Introduction
Hypoxia-inducible factor (HIF)-1 has been reported
as a transcription factor important for regulating cell energy,
angiogenesis, apoptosis, adhesion and growth (1–5). HIF-1
is a heterodimeric protein complex that is composed of α and β
subunits. The α subunit of HIF-1 is the determinant of HIF-1
transcriptional activity and acts as a regulatory subunit. The
regulation of HIF-1α is induced by a variety of factors, including
oxygen tension, cytokines and oncogenes (6–8). Upon
activation, HIF-1 interacts with the hypoxia response element of
HIF-1 target genes and promotes their transcription (9). HIF-1α has been found to be
overexpressed in several types of human cancer (10) and to enhance the adaptive response
of cancer to oxygen deprivation through upregulating the expression
of its target genes (11). Based on
the association between HIF-1α and malignant cancer phenotypes, the
inhibition of HIF-1α is becoming one of the most important
strategies for cancer treatment (12).
Prostate cancer (PCa) is one of the most common
types of malignant tumor worldwide. It has been reported that
HIF-1α may be a key factor for determining cancer malignancy and
prognosis (13). However, the role
of HIF-1α in PCa has yet to be elucidated. The present study aimed
to investigate the effect of HIF-1α on the biological
characteristics of PCa cells, including cell proliferation,
apoptosis and migration, through transfecting full length (fL) and
dominant-negative (dn) HIF-1α into the PCa PC3 cell line.
Materials and methods
Plasmids
pcDNA3.1 plasmids were purchased from Invitrogen
Life Technologies (Carlsbad, CA, USA). pcDNA3.1-fL HIF-1α (fL
HIF-1α) and pcDNA3.1-dn HIF-1 α (dn HIF-1α) plasmids were then
constructed.
Cell culture and transfection
The PCa PC3 cell line was purchased from the
American Type Culture Collection (Manassas, VA, USA) and cultured
in RPMI-1640 supplemented with 10% fetal bovine serum and 1%
L-glutamine. The cells were incubated at 37°C in 5% CO2
and 95% air. The cells were seeded onto six-well plates (Becton
Dickinson, Franklin Lakes, NJ, USA) and maintained overnight to
obtain ~80% confluence. Lipofectamine® 2000 (Invitrogen,
Rockville, MD, USA) was used for cell transfection according to the
manufacturer’s instructions. Following selection with 400 μg/ml
G418 (Invitrogen) for two weeks, separate positive clones of each
transfection were grown and termed PC3-pcDNA3.1-fL HIF-1α (fL
HIF-1α), PC3-pcDNA3.1-dn HIF-1α (dn HIF-1α) and PC3-pcDNA3.1
(pcDNA3.1). PC3-pcDNA3.1 and wild-type PC3 cells were used as
controls in subsequent experiments.
Western blot analysis
Cell lysates were obtained using
radioimmunoprecipitation assay buffer (Sigma-Aldrich, St. Louis,
MO, USA). A total of 10 μg protein was separated on 8% sodium
dodecyl sulfate-polyacrylamide gels (Beijing Solarbio Science and
Technology Co., Ltd., Beijing, China), transferred onto
polyvinylidene difluoride membranes (Roche, Basel, Switzerland) and
blocked using 5% blocking buffer (Wuhan Boster Biological
Technology, Ltd., Wuhan, China). The membranes were washed and
incubated with the following primary antibodies: Rabbit anti-human
HIF-1α polyclonal (dilution, 1:150; Santa Cruz Biotechnology, Inc.,
Santa Cruz, CA, USA), mouse anti-human VEGF monoclonal (dilution,
1:200; Santa Cruz Biotechnology, Inc.), rabbit anti-human EPO
polyclonal (dilution, 1:200; Santa Cruz Biotechnology, Inc.),
rabbit anti-human CXCR4 polyclonal (dilution, 1:150; Abnova,
Taipei, Taiwan) and rabbit anti-human β-actin polyclonal (dilution,
1:200; Santa Cruz Biotechnology, Inc.) for 1 h at room temperature
and overnight at 4°C. Subsequent to being washed with Tris-buffered
saline containing Tween 20, the membranes were incubated with
horseradish peroxidase-conjugated secondary antibodies (dilution,
1:3,000; Zsgb-Bio, Beijing, China). The signals from the bound
antibodies were identified using an enhanced chemiluminescence kit
(Amersham, Freiburg, Germany). The integrated density value (IDV)
of each band was assessed using a Gel-Pro Image Analyzer (Bio-Rad
Laboratories, Inc., Hercules, CA, USA), and the ratios of the
integrated density values (HIF-1α/β-actin, VEGF/β-actin,
EPO/β-actin and CXCR4/β-actin) were calculated to assess the
relative expression levels of the proteins.
Cell proliferation detected using MTT
assay
The PC3 cells were plated on 96-well plates and
maintained in normoxic conditions for five days. The viability of
the PC3 cells was assessed using an MTT assay. In brief, 20 μl MTT
solution (5 g/l; Sigma-Aldrich) was added to each well and
incubated for 4 h at 37°C. A total of 150 ml dimethyl sulfoxide
(Sigma-Aldrich) was then added to dissolve the crystal once the
growth medium was removed. The absorbance of 200 μl of solution
from each well was measured at 492 nm using a microplate reader
(Thermo Fisher Scientific, Waltham, MA, USA). Cell growth curves
were generated based on the corresponding optical density values at
492 nm.
Apoptosis assay
Exponential phase cells were split at a density of
5×104 cells per well on 96-well plates (Becton
Dickinson). Subsequent to 12 h of incubation, the RPMI-1640 medium
was replaced with RPMI-1640 containing 2 or 4 μmol/l docetaxol
(Aventis Pharma, Ltd., Mumbai, India) for 48 h. Cell apoptosis was
detected using an in situ terminal deoxynucleotidyl
transferase-mediated dUTP nick end labeling (TUNEL) kit (Roche)
according to the manufacturer’s instructions. A total of 10 fields
were chosen randomly at ×400 magnification to count the numbers of
apoptotic and total cells. The apoptotic index (AI) was calculated
as follows: AI = (number of apoptotic cells / total number counted)
× 100.
In vitro migration assay
The cells were harvested through trypsinization,
then counted and resuspended in RPMI-1640 at a concentration of
1×105/ml. Next, 0.5-ml aliquots of cell suspension were
added to the upper chamber of Millicell® Inserts
(Millipore Corporation, Billerica, MA, USA). The upper and lower
chambers were separated using a 12-mm pore polycarbonate membrane
that was coated with Matrigel™ (Becton Dickinson). Subsequent to 24
h of incubation at 37°C, the remaining cells on the upper side of
the chamber were removed using a cotton swab. The cells that had
migrated through the pores to the bottom side of the membrane were
fixed using 3.7% paraformaldehyde and stained with hematoxylin and
eosin. The number of migrated cells was counted in 10 randomly
selected fields using a microscope.
Statistical analysis
Student’s t-test was used to compare two groups.
Analysis of variance with Fisher’s post-hoc test was used for
comparing more than two groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
dn HIF-1α inactivates HIF-1 and
attenuates the expression of HIF-1 downstream genes
The effect of dn HIF-1α and fL HIF-1α on VEGF, EPO
and CXCR4 expression was analyzed using western blot analysis.
VEGF, EPO and CXCR4 were observed to be upregulated by fL HIF-1α,
while dn HIF-1α was found to downregulate the HIF-1 target genes,
with no impact on HIF-1α expression (Fig. 1).
 | Figure 1Expression of HIF-1α and its
downstream genes, VEGF, EPO and CXCR4. (A) Western blot analysis
was used to detect the effect of dn HIF-1α and fL HIF-1α on VEGF,
EPO and CXCR4 expression. β-actin was used as a loading control.
VEGF, EPO and CXCR4 were upregulated by fL HIF-1α, while dn HIF-1α
significantly downregulated the HIF-1 target genes. (B) The
relative protein expression of HIF-1α, VEGF, EPO and CXCR4 was
calculated as integrated density values. Data are presented as the
mean ± standard error of the mean from three independent
experiments. *P<0.05 vs. pcDNA3.1 or wild type. HIF,
hypoxia-inducible factor; VEGF, vascular endothelial growth factor;
EPO, erythropoietin; CXCR4, CXC chemokine receptor 4; fL, full
length; dn, dominant-negative; pc, pcDNA3.1; wt, wild-type. |
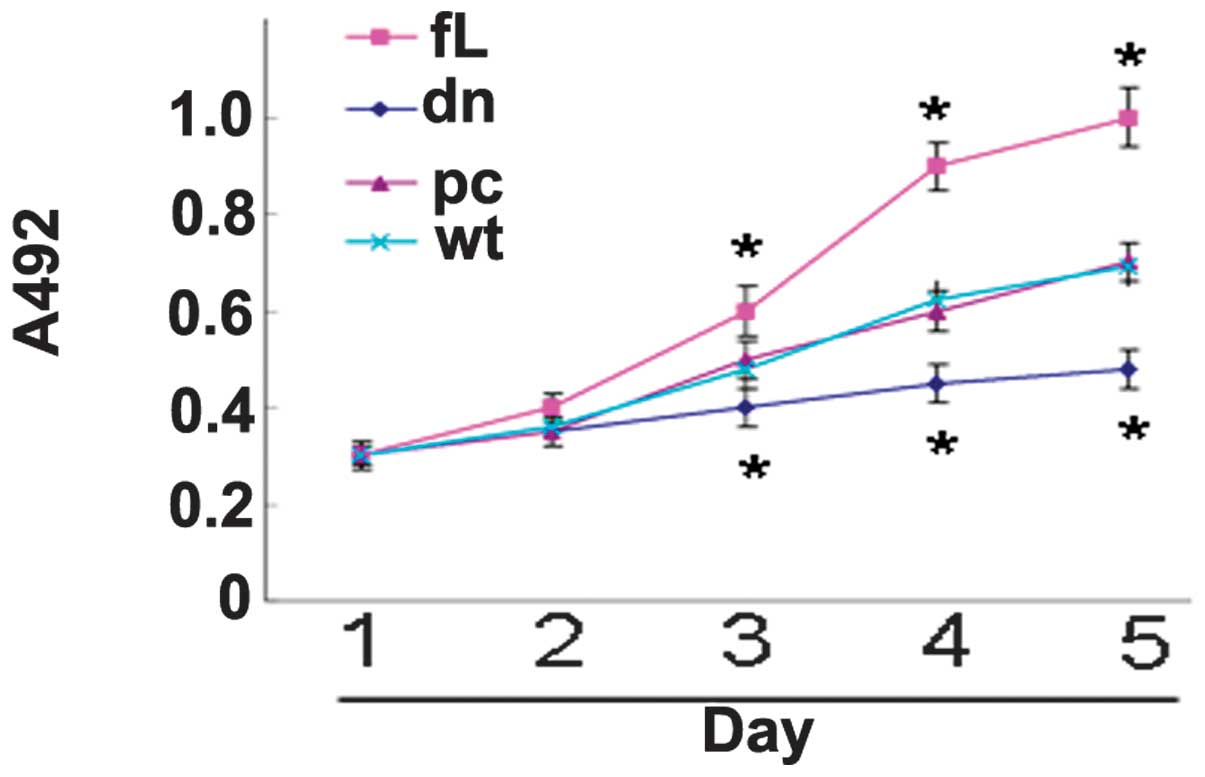
dn HIF-1α inhibits PC3 proliferation
Compared with the control cells, the proliferation
of the fL HIF-1α transfectants was observed to be significantly
enhanced, while the proliferation of the dn HIF-1α transfectants
was found to be significantly suppressed (Fig. 2).
HIF-1α attenuates docetaxol-induced PC3
cell apoptosis
A TUNEL assay was used to detect apoptosis in the
PC3 cells subjected to docetaxol treatment. Following treatment
with docetaxol for 48 and 72 h, dn HIF-1α was found to promote PC3
cell apoptosis, while fL HIF-1α was observed to have an
anti-apoptotic effect (Fig. 3).
HIF-1α enhances PC3 cell migration
A Boyden chamber assay was used to detect cell
migration, and revealed that the PC3 cells transfected with fL
HIF-1α migrated more rapidly compared with those transfected with
dn HIF-1α and the control cells (Fig.
4).
Discussion
dn HIF-1α is a truncated variant of HIF-1α that
functions in a dn manner. Through competing with normal fL HIF-1α
for the binding site of HIF-1β, dn HIF-1α inhibits the formation of
functional HIF-1 (14). In the
present study, a significant increase in HIF-1α expression was
observed in the pcDNA3.1-fL HIF-1α PC3 cells, but not in the
pcDNA3.1-dn HIF-1α PC3 cells. Furthermore, the expression of the
HIF-1α target genes, VEGF, EPO and CXCR4, was found to be increased
in the pcDNA3.1-fL HIF-1α-transfected PC3 cells, but decreased in
the pcDNA3.1-dn HIF-1α-transfected PC3 cells.
VEGF has a vital role in the growth of blood
vessels. Although the majority of studies have been focused on the
function of VEGF and VEGF receptors in angiogenesis and in
endothelial cells, the role of VEGF in carcinogenesis may be
another function that requires highlighting. VEGF has been reported
to have a role in the regulation of a number of functions in tumor
cells, including survival, adhesion, migration and invasion
(15). Similarly, EPO has also been
reported to have other effects in addition to its role in
erythropoiesis, including the promotion of tumor cell growth and
survival (16). The CXCR4 signaling
pathway is crucial in modulating cancer cell migration. The
activation of CXCR4 leads to cellular skeleton remolding and
pseudopodia formation, and consequently enhanced cell mobility and
migration (17). Therefore, the
HIF-1α-induced promotion of cell growth and migration and the
reversal of docetaxol-induced cell apoptosis, may be associated
with the overexpression of the HIF-1α downstream effectors VEGF,
EPO and CXCR4.
Docetaxol is one of the most common drugs for the
chemotherapeutic treatment of PCa (18). In the present study, following
treatment with docetaxol, dn HIF-1α was found to promote PC3
apoptosis, while fL HIF-1α exhibited an anti-apoptotic effect.
These findings indicate that dn HIF-1α may make PC3 cells more
susceptible to docetaxol-induced apoptosis and that fL HIF-1α may
rescue this susceptibility.
HIF-1α has a central role in regulating tumor
behavior, including proliferation, apoptosis and migration, thus
the inhibition of the HIF-1α pathway may be a promising strategy
for attenuating tumor progression. Several methods to inhibit
HIF-1α have been investigated, including using HIF-1α small
interfering RNA to inhibit HIF-1α transcription, agents to
accelerate the proteasome-dependent degradation of the HIF-1α
protein and small molecular inhibitors to block the transcriptional
activity of HIF-1α (19,20). The dn HIF-1α-induced inhibition of
HIF-1α has been reported in a number of types of cancer, including
malignant gliomas, gastric cancer and breast carcinoma, however,
the underlying mechanism has yet to be elucidated (21–23).
The present study showed that HIF-1α regulates certain biological
characteristics of PC3 cells and that dn HIF-1α may be a promising
biotherapy for PCa, providing novel insight into our understanding
of the mechanism underlying the carcinogenesis of PCa and a
potential therapy to treat PCa.
Acknowledgements
The present study was supported by the Major State
Basic Research Development Program (grant no. 2013CB967404), the
National Natural Science Foundation of China (grant nos. 81172174
and 81270724) and the Program for Outstanding Young Scholars of
Sichuan University (grant no. 2012SCU04B03).
References
|
1
|
Fukuda R, Zhang H, Kim JW, et al: HIF-1
regulates cytochrome oxidase subunits to optimize efficiency of
respiration in hypoxic cells. Cell. 129:111–122. 2007.
|
|
2
|
Hirota K and Semenza GL: Regulation of
angiogenesis by hypoxia-inducible factor 1. Crit Rev Oncol Hematol.
59:15–26. 2006.
|
|
3
|
Kilic M, Kasperczyk H, Fulda S and Debatin
KM: Role of hypoxia inducible factor-1 alpha in modulation of
apoptosis resistance. Oncogene. 26:2027–2038. 2007.
|
|
4
|
Esteban MA, Tran MG, Harten SK, et al:
Regulation of E-cadherin expression by VHL and hypoxia-inducible
factor. Cancer Res. 66:3567–3575. 2006.
|
|
5
|
Li J, Shi M, Cao Y, et al: Knockdown of
hypoxia-inducible factor-1alpha in breast carcinoma MCF-7 cells
results in reduced tumor growth and increased sensitivity to
methotrexate. Biochem Biophys Res Commun. 342:1341–1351. 2006.
|
|
6
|
Huang LE, Gu J, Schau M and Bunn HF:
Regulation of hypoxia-inducible factor 1alpha is mediated by an
O2-dependent degradation domain via the ubiquitin-proteasome
pathway. Proc Natl Acad Sci USA. 95:7987–7992. 1998.
|
|
7
|
Stiehl DP, Jelkmann W, Wenger RH and
Hellwig-Bürgel T: Normoxic induction of the hypoxia-inducible
factor 1alpha by insulin and interleukin-1beta involves the
phosphatidylinositol 3-kinase pathway. FEBS Lett. 512:157–162.
2002.
|
|
8
|
Semenza G: Signal transduction to
hypoxia-inducible factor 1. Biochem Pharmacol. 64:993–998.
2002.
|
|
9
|
Bárdos JI and Ashcrof M: Negative and
positive regulation of HIF-1: a complex network. Biochim Biophys
Acta. 1755:107–120. 2005.
|
|
10
|
Zhong H, De Marzo AM, Laughner E, et al:
Overexpression of hypoxia-inducible factor 1alpha in common human
cancers and their metastases. Cancer Res. 59:5830–5835. 1999.
|
|
11
|
Maxwell PH, Pugh CW and Ratcliffe PJ:
Activation of the HIF pathway in cancer. Curr Opin Genet Dev.
11:293–299. 2001.
|
|
12
|
Semenza GL: Targeting HIF-1 for cancer
therapy. Nat Rev Cancer. 3:721–732. 2003.
|
|
13
|
Semenza GL: Hypoxia-inducible factors:
mediators of cancer progression and targets for cancer therapy.
Trends Pharmacol Sci. 33:207–214. 2012.
|
|
14
|
Chen J, Zhao S, Nakada K, et al:
Dominant-negative hypoxia-inducible factor-1 alpha reduces
tumorigenicity of pancreatic cancer cells through the suppression
of glucose metabolism. Am J Pathol. 162:1283–1291. 2003.
|
|
15
|
Perrot-Applanat M and Di Benedetto M:
Autocrine functions of VEGF in breast tumor cells: adhesion,
survival, migration and invasion. Cell Adh Migr. 6:547–553.
2012.
|
|
16
|
Elliott S and Sinclair AM: The effect of
erythropoietin on normal and neoplastic cells. Biologics.
6:163–189. 2012.
|
|
17
|
Zheng H, Fu G, Dai T and Huang H:
Migration of endothelial progenitor cells mediated by stromal
cell-derived factor-1alpha/CXCR4 via PI3K/Akt/eNOS signal
transduction pathway. J Cardiovasc Pharmacol. 50:274–280. 2007.
|
|
18
|
Merseburger AS, Bellmunt J, Jenkins C, et
al: Perspectives on treatment of metastatic castration-resistant
prostate cancer. Oncologist. 18:558–567. 2013.
|
|
19
|
Zhang P, Wang Y, Hui Y, et al: Inhibition
of VEGF expression by targeting HIF-1alpha with small interference
RNA in human RPE cells. Ophthalmologica. 221:411–417. 2007.
|
|
20
|
Melillo G: Inhibiting hypoxia-inducible
factor 1 for cancer therapy. Mol Cancer Res. 4:601–605. 2006.
|
|
21
|
Jensen RL, Ragel BT, Whang K and Gillespie
D: Inhibition of hypoxia inducible factor-1alpha (HIF-1alpha)
decreases vascular endothelial growth factor (VEGF) secretion and
tumor growth in malignant gliomas. J Neurooncol. 78:233–247.
2006.
|
|
22
|
Stoeltzing O, McCarty MF, Wey JS, et al:
Role of hypoxia-inducible factor 1alpha in gastric cancer cell
growth, angiogenesis, and vessel maturation. J Natl Cancer Inst.
96:946–956. 2004.
|
|
23
|
Brown LM, Cowen RL, Debray C, et al:
Reversing hypoxic cell chemoresistance in vitro using genetic and
small molecule approaches targeting hypoxia inducible factor-1. Mol
Pharmacol. 69:411–418. 2006.
|