Introduction
Gastric cancer is one of the most common malignant
tumors in the digestive tract. In China, almost 300,000 individuals
succumb to gastric cancer and ~400,000 new cases are diagnosed each
year (1). The diagnosis, metastasis
and prognosis of tumors use various molecular markers, so the
search for molecular markers at different stages of tumor
development is of great clinical significance to the early
diagnosis, recurrence, metastasis prediction and prognosis of
tumors (2). Studies on tumor
markers have been carried out for numerous years, and there are
also a small number of markers used in the clinic, but their
sensitivity and specificity remain extremely limited (3,4).
Therefore, it is essential to study the molecular markers
associated with gastric cancer metastasis and prognosis. The
emergence of microRNA (miR/miRNA) provides a novel concept for
cancer research and the screening of molecular markers.
miRNAs are a class of endogenous non-coding small
molecule RNAs with regulatory function on genes, which are 22–28
nucleotides in length and widely exist in eukaryotes (5). A gene with miRNA encoded in the
nucleus is first transcribed into a pri-miRNA, i.e., the precursor
of miRNA (6). Specific miRNAs have
a close association with tumors. It has been confirmed that there
are ~500 miRNAs in the human genome, of which at least 200 miRNA
sequences are closely associated with tumors, and a number of them
can be used as tumor molecular markers (7). miRNAs as molecular markers have unique
advantages; they are more stable compared with mRNA, are not
affected by other physiological states of the body, can be
amplified and can be more easily detected compared with proteins
(8).
The present study aimed to conduct screening,
identification and validation of miRNAs in gastric cancer tissues
and cell lines, combined with an analysis on the
clinicopathological factors and survival data of gastric cancer
patients. The study also aimed to reveal the regulation mechanism
of miRNA expression in gastric cancer, in order to provide a
theoretical basis for miRNAs to become novel molecular markers and
therapeutic targets of the metastasis and prognosis of gastric
cancer.
Materials and methods
Materials
The following materials were used in the present
study: A normal gastric mucosal epithelial cell line, GES-1
(Shanghai Cell Bank Center, Chinese Academy of Medical Sciences,
Shanghai, China), a gel imager and electrophoresis apparatus
(Bio-Rad, Hercules, CA, USA) and pipettes (5, 10 and 200 μl and 1
ml; Eppendorf, Hamburg, Germany).
Subjects
A total of 46 patients who underwent gastric
resection in Xiangya Hospital of Central-South University
(Changsha, Hunan, China) between January and December 2012 were
selected. In total, thee study group consisted of 26 males and 20
females aged between 30 and 70 years old, with the mean age of
52.1±6.7 years old. The clinical data of the patients with gastric
cancer were recorded completely, including pathological type,
stage, metastasis, one-, two- and three-year survival rates and
other detailed information. The study was approved by the ethics
comittee of Xiangya Hospital of Central-South University. Consent
was obtained prior to examination for the enrolled patients
according to the Declaration of Helsinki and relevant laws in
China. All treatments were performed based on the best interests of
the patients.
RNA extraction
The gastric cancer cells were plated in 6-well
culture plates 24 h prior to transfection. When the cells reached
30–50% confluency, 10 μl antisense oligonucleotide liposome
(Promega Corporation, Madison, WI, USA) was taken correspondingly
and diluted to 250 μl by Opti-minimal essential medium (Invitrogen
China Limited, Beijing, China) for action at room temperature for
10 min. The two were then slowly mixed prior to leaving the mixture
stationary for 30 min and finally added it to the cells, which were
agitated and mixed well. Following 12 h of culture, the medium was
replaced with RPMI-1640 containing 10% fetal bovine serum for 48 h.
Subsequently, the medium was aspirated, the cells were washed with
phosphate-buffered saline and TRIzol (Invitrogen Life Technologies,
Carlsbad, CA, USA) was added. The samples were then stored at −70°C
(9,10).
Quantitative polymerase chain reaction
(qPCR)
The detection of the miRNA target and internal
control, U6, was conducted in a Rotor-Gene 3000 Real-time PCR
machine (Corbett Research, Mortlake, Australia). The 25 μl reaction
system included 2.5 μl dNTP, 2.5 μl 10× PCR buffer, 1.5 μl
MgCl2 aqueous solution, 1 unit Taq enzyme, SYBR-Green I
with a final concentration of 0.25X, 1 μl PCR specific primer, 1 μl
reverse transcriptase product and RNA-free enzyme water. The
reaction conditions were as follows: 95°C for 5 min, followed by
95°C for 10 sec and 60°C for 1 min for a total of 40 cycles. The
miRNA expression level was detected with the threshold cycle number
(Ct). The miRNA targets were relatively quantified by the ΔΔCt
method. ΔCt refers to the Ct value of the miRNA target minus the Ct
value of U6RNA, and ΔΔCt is the value of the ΔCt value of each
gastric tissue sample minus the ΔCt value of the normal gastric
tissue sample. The changing multiple was calculated using the
2−ΔΔCt formula (11).
Statistical analysis
All data were analyzed by SPSS, version 13.0 (SPSS,
Inc., Chicago, IL, USA). The measurement data were expressed as the
mean ± standard deviation and processed by paired t-test and
compatibility group design analysis of variance. P<0.05 was
considered to indicate a statistically significant difference.
Results
RNA extraction results
The integrity of RNA can be assessed by
electrophoresis on a denaturing agarose gel. Sharp 28S and 18S RNA
bands (eukaryotic samples) are produced by intact total RNA that is
run on a denaturing gel. An example for RNA electrophoresis on a
denaturing agarose gel is shown in Fig
1. The RNA electrophoresis showed that the 28S and 18S bands
were wider compared with the control (Fig. 1), indicating that the RNA was not
degraded.
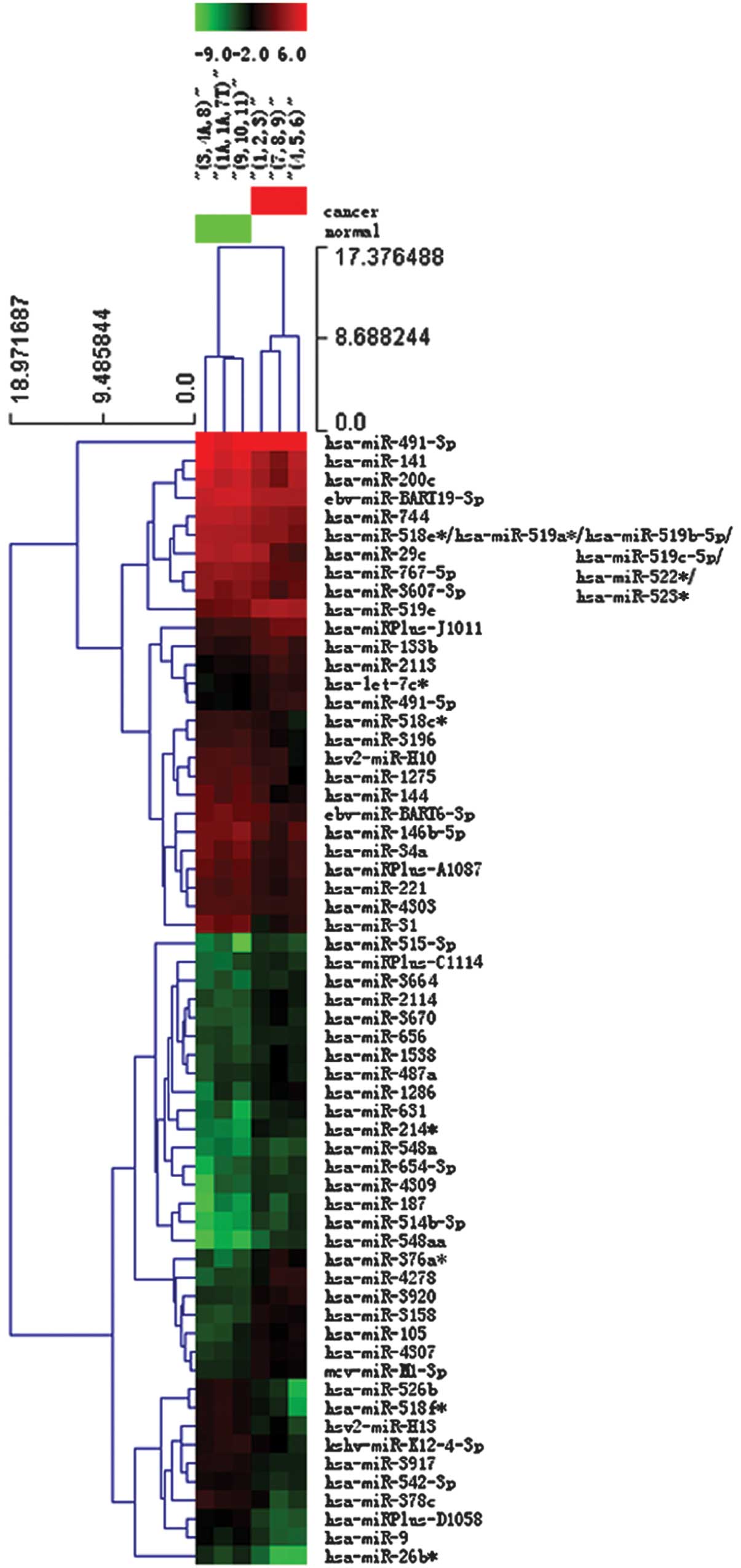
Heat map and hierarchical clustering
Two-way hierarchical clustering results for the
miRNA and samples are shown in Fig.
2 by the heat map diagram. One miRNA is represented per row,
and one sample per column. The left-hand side of the image shows
the miRNA clustering tree, while the sample clustering tree appears
at the top. Samples and miRNAs are arranged by cluster analysis
into groups based on their expression levels, which enables
hypotheses to be generated regarding the correlations between the
miRNAs and samples. Subsequently, hierarchical clustering was
performed based on the differentially-expressed miRNAs in a cancer
vs. normal pass volcano plot. The result of hierarchical clustering
shows distinguishable miRNA expression profiling among the samples
(Fig. 2).
miRNA expression in gastric cancer
tissues
The qPCR result showed that compared with the normal
gastric mucosa, the expression levels of miR-9, miR-433, miR-19b
and miR-370 were detected in the gastric cancer tissues and cells
(Table I and Fig. 3), and the electrophoretic bands were
located at 63, 64, 65 and 63 bp. Compared with levels in the normal
gastric mucosa, the miR-9, miR-433, miR-19b and miR-370 expression
levels were lowered (Figs.
4–7).
 | Table IExpression levels of miRNAs in gastric
cancer and normal gastric mucosa tissues. |
Table I
Expression levels of miRNAs in gastric
cancer and normal gastric mucosa tissues.
| Tissue | miR-9 | miR-19b | miR-433 | miR-370 |
|---|
| Cancer | 0.419±0.211a | 0.648±0.351a | 0.397±0.284a | 0.504±0.351a |
| Normal | 0.985±0.429 | 1.051±0.589 | 1.061±0.514 | 0.991±0.512 |
Association between miRNA expression in
gastric cancer and clinical pathological factors
Downregulated miR-9 expression was associated with
the size of the foci and lymph node metastasis (P=0.036 and 0.028,
respectively), while decreased miR-433 expression was associated
with the foci site and pathological grading (P=0.003 and 0.005,
respectively). Reduced miR-19b expression was correlated with the
foci site and pathological grading (P=0.002 and 0.009,
respectively), and downregulated miR-370 expression was associated
with the foci site, pathological grading and lymph node metastasis
(P=0.003, 0.004 and 0.004, respectively) (Table II).
 | Table IIAssociation between miRNA expression
of gastric cancer tissues and clinical pathological factors. |
Table II
Association between miRNA expression
of gastric cancer tissues and clinical pathological factors.
| | miR-9 | miR-19b | miR-433 | miR-370 |
|---|
| |
|
|
|
|
|---|
| Clinical pathological
factor | No. of cases | Low expression | High expression | P-value | Low expression | High expression | P-value | Low expression | High expression | P-value | Low expression | High expression | P-value |
|---|
| Gender, n | | | | 0.068 | | | 0.086 | | | 0.083 | | | 0.093 |
| Male | 26 | 23 | 3 | | 22 | 4 | | 23 | 3 | | 24 | 2 | |
| Female | 20 | 18 | 2 | | 18 | 2 | | 19 | 1 | | 18 | 2 | |
| Age, n | | | | 0.077 | | | 1.021 | | | 1.132 | | 1.324 | |
| <50 years | 24 | 22 | 2 | | 21 | 3 | | 22 | 2 | | 21 | 3 | |
| ≥50 years | 22 | 19 | 3 | | 19 | 3 | | 20 | 2 | | 21 | 1 | |
| Focus size, n | | | | 0.036 | | | 0.057 | | | 0.071 | | | 0.068 |
| <3 cm | 17 | 14 | 3 | | 14 | 3 | | 15 | 2 | | 15 | 2 | |
| ≥3 cm | 29 | 27 | 2 | | 26 | 3 | | 27 | 2 | | 27 | 2 | |
| Focus site, n | | | | 0.059 | | | 0.009 | | | 0.005 | | | 0.004 |
| Lesser gastric
curvature | 28 | 25 | 3 | | 27 | 1 | | 27 | 1 | | 27 | 1 | |
| Greater gastric
curvature | 18 | 16 | 2 | | 13 | 5 | | 15 | 3 | | 15 | 3 | |
| Pathological grading,
n | | | | 0.072 | | | 0.009 | | | 0.005 | | | 0.004 |
| High
differentiation | 19 | 17 | 2 | | 14 | 5 | | 16 | 3 | | 15 | 4 | |
| Low
differentiation | 27 | 24 | 3 | | 26 | 1 | | 26 | 1 | | 27 | 0 | |
| Lymphatic
metastasis, n | | | | 0.028 | | | 0.003 | | | 0.061 | | | 0.004 |
| Yes | 27 | 27 | 0 | | 27 | 0 | | 25 | 2 | | 27 | 0 | |
| No | 19 | 14 | 5 | | 13 | 6 | | 17 | 2 | | 15 | 4 | |
Discussion
There are various molecular markers that can be used
for tumor diagnosis, metastasis and prognosis, therefore, the
search for tumor molecular markers at these differing stages
provides an important link between basic and clinical research, and
a main way for basic research to serve a function in the clinic
(12–14). Studies have shown that the
metastasis and prognosis of gastric cancer is closely associated
with the connexins (Cx). The mRNA and protein levels of Cx32 and
Cx43 in gastric cancer cells and tissues are significantly reduced,
and thus miRNAs that regulate Cx are molecular markers of gastric
cancer (15,16). Through the process of looking for
miRNA-9, -433, -19b and -370 which regulate Cx and via the use of
statistical analysis on metastasis and prognosis in clinical
groups, combined with clinical data, the present study confirmed
that the miRNAs are good molecular markers. The present study is of
great significance in the theoretical basis of the development,
metastasis and prognosis of gastric cancer.
pri-miRNA is sheared into a length of ~70
nucleotides under the effect of the double-stranded RNA specific
nuclease-Drosha RNase, then pri-miRNA, having a hairpin structure,
is transferred from the nucleus into the cytoplasm with the action
of the transporter protein, exportin 5, and cut into
double-stranded miRNAs that are 21–25 nucleotides in length under
the effect of another double-stranded RNA-specific nuclease, the
Dicer enzyme (17–19). Subsequent to the unwinding of the
double helix, one of the two chains connects with the RNA-induced
silencing complex (RISC), functioning as an miRNA, and the other
chain is degraded immediately. The combination of miRNAs with
target mRNA can mediate the degradation of RISC into target
fragments or hinder the translation process, which depends on the
degree of mismatch between miRNA and the target mRNA 3′
untranslated region. If it is a complete or almost complete match,
the RNA interference pathway can be induced and mRNA will be
degraded. As the majority of miRNAs are not completely
complementary with target mRNAs, which only play a role in closing
mRNA targets and inhibiting the translation process so as to
regulate the expression of target genes, miRNA plays a vital role
in a variety of biological processes, including cell proliferation,
differentiation and apoptosis (20–22).
In addition, through the application of gene-chip
technology and bio-informatics methods, certain miRNAs that are
closely associated with gastric cancer have been found. The
experimental study by Li et al (23) showed that in 92% of gastric cancer
samples, miR-21 had a significantly high expression level,
indicating that miR-21 may serve as an effective diagnostic marker
of gastric cancer. He and Wang (24) identified that in gastric cancer cell
lines, the expression of the tumor-associated protein,
high-mobility group AT-hook 2 (HMGA2), was inhibited by miRNA
let27. In gastric cancer cells with a low HMGA2 expression level,
the expression level of miRNA let27a, let27b and let27c was
significantly higher compared with gastric cancer cells with a high
HMGA2 expression level, indicating that miRNAs may play a
biological role to promote or inhibit tumor development through the
interaction between tumor-associated proteins.
The main factor affecting the prognosis of gastric
cancer is the invasion and metastasis of gastric cancer, therefore,
it is of great significance to study particular miRNAs that are
closely associated with gastric cancer invasion, metastasis and
development, which can also provide clues for the screening of
miRNA molecular markers. A study by Li et al (25) analyzed the effect of miR-10a on the
migration and invasion of the gastric cancer BGC823 cell line, and
found that the transfection of miR-10a had no significant effect on
the proliferation and apoptosis of BGC823, but could clearly
promote its migration and invasion. Transfection with mature human
miR-210a could increase the miR-10a expression in BGC823 and
significantly promote the migration and invasion of BGC823
(26,27). Chen et al (28) studied the association between miRNA
and RPL23 (a lymph node metastasis-related gene) by creating an
miRNA expression plasmid that could express miR-155. This
effectively silenced the expression of the PRL23 target gene in the
gastric cancer SGC7901 cell line and significantly inhibited the
invasion and metastasis of the SGC7901 cells. Therefore, miRNAs are
closely associated with tumor proliferation and apoptosis, and
their correlation with tumor invasion and metastasis requires
further research.
To understand the carcinogenic mechanism of miRNAs,
systematic identification of the target taking effect in
vivo is required. The bioinformatics data show that each miRNA
can regulate hundreds of target genes. It is estimated that 30% of
human genes are subject to regulation by miRNAs, which also
indicates that miRNA may affect all the signaling pathways
(29). A study by Cao et al
(30) selected myocardial cells as
the study object and found that the regulatory targets of miR-1
existed in the GJA1-encoded Cx43, and miR-1 was overexpressed in
myocardial cells and inhibited the expression of GJA1. Cx43
expression is reduced accordingly in the myocardium, aggravating
arrhythmias (31). In this study,
the expression of miRNA in gastric cancer tissues and cells was
evaluated by analyzing the clinical pathological factors and
survival data of patients. The findings revealed the mechanism
regarding the mechanism of action, and provide theoretical evidence
for the use of miRNA as novel molecular markers and treatment
targets for the metastasis and prognosis of gastric cancer.
References
|
1
|
Tavazoie SF, Alarcón C, Oskarsson T, et
al: Endogenous human microRNAs that suppress breast cancer
metastasis. Nature. 451:147–152. 2008.
|
|
2
|
Waldman SA and Terzic A: MicroRNA
signature as diagnostic and therapeutic targets. Clin Chem.
54:943–944. 2008.
|
|
3
|
Ito T, Shimada Y, Kan T, et al: Pituitary
tumor-transforming 1 increases cell motility and promotes lymph
node metastasis in esophageal squamous cell carcinoma. Cancer Res.
68:3214–3224. 2008.
|
|
4
|
Inose H, Ochi H, Kimura A, et al: A
microRNA regulatory mechanism of osteoblast differentiation. Proc
Natl Acad Sci USA. 106:20794–20799. 2009.
|
|
5
|
Harquail J, Benzina S and Robichaud GA:
MicroRNAs and breast cancer malignancy: an overview of
miRNA-regulated cancer processes leading to metastasis. Cancer
Biomark. 11:269–280. 2012.
|
|
6
|
Huang XA and Lin H: The miRNA regulation
of stem cells. Wiley Interdiscip Rev Membr Transp Signal. 1:83–95.
2012.
|
|
7
|
Maccani MA and Marsit CJ: Exposure and
fetal growth-associated miRNA alterations in the human placenta.
Clin Epigenetics. 2:401–404. 2011.
|
|
8
|
Jethwa K, Wei J, McEnery K and Heimberger
AB: miRNA-mediated immune regulation and immunotherapeutic
potential in glioblastoma. Clin Investig (Lond). 1:1637–1650.
2011.
|
|
9
|
Lodewijk L, Prins AM, Kist JW, Valk GD,
Kranenburg O, Rinkes IH and Vriens MR: The value of miRNA in
diagnosing thyroid cancer: a systematic review. Cancer Biomark.
11:229–238. 2012.
|
|
10
|
Sanchez N, Lao N, Gallagher C, Clynes M
and Barron N: Engineering CHO cell growth by stable manipulation of
miRNA expression. BMC Proc. 5(Suppl 8): P222011.
|
|
11
|
Wang L, Liu W, Jiang W, Lin J, Jiang Y, Li
B and Pang D: A miRNA binding site single-nucleotide polymorphism
in the 3′-UTR region of the IL23R gene is associated with breast
cancer. PLoS One. 7:e498232012.
|
|
12
|
Hirata H, Ueno K, Shahryari V, Tanaka Y,
Tabatabai ZL, Hinoda Y and Dahiya R: Oncogenic miRNA-182-5p targets
Smad4 and RECK in human bladder cancer. PLoS One. 7:e510562012.
|
|
13
|
Srivastava K and Srivastava A:
Comprehensive review of genetic association studies and
meta-analyses on miRNA polymorphisms and cancer risk. PLoS One.
7:e509662012.
|
|
14
|
Agirre X, Martínez-Climent JÁ, Odero MD
and Prósper F: Epigenetic regulation of miRNA genes in acute
leukemia. Leukemia. 26:395–403. 2012.
|
|
15
|
Yue SB, Trujillo RD, Tang Y, O’Gorman WE
and Chen CZ: Loop nucleotides control primary and mature miRNA
function in target recognition and repression. RNA Biol.
8:1115–1123. 2011.
|
|
16
|
Guo Y, Chen Y, Carreon S and Qiang M:
Chronic intermittent ethanol exposure and its removal induce a
different miRNA expression pattern in primary cortical neuronal
cultures. Alcohol Clin Exp Res. 36:1058–1066. 2012.
|
|
17
|
Martin EC, Bratton MR, Zhu Y, Rhodes LV,
Tilghman SL, Collins-Burow BM and Burow ME: Insulin-like growth
factor-1 signaling regulates miRNA expression in MCF-7 breast
cancer cell line. PLoS One. 7:e490672012.
|
|
18
|
Devaraj S and Natarajan J: miRNA-mRNA
network detects hub mRNAs and cancer specific miRNAs in lung
cancer. In Silico Biol. 11:281–295. 2012.
|
|
19
|
Alshalalfa M: MicroRNA response
elements-mediated miRNA-miRNA interactions in prostate cancer. Adv
Bioinformatics. 2012:8398372012.
|
|
20
|
Arão TC, Guimarães AL, de Paula AM, Gomes
CC and Gomez RS: Increased miRNA-146a and miRNA-155 expressions in
oral lichen planus. Arch Dermatol Res. 304:371–375. 2012.
|
|
21
|
Staton AA and Giraldez AJ: Use of target
protector morpholinos to analyze the physiological roles of
specific miRNA-mRNA pairs in vivo. Nat Protoc. 6:2035–2049.
2011.
|
|
22
|
Fabbri E, Brognara E, Borgatti M, et al:
miRNA therapeutics: delivery and biological activity of peptide
nucleic acids targeting miRNAs. Epigenomics. 3:733–745. 2011.
|
|
23
|
Li SC, Liao YL, Ho MR, Tsai KW, Lai CH and
Lin WC: miRNA arm selection and isomiR distribution in gastric
cancer. BMC Genomics. 13(Suppl 1): S132012.
|
|
24
|
He M and Wang ZW: Current status and
development of miRNA and siRNA research on gastric cancer. Yi
Chuan. 33:925–930. 2011.(In Chinese).
|
|
25
|
Li C, Li JF, Cai Q, Qiu QQ, Yan M, Liu BY
and Zhu ZG: miRNA-199a-3p in plasma as a potential diagnostic
biomarker for gastric cancer. Ann Surg Oncol. 20(Suppl 3):
S397–S405. 2013.
|
|
26
|
Budak H and Akpinar A: Dehydration
stress-responsive miRNA in Brachypodium distachyon: evident by
genome-wide screening of microRNAs expression. OMICS. 15:791–799.
2011.
|
|
27
|
Rao E, Jiang C, Ji M, et al: The miRNA-17
~92 cluster mediates chemoresistance and enhances tumor growth in
mantle cell lymphoma via PI3K/AKT pathway activation. Leukemia.
26:1064–1072. 2012.
|
|
28
|
Chen W, Tang Z, Sun Y, et al: miRNA
expression profile in primary gastric cancers and paired lymph node
metastases indicates that miR-10a plays a role in metastasis from
primary gastric cancer to lymph nodes. Exp Ther Med. 3:351–356.
2012.
|
|
29
|
Tonevitsky AG, Maltseva DV, Abbasi A,
Samatov TR, Sakharov DA, Shkurnikov MU, et al: Dynamically
regulated miRNA-mRNA networks revealed by exercise. BMC Physiol.
13:92013.
|
|
30
|
Cao W, Fan R, Wang L, et al: Expression
and regulatory function of miRNA-34a in targeting survivin in
gastric cancer cells. Tumour Biol. 34:963–971. 2013.
|
|
31
|
Schnitger AK, Machova A, Mueller RU, et
al: Listeria monocytogenes infection in macrophages induces
vacuolar-dependent host miRNA response. PLoS One. 6:e274352011.
|