prevalent in various tumor types and are
found primarily at inflammatory sites in aggregates with other
immune cells. Yang et al (13) reported that B cells may provide a
contribution to a network with other cells in order to promote
STAT3-dependent tumor angiogenesis. Consistent with this, STAT3 has
been shown to be important in the regulation of the
multi-directional feed-forward loop between tumor-associated
myeloid cells, endothelial cells and tumor cells in tumor
angiogenesis (14). However the
association between JSI124 and STAT3 levels in B cells has yet to
be elucidated.
In this study, the expression of STAT3 in the B
cells of breast cancer patients was first detected and a mouse 4T1
breast cancer model was further applied for revealing a novel
mechanism of tumor suppression by JSI124.
Materials and methods
Cell culture and mice
4T1 mouse breast tumor cells were purchased from the
American Type Culture Collection (Manassas, VA, USA) and cultured
in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal
calf serum, 100 U/ml penicillin and 100 μg/ml streptomycin in a
humidified incubator at 37°C with 5% CO2.
BALB/c mice were purchased from the Experimental
Animal Center of Nanjing Medical University (Nanjing, China) and
maintained under pathogen-free conditions according to protocols
that were approved by the Jiangsu Province Animal Care and Use
Committee.
Human blood samples
Peripheral blood samples were obtained from nine
healthy individuals and 10 patients with breast cancer between 2011
and 2013. All the blood samples were collected subsequent to
obtaining written informed consent according to a protocol approved
by the Institutional Review Board of the First People’s Hospital of
Huai’an (Huai’an, China).
Western blot analysis
In brief, B cells that were purified from human
blood or mouse spleens were lysed and the proteins of the lysed
cells were separated on 12% polyacrylamide gels using SDS-PAGE. The
separated proteins were transferred onto nitrocellulose membranes
and western blot analysis was performed using phospho-Stat3
(Tyr705) (3E2) rabbit anti-mouse monoclonal antibody (Cell
Signaling Technology, Inc., Beverly, MA, USA) and a rabbit
anti-mouse β-actin polyclonal antibody as the control (Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA).
B-cell isolation
To prepare the B cells from the human blood samples,
the peripheral blood from nine healthy volunteers and 10 patients
with breast cancer was collected. CD19+ B cells from the
whole blood were isolated using Dynabeads® CD19
(Invitrogen Life Technologies, Carlsbad, CA, USA), according to the
manufacturer’s instructions.
In order to isolate the B cells from the mouse
spleens, splenocytes were prepared using homogenization. The red
blood cells were removed through lysis using
ammonium-chloride-potassium buffer, and following two washes in
pre-iced phosphate-buffered saline (PBS), the CD19+ B
cells were purified using Dynabeads CD19 (Invitrogen Life
Technologies).
In vivo tumor experiments
To confirm the anti-4T1 tumor function of JSI124
in vivo, 2×105 4T1 cells were subcutaneously
injected into the Balb/c mice. Tumor growth was assessed every five
days and the expression of STAT3 was analyzed in the B cells
(1×106) from the PBS- and JSI124-treated mice using
western blot analysis.
To investigate the effect of B cells on 4T1 tumor
growth, 4T1-bearing Balb/c mice were treated with normal Balb/c
mouse-derived B cells, 4T1-bearing mouse-derived B cells or
JSI124-treated 4T1-bearing mouse-derived B cells (2×105
cells each time) every three days, for five times. Tumor growth was
determined by measuring the tumor volume.
To further investigate the function of
JSI124-treated B cells on tumor growth, B cells were treated with
JSI124 or incubated with 4T1 cells in vitro for 24 h, then
the B cells were intravenously injected into 4T1-implanted mice and
the tumor volume was measured every five days.
Results
STAT3 expression is increased in B cells
from the peripheral blood of patients with breast cancer
STAT3 expression was assessed in the B cells from
the peripheral blood of nine healthy individuals and 10 patients
with breast cancer. As shown in Fig.
1, STAT3 expression was upregulated approximately two-fold in
the B cells from the patients with breast cancer compared with
those in the healthy individuals (P=0.0015).
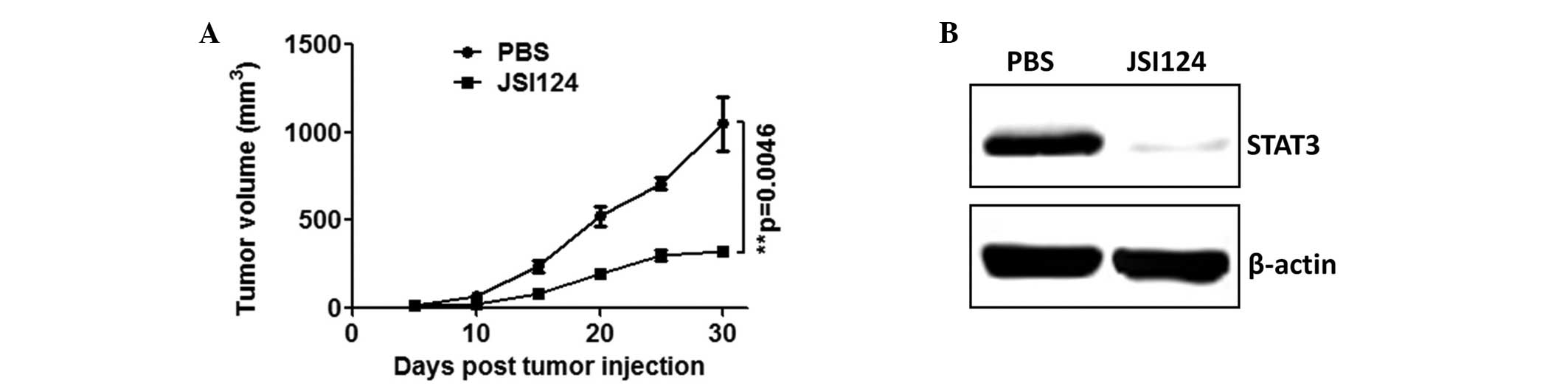
JSI124 suppresses 4T1 tumor growth
through the inhibition of STAT3 expression in B cells
JSI124 was used to treat the 4T1 tumor-bearing mice
and tumor growth was measured every five days. As shown in Fig. 2A, JSI124 was found to significantly
inhibit 4T1 tumor growth (P=0.0046). The expression of STAT3 in the
B cells of the JSI124- and PBS-treated mice was assessed using
western blot analysis. As shown in Fig.
2B, STAT3 expression was observed to be markedly downregulated
in the B cells of the 4T1-bearing mice treated with JSI124 compared
with those treated with PBS.
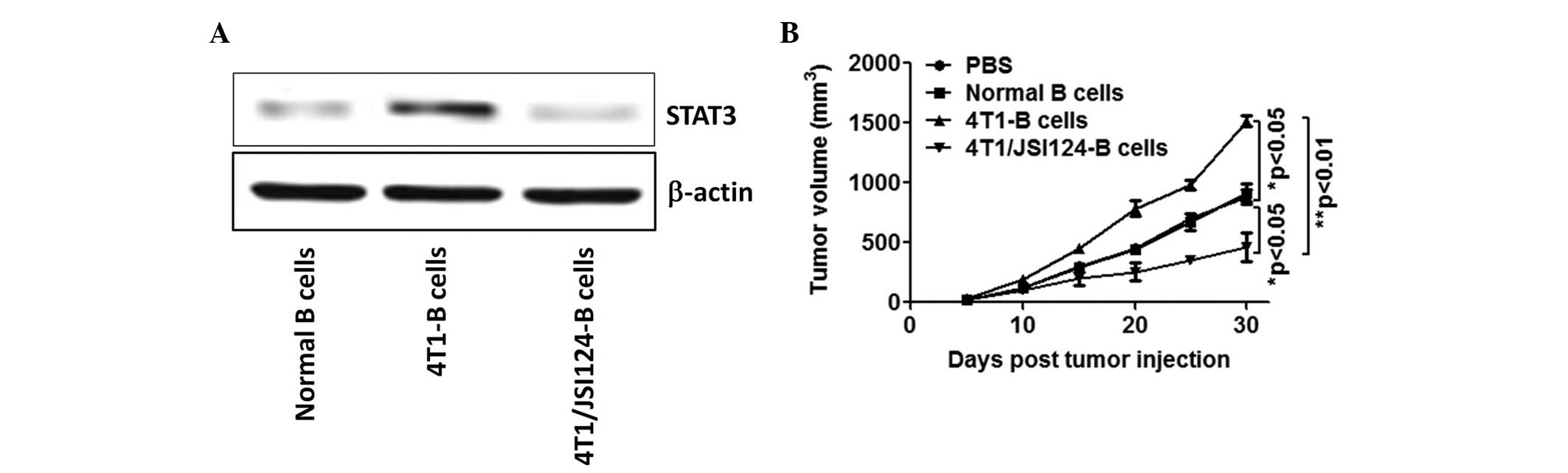
JSI124-treated 4T1 mouse-derived B cells
suppress 4T1 tumor growth
Previous studies have reported that STAT3 levels in
B cells are associated with tumor growth (13), therefore, the present study
investigated the effect of JSI124-treated 4T1 mouse-derived B cells
on 4T1 tumor growth in vivo. As shown in Fig. 3A, the expression of STAT3 in the
JSI124-treated 4T1 mouse-derived B cells was significantly
downregulated compared with the B cells from the 4T1-bearing mice.
In vivo tumor volume data revealed that the B cells from the
4T1-bearing mice promoted tumor growth (P<0.05); however, the
JSI124-treated 4T1 mouse-derived B cells suppressed tumor growth
(P<0.05; Fig. 3B).
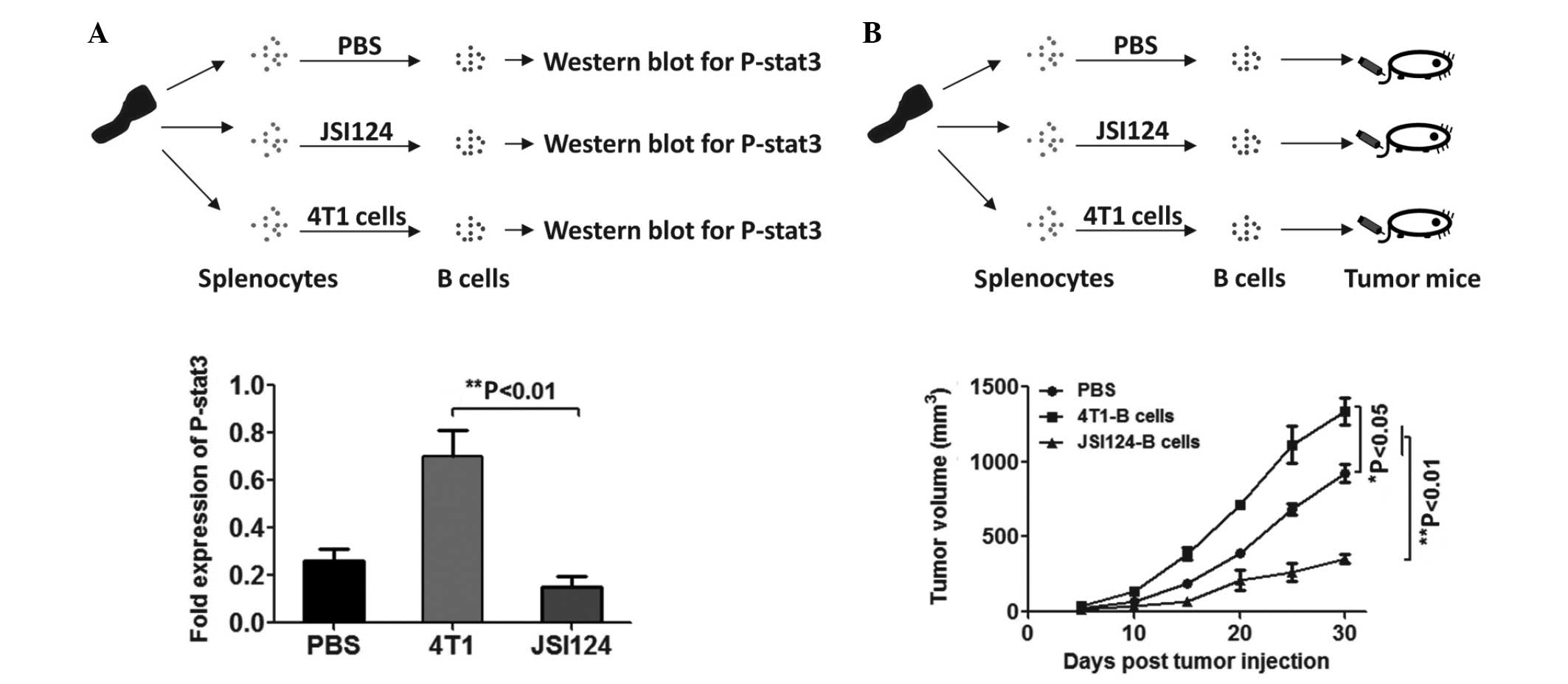
JSI124-treated normal B cells inhibit 4T1
tumor growth
To further confirm the tumor inhibitory effect of
JSI124-treated B cells, B cells from normal Balb/c mice were
treated with JSI124 or co-cultured with 4T1 cells, then the B cells
were purified and intravenously injected into 4T1-bearing mice. As
shown in Fig. 4A, STAT3 expression
was observed to be upregulated in the B cells following co-culture
with 4T1 cells, and downregulated in B cells following treatment
with JSI124. An in vivo tumor therapeutic model revealed
that JSI124-treated B cells in vitro have a tumor suppressor
function in vivo (Fig.
4B).
Discussion
The STAT proteins are a family of transcription
factors that consist of seven different members that have roles in
normal cellular events, including differentiation, apoptosis,
proliferation and the regulation of hematopoietic cell function
(15).
The transcription factors of the STAT family are
activated by Janus kinase, and the downregulation of this pathway
is frequently observed in primary tumors and leads to increased
angiogenesis, enhanced tumor cell survival and immunosuppression.
Specifically, activated STAT3 promotes tumor cell proliferation
(16), survival and invasion
(17), and inhibits antitumor
immune responses.
Previous studies have shown that STAT3 is a key
regulator of tumor growth, metastasis and tumor-associated
immunosuppression in patients with malignancies such as breast
cancer. More than half of all primary breast tumors and
tumor-derived cell lines express constitutively activated STAT3
(18,19). Furthermore, high levels of STAT3 are
a poor survival predicator in patients with breast cancer with
lymph node metastasis (20). A
number of studies have demonstrated that STAT3 inhibitors,
including cepharanthine (21),
niclosamide (22), cryptotanshinone
(23) and JSI124, suppress breast
tumor growth. As a STAT3 inhibitor, JSI124 has been extensively
used for the treatment of various types of tumor cells, including
those of breast cancer. Blaskovich et al (2) showed that JSI124 strongly inhibited
the growth of MDA-MB-468 human carcinoma cells through targeting
the Janus kinase/signal transducer and activator of transcription 3
signaling pathway (2). Lopez-Haber
and Kazanietz (9) found that JSI124
also suppresses breast cancer cells by inhibiting Rac1 activation
through a reactive oxygen species-mediated and Janus tyrosine
kinase 2- and P-Rex1-independent mechanism. However, there have
been no studies with regard to the correlation between JSI124 and
breast cancer-associated B cells. Olkhanud et al (24) reported that B cells evoked by tumors
are able to promote breast cancer metastasis by the conversion of
resting CD4+ T cells into T-regulatory cells. The
present study focused on the mechanism of the effect of JSI124 on
4T1 tumor growth. The present study showed that JSI124 suppresses
mouse breast 4T1 tumor growth. However, in contrast to these
studies, B cells were used as the target of JSI124. In the present
study, STAT3 expression in tumor associated-B cells was found to be
significantly inhibited by JSI124. Based on a previous study that
reported that B cells promote tumor angiogenesis in a
STAT3-dependent manner (13), the
present study investigated the function of B cells in 4T1 mice,
JSI124-treated 4T1 mice, 4T1-cocultured B cells and JSI124-treated
B cells in vitro. In accordance with the findings of Yang
et al (13), in the present
study, 4T1 tumor-associated B cells were observed to accelerate
tumor growth in a STAT3-dependent manner and this acceleration was
inhibited by JSI124. In conclusion, the present study has provided
a novel tumor suppressor mechanism of JSI124 for the inhibition of
mouse breast cancer.
References
|
1
|
Jing N and Tweardy DJ: Targeting Stat3 in
cancer therapy. Anticancer Drugs. 16:601–607. 2005.
|
|
2
|
Blaskovich MA, Sun J, Cantor A, Turkson J,
Jove R and Sebti SM: Discovery of JSI-124 (cucurbitacin I), a
selective Janus kinase/signal transducer and activator of
transcription 3 signaling pathway inhibitor with potent antitumor
activity against human and murine cancer cells in mice. Cancer Res.
63:1270–1279. 2003.
|
|
3
|
Lirdprapamongkol K, Sakurai H, Abdelhamed
S, Yokoyama S, Athikomkulchai S, Viriyaroj A, Awale S, Ruchirawat
S, Svasti J and Saiki I: Chrysin overcomes TRAIL resistance of
cancer cells through Mcl-1 downregulation by inhibiting STAT3
phosphorylation. Int J Oncol. 43:329–337. 2013.
|
|
4
|
Gheeya JS, Chen QR, Benjamin CD, Cheuk AT,
Tsang P, Chung JY, Metaferia BB, Badgett TC, Johansson P, Wei JS,
Hewitt SM and Khan J: Screening a panel of drugs with diverse
mechanisms of action yields potential therapeutic agents against
neuroblastoma. Cancer Biol Ther. 8:2386–2395. 2009.
|
|
5
|
Wang Q, Zhuang X, Mu J, Deng ZB, Jiang H,
Zhang L, Xiang X, Wang B, Yan J, Miller D and Zhang HG: Delivery of
therapeutic agents by nanoparticles made of grapefruit-derived
lipids. Nat Commun. 4:18672013.
|
|
6
|
Molavi O, Ma Z, Hamdy S, Lai R,
Lavasanifar A and Samuel J: Synergistic antitumor effects of CpG
oligodeoxynucleotide and STAT3 inhibitory agent JSI-124 in a mouse
melanoma tumor model. Immunol Cell Biol. 86:506–514. 2008.
|
|
7
|
Ishdorj G, Johnston JB and Gibson SB:
Cucurbitacin-I (JSI-124) activates the JNK/c-Jun signaling pathway
independent of apoptosis and cell cycle arrest in B leukemic cells.
BMC Cancer. 11:2682011.
|
|
8
|
McFarland BC, Gray GK, Nozell SE, Hong SW
and Benveniste EN: Activation of the NF-κB pathway by the STAT3
inhibitor JSI-124 in human glioblastoma cells. Mol Cancer Res.
11:494–505. 2013.
|
|
9
|
Lopez-Haber C and Kazanietz MG:
Cucurbitacin I inhibits Rac1 activation in breast cancer cells by a
reactive oxygen species-mediated mechanism and independently of
Janus tyrosine kinase 2 and P-Rex1. Mol Pharmacol. 83:1141–1154.
2013.
|
|
10
|
Hsu HS, Huang PI, Chang YL, Tzao C, Chen
YW, Shih HC, Hung SC, Chen YC, Tseng LM and Chiou SH: Cucurbitacin
I inhibits tumorigenic ability and enhances radiochemosensitivity
in nonsmall cell lung cancer-derived CD133-positive cells. Cancer.
117:2970–2985. 2011.
|
|
11
|
Knecht DA, LaFleur RA, Kahsai AW, Argueta
CE, Beshir AB and Fenteany G: Cucurbitacin I inhibits cell motility
by indirectly interfering with actin dynamics. PLoS One.
11:e140392010.
|
|
12
|
Su Y, Li G, Zhang X, Gu J, Zhang C, Tian Z
and Zhang J: JSI-124 inhibits glioblastoma multiforme cell
proliferation through G(2)/M cell cycle arrest and apoptosis
augment. Cancer Biol Ther. 7:1243–1249. 2008.
|
|
13
|
Yang C, Lee H, Pal S, Jove V, Deng J,
Zhang W, Hoon DS, Wakabayashi M, Forman S and Yu H: B cells promote
tumor progression via STAT3 regulated-angiogenesis. PLoS One.
8:e641592013.
|
|
14
|
Kujawski M, Kortylewski M, Lee H, Herrmann
A, Kay H and Yu H: Stat3 mediates myeloid cell-dependent tumor
angiogenesis in mice. J Clin Invest. 118:3367–3377. 2008.
|
|
15
|
Calò V, Migliavacca M, Bazan V, Macaluso
M, Buscemi M, Gebbia N and Russo A: STAT proteins: from normal
control of cellular events to tumorigenesis. J Cell Physiol.
197:157–168. 2003.
|
|
16
|
Ashizawa T, Miyata H, Iizuka A, Komiyama
M, Oshita C, Kume A, Nogami M, Yagoto M, Ito I, Oishi T, Watanabe
R, Mitsuya K, Matsuno K, Furuya T, Okawara T, Otsuka M, Ogo N, Asai
A, Nakasu Y, Yamaguchi K and Akiyama Y: Effect of the STAT3
inhibitor STX-0119 on the proliferation of cancer stem-like cells
derived from recurrent glioblastoma. Int J Oncol. 43:219–227.
2013.
|
|
17
|
Subramaniam A, Shanmugam MK, Perumal E, Li
F, Nachiyappan A, Dai X, Swamy SN, Ahn KS, Kumar AP, Tan BK, Hui KM
and Sethi G: Potential role of signal transducer and activator of
transcription (STAT)3 signaling pathway in inflammation, survival,
proliferation and invasion of hepatocellular carcinoma. Biochim
Biophys Acta. 1835:46–60. 2013.
|
|
18
|
Diaz N, Minton S, Cox C, Bowman T, Gritsko
T, Garcia R, Eweis I, Wloch M, Livingston S, Seijo E, Cantor A, Lee
JH, Beam CA, Sullivan D, Jove R and Muro-Cacho CA: Activation of
stat3 in primary tumors from high-risk breast cancer patients is
associated with elevated levels of activated SRC and survivin
expression. Clin Cancer Res. 12:20–28. 2006.
|
|
19
|
Gritsko T, Williams A, Turkson J, Kaneko
S, Bowman T, Huang M, Nam S, Eweis I, Diaz N, Sullivan D, Yoder S,
Enkemann S, Eschrich S, Lee JH, Beam CA, Cheng J, Minton S,
Muro-Cacho CA and Jove R: Persistent activation of stat3 signaling
induces survivin gene expression and confers resistance to
apoptosis in human breast cancer cells. Clin Cancer Res. 12:11–19.
2006.
|
|
20
|
Chen Y, Wang J, Wang X, Liu X, Li H, Lv Q,
Zhu J, Wei B and Tang Y: STAT3, a poor survival predicator, is
associated with lymph node metastasis from breast cancer. J Breast
Cancer. 16:40–49. 2013.
|
|
21
|
Ono M, Tanaka N and Orita K: Positive
interactions between human interferon and cepharanthin against
human cancer cells in vitro and in vivo. Cancer Chemother
Pharmacol. 35:10–16. 1994.
|
|
22
|
Lu W, Lin C, Roberts MJ, Waud WR, Piazza
GA and Li Y: Niclosamide suppresses cancer cell growth by inducing
Wnt co-receptor LRP6 degradation and inhibiting the Wnt/β-catenin
pathway. PLoS One. 6:e292902011.
|
|
23
|
Nizamutdinova IT, Lee GW, Son KH, Jeon SJ,
Kang SS, Kim YS, Lee JH, Seo HG, Chang KC and Kim HJ: Tanshinone I
effectively induces apoptosis in estrogen receptor-positive (MCF-7)
and estrogen receptor-negative (MDA-MB-231) breast cancer cells.
Int J Oncol. 33:485–491. 2008.
|
|
24
|
Olkhanud PB, Damdinsuren B, Bodogai M,
Gress RE, Sen R, Wejksza K, Malchinkhuu E, Wersto RP and Biragyn A:
Tumor-evoked regulatory B cells promote breast cancer metastasis by
converting resting CD4+ T cells to T-regulatory cells.
Cancer Res. 71:3505–3515. 2011.
|