Introduction
The dissemination of gastric neoplasms commonly
occurs due to hematogenous spread, lymphatic metastases, direct
local invasion of adjacent organs and peritoneal or transcoelomic
spread (1). Metastases are found at
the sites of the regional lymph nodes, peritoneum, liver, lungs and
bones (2). The criteria for the
diagnosis of metastatic tumors are well documented. Firstly, the
primary tumor must be known and histologically confirmed. Secondly,
the metastatic tumor must be of the same histological type as the
primary tumor. Finally, the possibility of direct local spread from
the primary tumor must be excluded (3). Colonic metastases are uncommon and
usually originate from carcinomas of the breast, stomach, skin
(melanomas), kidney, prostate, or ovaries (4). Colonic metastases from gastric
adenocarcinoma usually present as ‘linitis plastica’ or as an
annular stricture (5). Gastric, or
gastric stump, carcinoma may metastasize to the colon and present
as solitary or multiple colonic polyps, which is an extremely rare
condition with <10 cases described in the literature before
August 20, 2012 (www.ncbi.nlm.nih.gov/pubmed), with the first case
reported by Metayer et al (6) in 1991, and subsequently by Ogiwara
et al (4) in 1994. The
present study reports a case of poorly-differentiated
adenocarcinoma with diffuse signet ring cells of gastric stump
adenocarcinoma and mucosal metastases in multiple colonic polyps.
The patient provided written informed consent.
Case report
An 80-year-old male patient who presented with the
symptoms of diarrhea, weight loss, anorexia and lower abdominal
pain was admitted to the Department of Geriatric Medicine (Beijing
Shijitan Hospital, Beijing, China). The patient had previously
undergone a gastrectomy due to the perforation of a benign gastric
ulcer 48 years previously. A physical examination revealed paleness
and no significant cervical or supraclavicular lymphadenopathy was
noted. Breath sounds were normal and a grade 2/6 systolic apical
murmur was detected upon auscultation. The laboratory examination
showed a hemoglobin level of 9.9 g/dl, a lactate dehydrogenase
level of 1,756 mmol/l (normal range, 40–240 mmol/l) and
hydroxybutyrate dehydrogenase levels of 1,383 mmol/l (normal range,
80–200 mmol/l). The serum carcinoembryonic antigen level was 416.4
ng/ml (normal, ≤5.0 ng/ml), the carbohydrate antigen (CA)72.4 level
was >300 U/ml (normal, ≤6.9 U/ml) and the CA19-9 level was
272.82 U/ml (normal, ≤37 U/ml). All other biochemical and
hematological tests were normal.
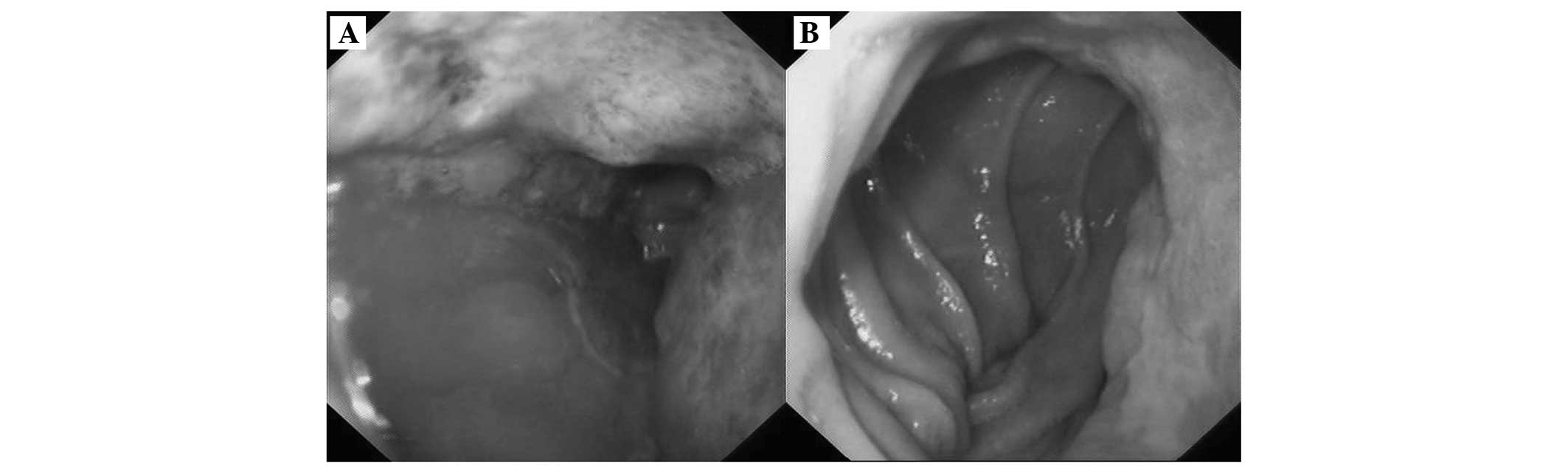
Gastroscopy detected multifocal ulcerated lesions in
the remnant stomach from the cardia (Fig. 1A) to the gastrointestinal
anastomosis (Fig. 1B), however, the
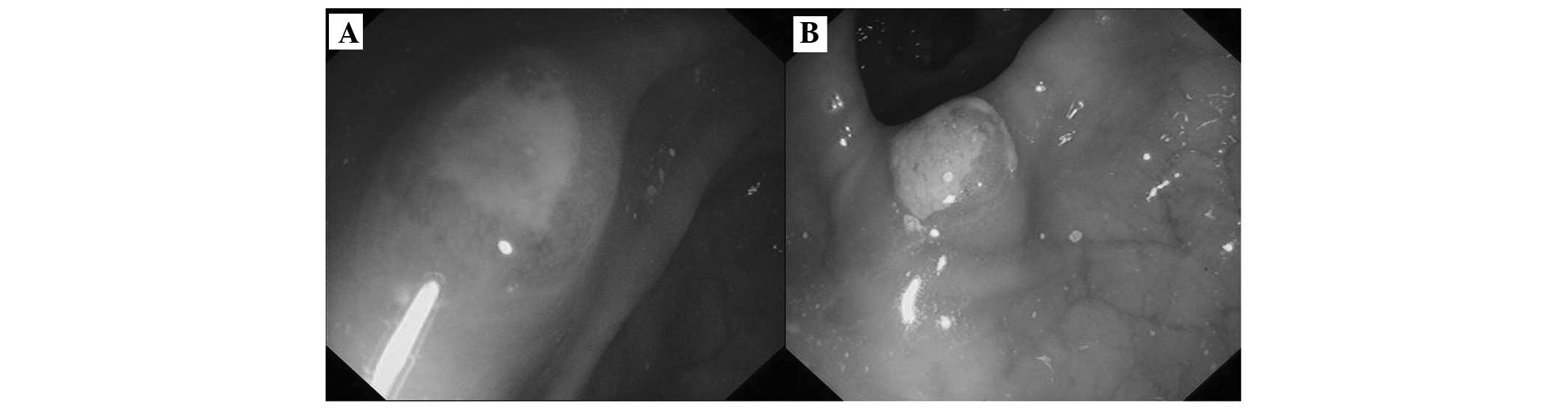
boundaries of certain lesions were unclear. Colonoscopy revealed
that >10 multifocal polypoid lesions measuring 6–10 mm in
diameter were scattered throughout the entire colon, except in the
rectum (Fig. 2A, transverse colon;
and Fig. 2B, descending colon).
Each lesion had either erosion or a depression at the top, and
several were covered with a white fur-like substance. Abdominal
magnetic resonance imaging revealed diffuse thickening of the
remnant stomach wall and multiple enlarged lymph nodes on the
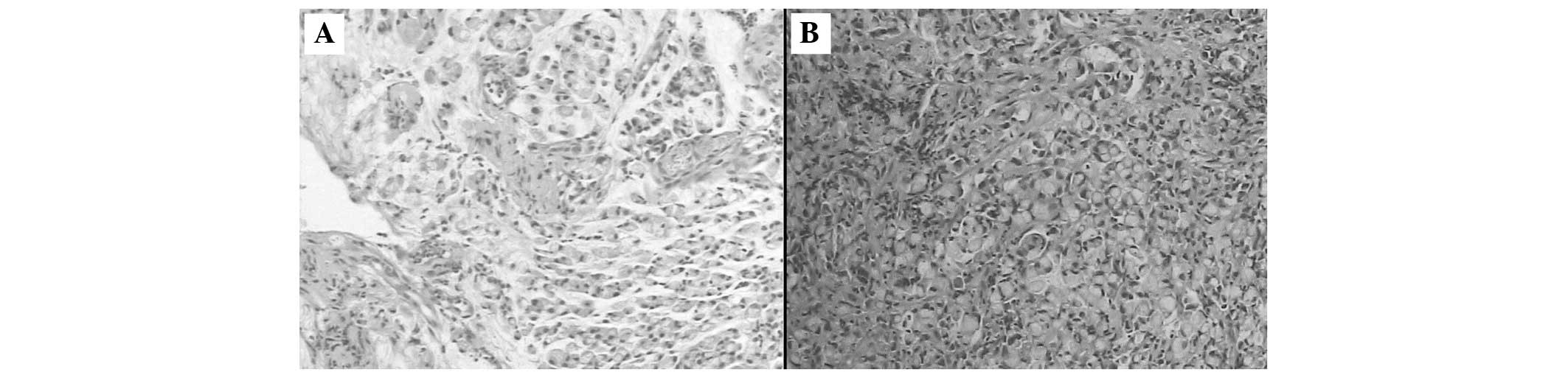
lesser curvature and retroperitoneum. The biopsy specimens from the
stomach showed a poorly-differentiated adenocarcinoma with
scattered signet ring cells (Fig.
3A), and the colonoscopy-guided biopsy revealed a signet ring
cell adenocarcinoma (Fig. 3B).
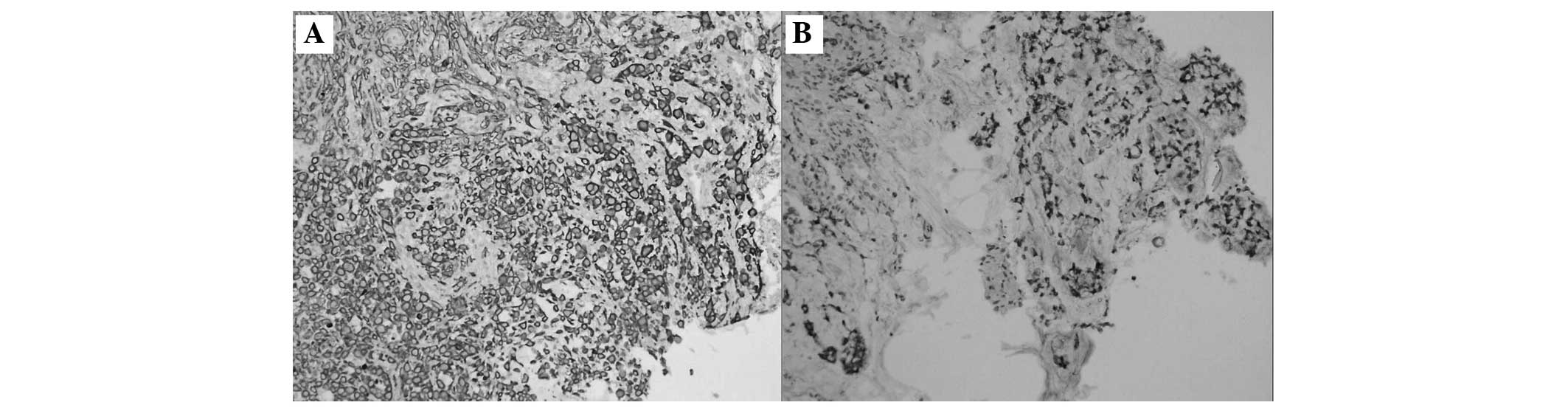
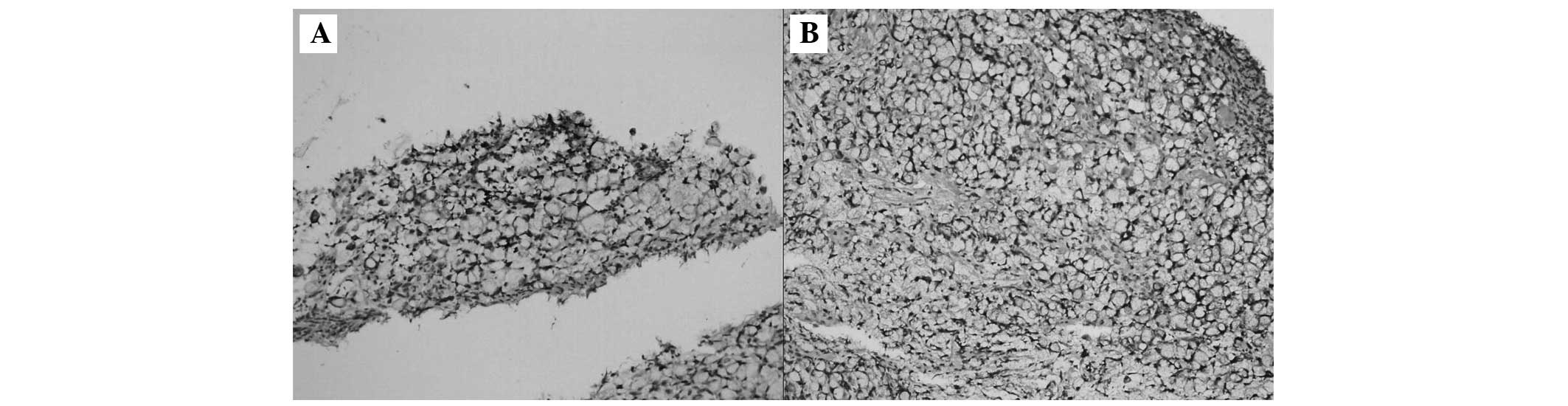
Immunohistochemical staining of the gastric stump mucosa (Fig. 4A and B) and colon mucosa (Fig. 5A and B) was positive for cytokeratin
(CK)7 and CK20. Thus, the actual colonic lesions were corresponding
with the mucosal spread of the primary gastric carcinoma.
The patient was referred to the Oncology unit for
assessment, and chemotherapy consisting of 1,000 mg Xeloda was
administered twice a day for one period. The patient succumbed to
upper gastrointestinal hemorrhage and pneumonia after three
months.
Discussion
Gastric stump cancer occurs more frequently at the
site of anastomosis, and poorly-differentiated carcinoma is the
most common histological type (7).
Gastric cancer spreads via several routes, including hematogenous
spread, which is the most frequent mechanism by which distant
metastases arise. The liver, lung and pancreas are the most common
sites for gastric metastases, and direct local invasion of adjacent
organs, peritoneal or trans-coelomic spread and lymphatic
metastases can also occur (8).
Colonic metastases from gastric cancer are extremely rare. The
predominant route is known to be hematogenous, whereby metastatic
deposits invade the submucosal lymphatics and extend to form a
linitis plastica appearance or an annular stricture (5). The overlying mucosa may give the
impression of being normal and test negative for malignancy upon
mucosal biopsy, as observed in the study by Lim et al
(9). Polypoid colonic metastases
from gastric cancer have been reported in <10 cases. One such
case occurred 11 years after a total gastrectomy for a
poorly-differentiated adenocarcinoma of the stomach (4). A second case occurred at the colonic
anastomosis, with colonic polyp mucosal metastasis of a signet ring
cell gastric adenocarcinoma developing one year after a
sigmoidectomy with termino-terminal anastomosis for sigmoid
adenocarcinoma (2). Two cases
presented with colonic metastasis at the time of the diagnosis of
gastric cancer; however, yet another case was recorded by
postmortem investigation (6,10–12).
In the present study, the patient had undergone a partial
gastrectomy for a perforated gastric ulcer 48 years previously.
Polypoid colonic metastasis arising from gastric carcinoma has been
recorded with the following clinical pathological characteristics:
i) Poorly-differentiated cancer or differentiation of signet ring
cells as the common histological type; ii) colonoscopy or barium
enema revealing a solitary adenomatous colonic polyp (11–14) or
polymorphic polyps (4,6,10)
ranging in diameter from 2 to 15 mm, with a sessile or
semi-pedunculated nature; iii) nodules scattered throughout the
colon, with either erosion or a depression at the top of each; and
iv) weight loss, diarrhea, melena and anorexia as the common
symptoms. In addition, the primary tumor on the stomach is always a
large ulcer.
In total, >96% of signet ring cell carcinoma
cases originate in the stomach, with the remaining cases occurring
in the colon, rectum, gallbladder, pancreas, urinary bladder and
breast (15). The incidence of
signet ring cell cancer in the colorectum is 0.1–2.4%, and the
clinical characteristics include an advanced stage at diagnosis, a
large tumor size, a proximal location, a young patient age, a
propensity for lymphovascular invasion and peritoneal seeding
(16).
As colon signet ring cell adenocarcinomas are rare,
the differential diagnosis of a primary colon or metastatic gastric
cancer is debated when a signet ring cell carcinoma is diagnosed
via colonoscopy. Immunohistochemical analyses are performed to
differentiate between a gastric and colonic primary tumor, with CK7
and CK20 commonly used as tumor markers. CK7 expression has been
observed in the majority of carcinoma cases, with the exception of
those cases in which the cancers originated from the prostate,
colon, thymus and kidney, in carcinoid tumors originating from the
lungs and gastrointestinal tract and in Merkel cell tumors of the
skin. CK20-positive staining has been found in almost all
colorectal carcinoma and Merkel cell tumor cases, as well as a high
percentage of patients with pancreatic carcinoma (62%), gastric
carcinoma (50%), cholangiocarcinoma (43%) and transitional cell
carcinoma (29%). It has been hypothesized that when a signet ring
cell adenocarcinoma is revealed on colon biopsy, the diagnosis of a
colonic origin is supported by the presence of a
CK7−/CK20+ staining pattern in the neoplastic
cells, while a gastric origin is diagnosed when the cells have a
CK7+/CK20 staining pattern (15). However, Chu et al (18) reported that 13% (1/8) of cases of
gastric carcinomas and 5% (1/20) of colorectal carcinomas were
CK7+/CK20+. In addition, Wang et al
(19) reported that 38% (11/29) of
gastric adenocarcinomas and 10% (4/40) of colorectal
adenocarcinomas were CK7+/CK20+; thus,
CK7+/CK20+ staining pattern is more common in
gastric adenocarcinomas than in colorectal cancer. In the present
case, the biopsy specimens were positively stained for CK7 and
CK20. The colonic lesions were multifocal, therefore the actual
colonic lesions corresponded with the mucosal spread of the primary
gastric cancer. A previous study has hypothesized that tissues of
chronic inflammation may provide a spectrum of mitogen and trophic
signals that make this area more favorable for the establishment of
tumor metastasis (2). However, the
routes by which lymphatic or hematogenous metastases occur could
not be excluded in the present study. There were certain
limitations to the study, as an endoscopic ultrasound was not
performed for colonic lesions, therefore the source of the lesions
was not found.
In conclusion, gastric or gastric stump carcinoma
may metastasize to the colon and present as solitary or multiple
colonic polyps. This carcinoma is an extremely rare condition with
<10 cases described in the literature up until August 20, 2012
(www.ncbi.nlm.nih.gov/pubmed). Therefore,
it is important to consider gastric carcinoma as a possible
diagnosis, as colon metastases may mimic solitary or multiple
colonic polyps, which are more commonly observed. In such
complicated cases, a differential diagnosis is required.
Acknowledgements
The authors would like to thank the International
Center of Papertrans (http://www.papertrans.cn/) for editing the composition
and language of the original manuscript.
References
|
1
|
Batson OV: The function of the vertebral
veins and their role in the spread of metastases. Ann Surg.
112:138–149. 1940.
|
|
2
|
Rodríguez SN, González PC, Rivera T, et
al: Colonic anastomosis and colonic polyp mucosal metastasis of
signet ring cell gastric adenocarcinoma. Clin Transl Oncol.
12:238–239. 2010.
|
|
3
|
Kwon MS, Ko SO, Cho NP, et al: Gastric
signet-ring cell adenocarcinoma metastatic to the gingiva: a case
report. Oral Surg Oral Med Oral Pathol Oral Radiol Endod.
102:62–66. 2006.
|
|
4
|
Ogiwara H, Konno H, Kitayama Y, Kino I and
Baba S: Metastases from gastric adenocarcinoma presenting as
multiple colonic polyps: report of a case. Surg Today. 24:473–475.
1994.
|
|
5
|
Feczko PJ, Collins DD and Mezwa DG:
Metastatic disease involving the gastrointestinal tract. Radiol
Clin North Am. 31:1359–1373. 1993.
|
|
6
|
Metayer P, Antonietti M, Oumrani M, et al:
Metastases of a gastric adenocarcinoma presenting as colonic
polyposis. Report of a case. Dis Colon Rectum. 34:622–623.
1991.
|
|
7
|
Hu X, Tian DY and Cao L:
Clinicopathological features and outcome of patients with remnant
gastric cancer. Zhonghua Wei Chang Wai Ke Za Zhi. 12:581–583.
2009.(In Chinese).
|
|
8
|
Sauerborn D, Vidakovic B, Baranovic M, et
al: Gastric adenocarcinoma metastases to the alveolar mucosa of the
mandible: a case report and review of the literature. J
Craniomaxillofac Surg. 39:645–648. 2011.
|
|
9
|
Lim SW, Huh JW, Kim YJ and Kim HR:
Laparoscopic low anterior resection for hematogenous rectal
metastasis from gastric adenocarcinoma: a case report. World J Surg
Oncol. 9:1482011.
|
|
10
|
Tomikashi K, Mitsufuji S, Kanemasa H, et
al: Gastric cancer metastatic to the colon. Gastrointest Endosc.
55:5612002.
|
|
11
|
Tiszlavicz L: Metastasis of a stomach
carcinoma in a solitary adenomatous cecal polyp. Zentralbl Allg
Pathol. 136:277–282. 1990.
|
|
12
|
Tiszlavicz L: Stomach cancer metastasizing
into a solitary adenomatous colonic polyp. Orv Hetil.
131:1259–1261. 1990.
|
|
13
|
Niimi K, Matsuki K, Tomoda S, et al: 2
casesa of solitary metastasis to the large intestine from gastric
carcinoma. Gan No Rinsho. 30:1720–1725. 1984.
|
|
14
|
McKay J: A case of intestinal-type gastric
adenocarcinoma metastatic to a caecal tubulovillous polyp. N Z Med
J. 123:86–87. 2010.
|
|
15
|
Tung SY, Wu CS and Chen PC: Primary signet
ring cell carcinoma of colorectum: an age- and sex-matched
controlled study. Am J Gastroenterol. 91:2195–2199. 1996.
|
|
16
|
Chen JS, Hsieh PS, Chiang JM, et al:
Clinical outcome of signet ring cell carcinoma and mucinous
adenocarcinoma of the colon. Chang Gung Med J. 33:51–57. 2010.
|
|
17
|
Sim HL, Tan KY, Poon PL and Cheng A:
Primary rectal signet ring cell carcinoma with peritoneal
dissemination and gastric secondaries. World J Gastroenterol.
14:2118–2120. 2008.
|
|
18
|
Chu P, Wu E and Weiss LM: Cytokeratin 7
and cytokeratin 20 expression in epithelial neoplasms: a survey of
435 cases. Mod Pathol. 13:962–972. 2000.
|
|
19
|
Wang NP, Zee S, Zarbo RJ, Bacchi CE and
Gown AM: Coordinate expression of cytokeratins 7 and 20 defines
unique subsets of carcinomas. Appl Immunohistochem. 3:99–107.
1995.
|