Introduction
Odontogenic myxomas (OMs) are benign, slow-growing,
locally aggressive and non-metastasizing neoplasms of the jaw bone.
OM is derived from embryonic mesenchymal elements of the dental
anlage (1–3). According to the World Health
Organization, OM is a benign tumor of ectomesenchymal origin with
or without odontogenic epithelium (4). OM accounts for 0.5–20% of all
odontogenic tumors (5,6). OM often occurs in individuals who are
between their second and forth decades, has a slight predilection
for females and is rarely found in children and the elderly
(3). The majority of lesions that
are without pain reach a large size and cause displacement of the
teeth and asymmetry of the mandible or maxilla prior to discovery
(7).
OM of the maxilla was first reported by Thoma and
Goldman in 1947 (8). While OMs
predominantly involve the mandible, maxillary tumors are more
aggressive than those in the mandible (9). Certain lesions spread with progressive
pain through the maxillary sinus and nasal cavity, and severe cases
result in exopthalmus, nasal obstruction and neurological
disturbance (7,10). The surgical treatment of OM,
including curettage and radical resection, is controversial due to
the varying recurrence rates (9,11).
The present study reports the case of a male patient
with a large maxillary OM. Although the mass was removed
successfully, repairing the defect in the maxilla was a complex
process. Patient provided written informed consent.
Case report
Patient presentation
A 37-year-old male presented with a mass on the
right side of the face, which had persisted for five years, with
accelerated growth for one year, causing serious facial deformity
and difficulty in eating. The painless mass was ~16×16 cm and
occupied the right maxilla and the nose, extending superoinferiorly
from the right orbit and infraorbital rim to the bilateral alveolar
bone and hard palate. Part of the mass displaced the teeth and
protruded outward from the mouth, with a rough surface that
released a purulent discharge upon palpation. No superficial
ulceration, sinuses or fistulas were observed on the overlying
skin, which did not adhere to the mass. The patient had bilateral
nasal obstruction with effluvial secretion and normal vision, even
though the infraorbital rim disappeared, as the mass displaced the
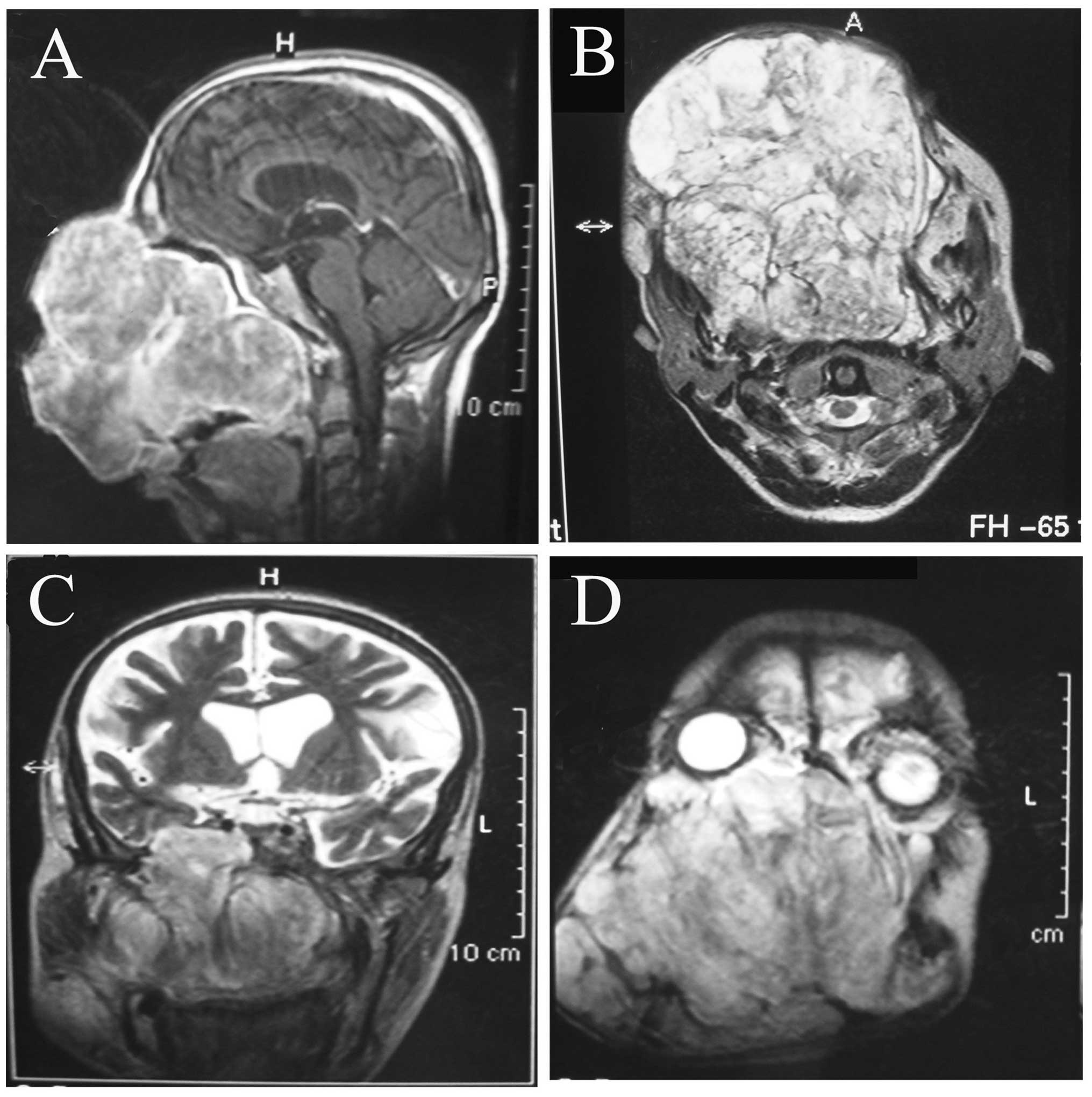
optic nerve (Fig. 1).
Clinical and imaging analyses
Enhanced computed tomography revealed that an
irregular and low-density shadow without obvious enhancement was
displaced in the maxillae, and that a high-density shadow was
interspersed in it. Magnetic resonance imaging identified that the
well-circumscribed tumor had already obliterated the maxillae, the
maxillary process of the right zygomatic bone, part of the left
maxilla, the ethmoidal sinuses, the sphenoid sinus and the nasal
cavity. The nasopharyngeal cavity and the left maxillary sinus were
observed to be narrow, and the turbinate bones and the nasal septum
had been resorbed due to the tumor pressure. Although the large
mass grew into the intracranial cavity through a bone defect in the
sphenoid wing caused by tumor pressure-induced resorption, the
tumor was present with well-defined borders under the dura mater
(Fig. 2). According to the clinical
and imaging examinations, it was hypothesized that the tumor may be
a mesenchymal benign tumor with malignant transformation.
Surgery
Following a tracheostomy, the patient underwent
right radical maxillectomy and left partial maxillectomy, which
included the partial right zygomatic bone and zygomatic alveolar
ridge of the left maxilla, with a 1-cm healthy margin. Moreover,
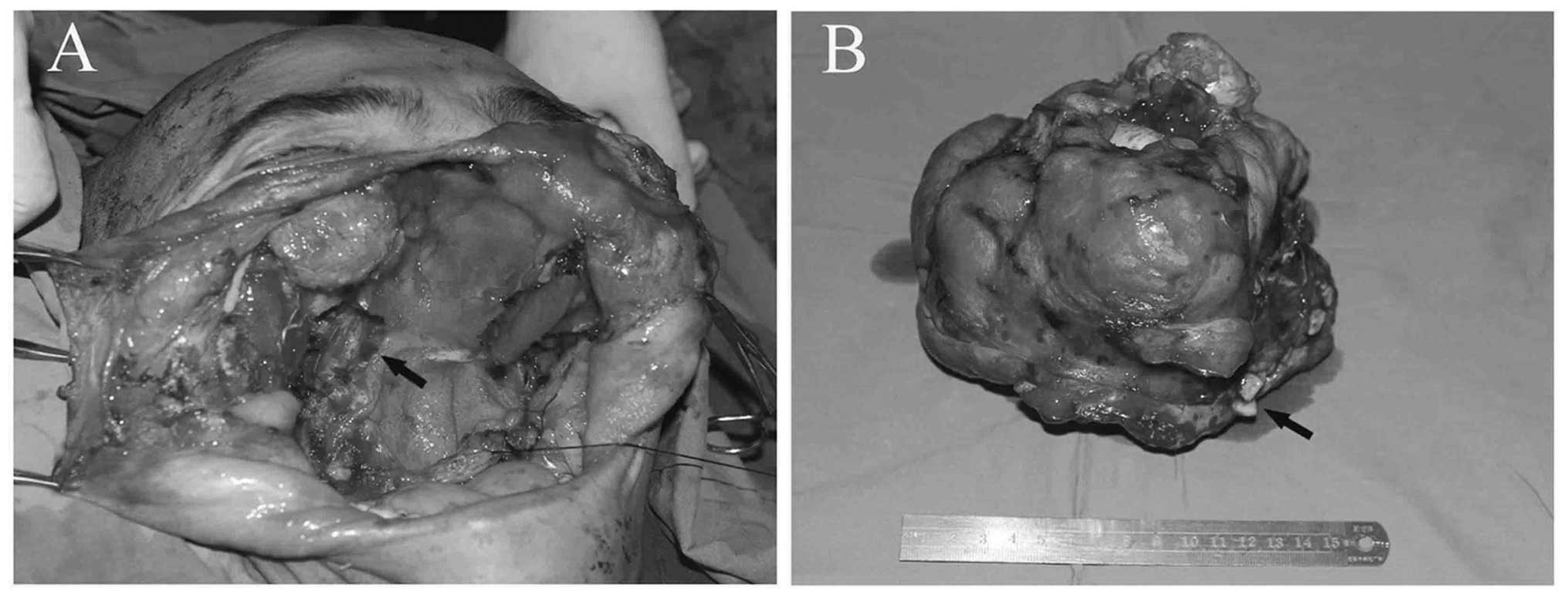
the mass was enucleated from the soft tissues around its capsule. A
small quantity of bone surrounding the bone defect in the skull
base, as well as the turbinate mucosa and the thin skin covering
the mass were also resected. The medial rectus in the right eye,
the septum mucosa, the left nasal mucosa, the thin bone of the left
infraorbital rim and the residual posterior wall of the maxillary
sinus were retained. Subsequent to tumor removal, the cavernous,
ethmoid and left maxillary sinuses were exposed (Fig. 3). The patient did not undergo repair
using soft-tissue flaps due to the risk of complications and
financial constraints. Thus, the wound surface was covered with
Heal-All Rehabilitation Membrane® (heterogeneous
acellular dermal matrix of cattle; Zhenghai Biological Co., Ltd.,
Shandong, China) and the defect cavity was filled with large
numbers of staple slivers with iodoform. No intracranial infection
or cerebrospinal leakage were observed. No clinical or
radiographical signs of recurrence were observed, and the soft- and
hard-tissue defects were covered with compact mucosa after one year
of post-operative control. The right side of the patient’s face and
nasal bridge collapsed and the right eyeball moved down, as the
defects were not repaired using soft flaps.
Diagnosis
Macroscopically, the surgically resected mass
measured ~15×16×16 cm and appeared as a completely encapsulated
whitish-grey, lobulated, smooth and hard mass. Microscopically, the
tumor was composed of a faintly basophilic myxomatous ground
substance and a mount of spindle- and stellate-shaped cells.
Variable quantities of fibrous tissues were found throughout the
mucoid-rich matrix. Furthermore, minimal and inconspicuous
thin-walled vessels and residual bone fragments were interspersed
within the tumor. Islands of odontogenic epithelium, hyalinization
and calcification were not found. The mass was diagnosed as an
OM.
Discussion
An OM is a mesenchymal benign tumor with the
potential for extensive bone destruction, cortical expansion and a
relatively high recurrence rate (8,12). It
has been found that OM accounts for ~7.2% of all odontogenic tumors
and has no marked predilection in terms of gender and location
(mandible or maxilla) in the Chinese population (12,13).
By contrast, other studies have reported a male:female ratio of
between 1:2 and 1:3 and a mandible:maxilla ratio of between 2:1 and
2.5:1, particularly in African countries (12,13).
OM has been reported in individuals of a wide range of ages, with
an average age of 25.3 years old. A total of eight cases <10
years old and none >60 years old have been reported at the West
China Hospital of Stomatology (Chengdu, China) (13). The majority of patients with OM
present with a slowly increasing swelling and facial asymmetry with
infiltration. The mass is locally aggressive, particularly in the
maxilla (4,14). In the present case, the growth of
the mass had accelerated for one year prior to surgery due to the
accumulation of mucoid ground substance. Moreover, the tumor
contained residual bone fragments and nasal mucosa with tumor
infiltration.
While surgical intervention is recommended, no
consensus has been reached with regard to the surgical method for
the treatment of OM due to its aggressive nature and high rate of
recurrence, which is between 10 and 43%, particularly in patients
who are treated with curettage or local surgical excision (15). Boffano et al (11) reported that conservative surgery,
such as curettage and enucleation, should be performed when tumors
are <3 cm, while radical surgery should be performed when tumors
are large and aggressive (11,16,17).
Locally aggressive OM should be subjected to radical resection with
a margin of 0.5–1.0 cm or 1.0–1.5 cm of healthy bone (11,18).
In the present case, based on the absorption of the bilateral
maxillae and the partial right sphenoid wing, an extended resection
of the mass was performed, with 1 cm margins in the right zygomatic
bone and the zygomatic alveolar ridge of the left maxilla to
prevent recurrence. The tumor was enucleated around its complete
envelope in the soft tissue. Moreover, the thin skin covering the
mass was resected and was not found to be infiltrated in the frozen
histopathology report. A partial or radical resection was
hypothesized to be the best treatment choice for maxillary OM,
rather than conservative surgery, in order to reduce the recurrence
rate.
The development of microsurgery and reconstructive
surgery techniques have enabled the basic functional and aesthetic
aims of maxillary reconstruction to be achieved. Various methods
have been used to reconstruct maxillary defects, including
vascularized soft- or hard-tissue grafts from the radial forearm,
rectus abdominis, anterolater thigh, scapular and fibular, iliac
crest flaps, vascularized free fibular flaps and cranial bone
flaps, as well as material implants using, for example, titanium
mesh (19–23). According to the Brown classification
system for maxillary defects (24),
the patient described in the present study had defects higher than
class IV (Fig. 4), with the large
soft- and hard-tissue defects being difficult to reconstruct. In
this case, the residual thinning bones and soft tissue had
insufficient strength to support the weight of a composite bone
flap with titanium miniplates. Peng et al (20) reported that the composite fibular
flap is inadequate for restoring the maxilla and the infraorbital
area. Smolka and Iizuka (25) used
a latissimus dorsi flap/rectus abdominis flap and free iliac crest
graft to repair a class IVa defect with the highest rate of
transplant loss. Although vascularized soft-tissue flaps are not
capable of restoring the maxillary buttress, they have been
reported to fill the partial large cavity of midfacial defects and
rehabilitate the basic swallowing function of patients. In the
present case, the use of a latissimus dorsi myocutaneous flap and
an anterolateral femoral skin flap was proposed for the repair of
the skull base bone and maxillary defects in order to alleviate the
symptoms of right facial collapse. Secondary reconstruction with a
prosthesis was also proposed. However, the patient refused
aesthetic reconstruction.
 | Figure 4Brown’s classification system for
maxillary defects (24). Vertical
component; Classes I, maxillectomy with no oroantral fistula; II,
low maxillectomy III, high maxillectomy; and IV, radical
maxillectomy. Horizontal component: I, unilateral alveolar maxilla
and resection of the hard palate; a, resection of less than or
equal to half of the alveolar and hard palate, not involving the
nasal septum or crossing the midline; b, resection of the bilateral
alveolar maxilla and hard palate, including a smaller resection
that crosses the midline of the alveolar bone, including the nasal
septum; and c, removal of the entire alveolar maxilla and hard
palate. |
To the best of our knowledge, the present study is
the first report of such a large maxillary OM. In the present
study, without surgical intervention, the patient had a high risk
of succumbing due to increasing intracranial pressure. The mass was
removed successfully without any complications and did not recur
within one year despite the unsatisfactory results with regard to
the external facial features and their function. Due to the
structure of the maxillae and the local infiltration of OM, radical
resection may the best treatment choice for maxillary OM in hard
tissue and local resection may be best around the envelope in soft
tissue. It is difficult to restore the maxillary buttress and
achieve an optimal aesthetic appearance using current methods for
reconstructing midfacial defects due to the lack of suitable
conditions for oral rehabilitation with good dentition. Further
investigations are required to develop appropriate, satisfactory
reconstruction methods for midfacial defects.
Acknowledgements
The authors would like to thank Professor Zhixiu He
(Department of Pathology, West China Hospital of Stomatology,
Sichuan University, Chengdu, China) for the pathological diagnosis
of the patient.
References
|
1
|
Buchner A, Merrell PW and Carpenter WM:
Relative frequency of central odontogenic tumors: a study of 1,088
cases from Northern California and comparison to studies from other
parts of the world. J Oral Maxillofac Surg. 64:1343–1352. 2006.
|
|
2
|
Farman AG, Nortjé CJ, Grotepass FW, Farman
FJ and Van Zyl JA: Myxofibroma of the jaws. Br J Oral Surg.
15:3–18. 1977.
|
|
3
|
Abiose BO, Ajagbe HA and Thomas O:
Fibromyxomas of the jawbones - a study of ten cases. Br J Oral
Maxillofac Surg. 25:415–421. 1987.
|
|
4
|
Kramer IRH, Pindborg JJ and Shear M:
Histological typing of odontogenic tumours. 2nd edition. Springer
Verlag; Berlin: 1991
|
|
5
|
Barnes L, Eveson J, Reichart P and DS:
World health organization classification of tumors. Pathology and
genetics of head and neck tumors. IARC Press; Lyon: 2006
|
|
6
|
Simon EN, Merkx MA, Vuhahula E, Ngassapa D
and Stoelinga PJ: Odontogenic myxoma: a clinicopathological study
of 33 cases. Int J Oral Maxillofac Surg. 33:333–337. 2004.
|
|
7
|
Shah A, Lone P, Latoo S, et al:
Odontogenic myxoma of the maxilla: A report of a rare case and
review on histogenetic and diagnostic concepts. Natl J Maxillofac
Surg. 2:189–195. 2011.
|
|
8
|
Thoma KH and Goldman HM: Central myxoma of
the jaw. Am J Oral Surg Orthod. 33:1947.
|
|
9
|
Deron PB, Nikolovski N, den Hollander JC,
Spoelstra HA and Knegt PP: Myxoma of the maxilla: a case with
extremely aggressive biologic behavior. Head Neck. 18:459–464.
1996.
|
|
10
|
Kaffe I, Naor H and Buchner A: Clinical
and radiological features of odontogenic myxoma of the jaws.
Dentomaxillofac Radiol. 26:299–303. 1997.
|
|
11
|
Boffano P, Gallesio C, Barreca A, et al:
Surgical treatment of odontogenic myxoma. J Craniofac Surg.
22:982–987. 2011.
|
|
12
|
Li TJ, Sun LS and Luo HY: Odontogenic
myxoma: a clinicopathologic study of 25 cases. Arch Pathol Lab Med.
130:1799–1806. 2006.
|
|
13
|
Jing W, Xuan M, Lin Y, et al: Odontogenic
tumours: a retrospective study of 1642 cases in a Chinese
population. Int J Oral Maxillofac Surg. 36:20–25. 2007.
|
|
14
|
Brannon RB: Central odontogenic fibroma,
myxoma (odontogenic myxoma, fibromyxoma), and central odontogenic
granular cell tumor. Oral Maxillofac Surg Clin North Am.
16:359–374. 2004.
|
|
15
|
Lo Muzio L, Nocini P, Favia G, Procaccini
M and Mignogna MD: Odontogenic myxoma of the jaws: a clinical,
radiologic, immunohistochemical, and ultrastructural study. Oral
Surg Oral Med Oral Pathol Oral Radiol Endod. 82:426–433. 1996.
|
|
16
|
Pahl S, Henn W, Binger T, Stein U and
Remberger K: Malignant odontogenic myxoma of the maxilla: case with
cytogenetic confirmation. J Laryngol Otol. 114:533–535. 2000.
|
|
17
|
Ogütcen-Toller M, Sener I, Kasap V and
Cakir-Ozkan N: Maxillary myxoma: surgical treatment and
reconstruction with buccal fat pad flap: a case report. J Contemp
Dent Pract. 7:107–116. 2006.
|
|
18
|
Leiser Y, Abu-El-Naaj I and Peled M:
Odontogenic myxoma - a case series and review of the surgical
management. J Craniomaxillofac Surg. 37:206–209. 2009.
|
|
19
|
González-García R, Naval-Gías L,
Rodríguez-Campo FJ, Muñoz-Guerra MF and Sastre-Pérez J:
Vascularized free fibular flap for the reconstruction of mandibular
defects: clinical experience in 42 cases. Oral Surg Oral Med Oral
Pathol Oral Radiol Endod. 106:191–202. 2008.
|
|
20
|
Peng X, Mao C, Yu GY, et al: Maxillary
reconstruction with the free fibula flap. Plast Reconstr Surg.
115:1562–1569. 2005.
|
|
21
|
Swartz WM, Banis JC, Newton ED, et al: The
osteocutaneous scapular flap for mandibular and maxillary
reconstruction. Plast Reconstr Surg. 77:530–545. 1986.
|
|
22
|
Chrcanovic BR, do Amaral MB, Marigo Hde A
and Freire-Maia B: An expanded odontogenic myxoma in maxilla.
Stomatologija. 12:122–128. 2010.
|
|
23
|
Sun J, Shen Y, Li J and Zhang ZY:
Reconstruction of high maxillectomy defects with the fibula
osteomyocutaneous flap in combination with titanium mesh or a
zygomatic implant. Plast Reconstr Surg. 127:150–160. 2011.
|
|
24
|
Brown JS, Jones DC, Summerwill A, et al: A
modified classification for the maxillectomy defect. Head Neck.
22:17–26. 2000.
|
|
25
|
Smolka W and Iizuka T: Surgical
reconstruction of maxilla and midface: clinical outcome and factors
relating to postoperative complications. J Craniomaxillofac Surg.
33:1–7. 2005.
|