Introduction
Undifferentiated embryonal liver sarcoma (UELS) is a
rare and highly malignant hepatic tumor of mesenchymal origin,
which often occurs in individuals between 5 and 10 years old, as
well as in young adults (1–3). Patients with UELS usually present with
a painful right upper quadrant mass, fever and other symptoms,
including weight loss, vomiting, nausea, anorexia and jaundice
(4–6). Ultrasonography (US), computed
tomography (CT), magnetic resonance imaging (MRI) and biopsy are
used as diagnostic tools to identify other liver tumors. However,
the typical radiological findings of liver tumors are also observed
in other liver diseases, including cystic hydatid disease (5). Previous studies have shown that
complete tumor resection followed by adjuvant chemotherapy and/or
radiation improves survival and reduces recurrence in patients with
UELS, and this has now become a current standard therapy for UELS
(7–9). The present study presents a childhood
case of recurrent UELS that was treated with surgical resection
without chemotherapy, and discusses the clinical characteristics,
laboratory test results, immunohistochemical findings and treatment
for this rare disease. Written informed consent was obtained from
the parents of the patient.
Case report
Clinical characteristics, laboratory
findings and imaging
A 9-year-old, previously healthy female was admitted
to The First Affiliated Hospital of Wenzhou Medical University
(Wenzhou, China), and presented with abdominal pain and fever. Upon
examination, the patient’s temperature was 38°C, the pulse was 86
beats per minute, the blood pressure was 103/53 mmHg and the
respiratory rate was 20 breaths per minute. Abdominal tenderness
was identified in the right upper quadrant without rebound
tenderness. The remainder of the examination was normal and the
results are shown in Table I.
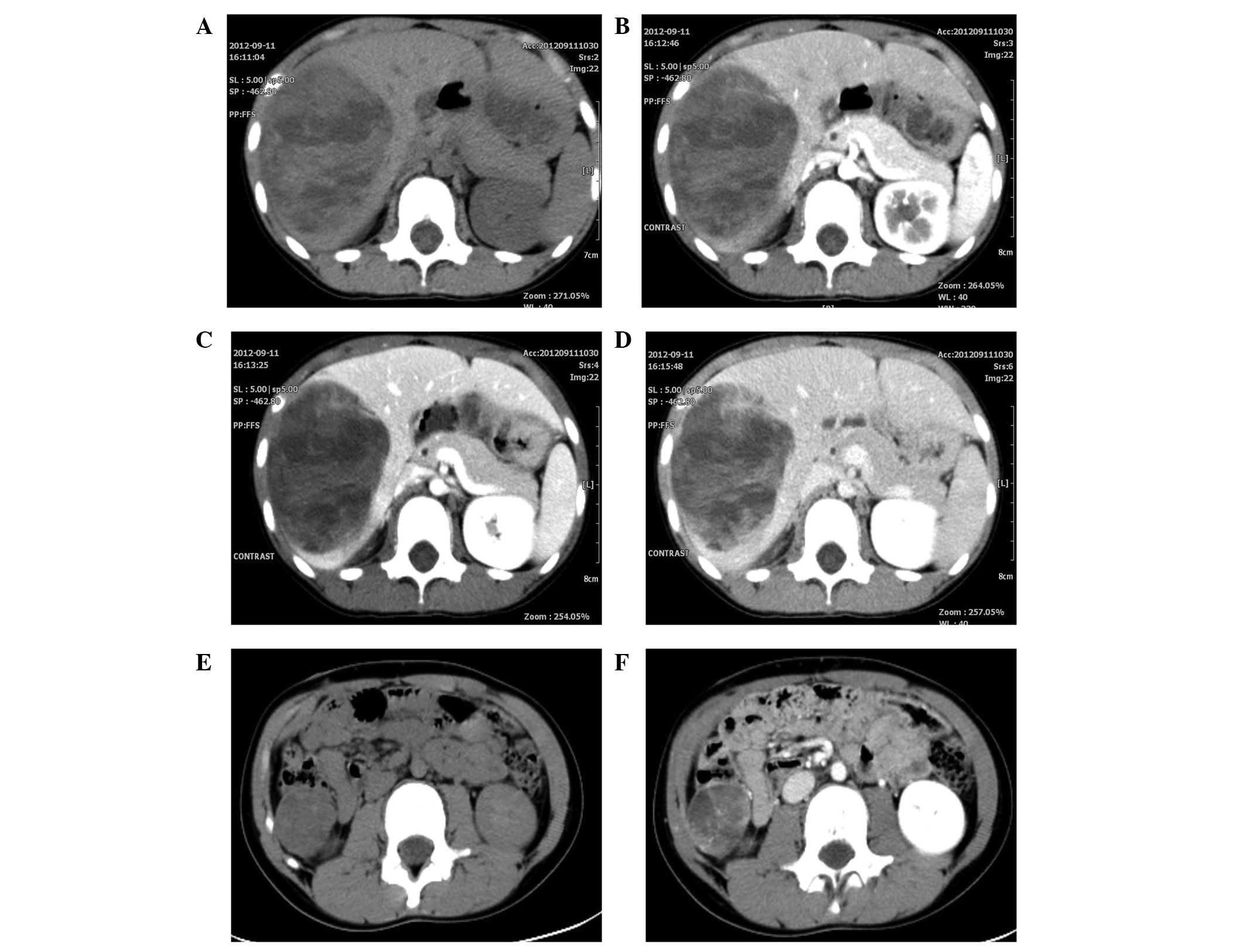
Abdominal CT scan revealed a cystic and solid mass with a size of
11.5×8.3×6.7 cm3 and a well-defined border, which
involved the right lobe of the liver and had a non-homogeneous
density (Fig. 1A). The density was
marginally and heterogeneously increased in the enhanced CT scan
(Fig. 1B–D). The clinical
impression was that this mass represented a hepatoblastoma.
 | Table ILaboratory data from a pediatric
patient with undifferentiated embryonal liver sarcoma. |
Table I
Laboratory data from a pediatric
patient with undifferentiated embryonal liver sarcoma.
| Parameter | First admission | Second admission |
|---|
| Blood |
| White cell count
(per mm3) | 14,200 | 7,880 |
| Differential count
(%) |
| Neutrophils | 75.9 | 54.8 |
| Eosinophils | 0.2 | 0.9 |
| Band forms | 0.2 | 0.4 |
| Monocytes | 9.4 | 7.9 |
| Lymphocytes | 14.2 | 36 |
| Erythrocyte count
(per mm3) | 3,860,000 | 4,770,000 |
| Hemoglobin
(g/dl) | 11.5 | 14.3 |
| Hematocrit (%) | 33.8 | 41.4 |
| Platelet count (per
mm3) | 323,000 | 192,000 |
| Prothrombin time
(sec) | 14.3 | 13.8 |
| Activated partial
thromboplastin time (sec) | 41.8 | 53.9 |
| Serum AFP level
(μg/l) | 1.3 | 1.93 |
| Serum CA-125 level
(μU/ml) | 30.7 | - |
| Serum CA-19-9 level
(U/ml) | - | 5.0 |
| Serum CEA level
(μg/l) | 0.8 | 1.2 |
| Total bilirubin
(μmol/l) | 7 | 7 |
| Direct bilirubin
(μmol/l) | 3 | 4 |
| Indirect bilirubin
(μmol/l) | 4 | 3 |
| Total protein
(g/l) | 59.6 | 67.1 |
| Albumin (g/l) | 32.1 | 41.0 |
| Alanine
aminotransferase (U/l) | 38 | 10 |
| Aspartate
aminotransferase (U/l) | 45 | 24 |
| Alkaline phosphatase
(U/l) | 208 | 296 |
| γ-glutamyl
transferase (U/l) | 50 | 10 |
| Lactate dehydrogenase
(U/l) | 377 | 291 |
| Creatine kinase
(U/l) | 20 | 144 |
| Glucose (mmol/l) | 5.2 | 5.0 |
| Sodium (mmol/l) | 138 | 137 |
| Potassium
(mmol/l) | 4.25 | 3.97 |
| Chloride
(mmol/l) | 101 | 104 |
| Urea nitrogen
(mmol/l) | 7.0 | 3.4 |
| Creatinine
(μmol/l) | 40 | 40 |
| Uric acid
(μmol/l) | 177 | 307 |
Treatment
Surgery was performed to resect the tumor. A right
subcostal incision was made and, following entry into the abdomen,
the ligamentum teres hepatis and falciform were incised.
Examination of the future liver remnant and abdominal cavity
revealed no evidence of tumor metastasis. The tumor had ruptured at
the diaphragmatic surface and had adhered to the diaphragm. The
surgical margin distance from the tumor was at ≥1 cm. The tumor was
resected following the margins, until it was completely removed. A
drainage tube was inserted under the right diaphragm and exited the
body through the right abdominal wall. The abdominal wall was
closed using standard procedures. During surgery, the patient had
300 ml bleeding and was transfused with 1.5 units of red blood
cells.
Pathologic findings
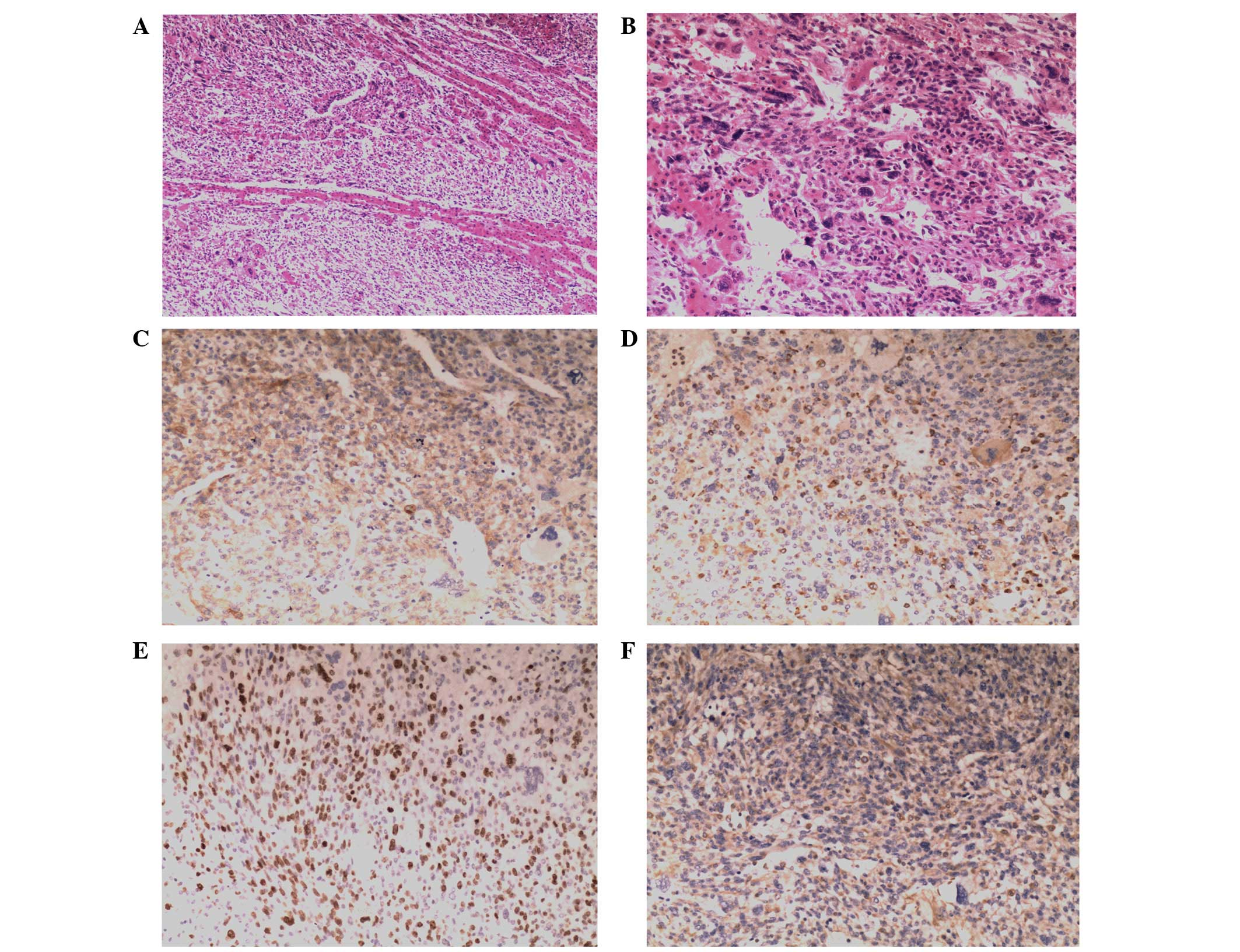
Histological examination revealed pleomorphically
shaped tumor cells with marked nuclei and clear to the eosinophilic
cytoplasm (Fig. 2A–B). All the
primary antibodies used in the present study are shown in Table II. Immunohistochemical staining of
the tumor cells showed positive expression of caldesmon (Fig. 2C), cluster of differentiation (CD)
68 (Fig. 2D), Ki67 (Fig. 2E) and vimentin (Fig. 2F), but negative expression of
α-fetoprotein (AFP), creatine kinase (CK), desmin, hepatocyte,
muscle specific actin (MSA), myogenic differentiation (myod) 1,
myoglobin and smooth muscle actin (SMA). On the basis of these
findings, the patient was diagnosed with UESL, which was confirmed
by two surgical pathologists.
 | Table IIPrimary antibodies used in the present
study. |
Table II
Primary antibodies used in the present
study.
| Antibody | Clone | Source | Dilution |
|---|
| AFP (M) | ZSA06 | Zhongshan Gold Bridge
Biological Technology Co. (Beijing, China) | 1:100 |
| Caldesmon (M) | EP19 | Zhongshan Gold Bridge
Biological Technology Co. (Beijing, China) | 1:100 |
| CD68 (M) | KP1 | Zhongshan Gold Bridge
Biological Technology Co. (Beijing, China) | 1:100 |
| CK (M) | AE1 | Zhongshan Gold Bridge
Biological Technology Co. (Beijing, China) | 1:100 |
| Desmin (M) | ZC18 | Zhongshan Gold Bridge
Biological Technology Co. (Beijing, China) | 1:100 |
| Hepatocyte (M) | OCH1E5 | Zhongshan Gold Bridge
Biological Technology Co. (Beijing, China) | 1:100 |
| Ki67 (M) | K-2 | Zhongshan Gold Bridge
Biological Technology Co. (Beijing, China) | 1:100 |
| MSA (M) | HHF35 | Zhongshan Gold Bridge
Biological Technology Co. (Beijing, China) | 1:100 |
| Myodl (M) | 5.2F | Zhongshan Gold Bridge
Biological Technology Co. (Beijing, China) | 1:100 |
| Myoglobin (M) | Z001 | Zhongshan Gold Bridge
Biological Technology Co. (Beijing, China) | 1:100 |
| SMA (M) | IA4 | Zhongshan Gold Bridge
Biological Technology Co. (Beijing, China) | 1:100 |
| VIM (M) | V9 | Zhongshan Gold Bridge
Biological Technology Co. (Beijing, China) | 1:100 |
Clinical course
The patient was recommended for further treatment,
which was refused due to socioeconomic and psychological reasons.
Thus, adjuvant chemotherapy was not initiated. Following complete
tumor resection, the patient was regularly examined. Abdominal CT
scans and US were performed every 3 months to monitor tumor
recurrence. At the 6-month follow-up, the patient was surviving
free of disease; however, at the 9-month follow-up, unenhanced CT
revealed a right, low-density prerenal recurrent mass with a size
of ~2.7×2.4 cm2 and a well-defined border (Fig. 1E). Enhanced CT showed a marginally
and heterogeneously increased density (Fig. 1F). The patient did not accept any
further treatment. Two months later, CT scan demonstrated that the
mass had enlarged to ~3.3×3.4 cm2. A right prerenal
dissemination of UESL was suspected. Based on the patient’s
condition, a second surgical procedure was required.
Upon second admission, the patient was comfortable
and physical examination revealed there was no abnormal condition.
The laboratory results are shown in Table I. Complete prerenal tumor resection
was performed. There was tumor hemorrhage inside. Microscopic
analysis showed that the tumor was evidence of undifferentiated
embryonal sarcoma recurrence. However, the patient was comfortable
and physical examination revealed no abnormal conditions. In
addition, the laboratory results were normal. Abdominal computed
tomography scan and ultrasound were performed every 3 months to
monitor the tumor recurrence. At the time of writing, it has been 6
months after the second surgical procedure and there has been no
appearence of abnormalities.
Discussion
UELS is a rare, aggressive neoplasm with a poor
prognosis, which predominantly affects children without gender
predilection (1). UELS normally
occurs in childhood, but has also been reported in adults (7). UELS typically presents with various
non-specific tumor-associated symptoms, including right upper
abdominal pain, distention, mass, nausea, swelling, vomiting,
fever, weight loss, fatigue and jaundice (3,10,11).
Occasionally, patients also have shoulder pain (10,12).
Furthermore, physical examination occasionally reveals right upper
abdominal tenderness. Laboratory studies are non-specific and have
identified that patients with UELS exhibit low albumin, elevated
lactic dehydrogenase, anemia and abnormal liver function (4,13,14).
Moreover, serum assays for tumor markers, including AFP, cancer
antigen 199 and carcinoembryonic antigen yield normal results.
The typical radiological finding of UELS is a large
mass in the right liver lobe and occasionally in the left liver
lobe. Abdominal CT images show large (range, 10–30 cm in size),
cystic, solitary and well-circumscribed masses with variable areas
of necrosis and hemorrhage (15,16).
On enhanced CT, during the phase of the filling of the hepatic
portal vein, a tumor was observed (15). Abdominal US reveals large
multilocular (17) or unilocular
(18) cystic and solid liver
masses. The cystic region exhibits a large, mixed and disorderly
low level echo (3,19), and the solid areas of the mass
demonstrate a mixture of high and low level echos (19). Fluorine-18 fluorodeoxyglucose
positron emission tomography (F-18 FDG PET)/CT may become
increasingly important for the examination and treatment of UELS.
F-18 FDG PET/CT may be a feasible and valuable imaging modality for
further analysis of distant metastasis, as UELS and its metastases
have strong F-18 FDG uptake (20).
Microscopically, UELS is characterized by cellular
proliferation of oval, spindle or stellate pleomorphic cells with
poorly defined cell borders (2,3).
Immunocytochemically, the indices of the present patient were as
follows: Caldesmon(+), CD68(+), Ki67(+), vimentin(+), AFP(−),
CK(−), desmin(−), hepatocyte(−), MSA(−), myod1(−), myoglobin(−) and
SMA(−). The outcome was approximately the same as that described
previously (2). As in the present
case, certain cases of UELS are positive for CD68 (21,22)
and vimentin (23), which implies a
mesenchymal origin. Moreover, in the present case, positive
expression was found for caldesmon (24–26),
which suggests sarcous origin. The positive expression of the
proliferation marker Ki67 suggests it is an aggressive tumor
(27,28).
The prognosis of UELS is poor even if the tumor is
completely resected. Previous reports have demonstrated that
treatment with surgery and adjuvant chemotherapy is curative for
UELS in certain cases (1,9,29). May
et al (4) reported five
pediatric patients with UELS who were treated with radical
resection followed by adjuvant chemotherapy or radiation and were
alive without evidence of recurrence at a median of 53 months
(4). Kim et al (9) reported that five pediatric patients
who received similar therapy had a comparable prognosis, and
proposed that a combined therapy of surgery and chemotherapy
improves prognosis (9). Liver
transplantation may be a potential treatment option for pediatric
patients with UELS if it is feasible. Liver transplantation has
been reported in pediatric patients with UELS (12,30,31).
One study reported the case of a 6-year-old male who received a
liver transplant combined with chemotherapy and was alive 6.5 years
following surgery (30).
Furthermore, another study reported a male who has been in good
health and disease-free for 5 years following treatment with
chemotherapy, surgical resection and liver transplantation
(12). However, reports of liver
transplantation to treat UELS are limited.
In conclusion, complete tumor resection combined
with adjuvant chemotherapy may reduce the risk of recurrence and
improve the survival time in patients with UELS. Further
investigations are required to identify more effective strategies
for treating pediatric patients with UELS.
References
|
1
|
Bisogno G, Pilz T, Perilongo G, et al:
Undifferentiated sarcoma of the liver in childhood: a curable
disease. Cancer. 94:252–257. 2002.
|
|
2
|
Wei ZG, Tang LF, Chen ZM, Tang HF and Li
MJ: Childhood undifferentiated embryonal liver sarcoma: clinical
features and immunohistochemistry analysis. J Pediatr Surg.
43:1912–1919. 2008.
|
|
3
|
Li XW, Gong SJ, Song WH, et al:
Undifferentiated liver embryonal sarcoma in adults: a report of
four cases and literature review. World J Gastroenterol.
16:4725–4732. 2010.
|
|
4
|
May LT, Wang M, Albano E, Garrington T,
Dishop M and Macy ME: Undifferentiated sarcoma of the liver: a
single institution experience using a uniform treatment approach. J
Pediatr Hematol Oncol. 34:e114–e116. 2012.
|
|
5
|
Oral A, Yigiter M, Demirci E, Yildirim ZK,
Kantarci M and Salman AB: A case of undifferentiated embryonic
liver sarcoma mimicking cystic hydatid disease in an endemic region
of the world. J Pediatr Surg. 46:e5–e9. 2011.
|
|
6
|
Stocker JT and Ishak KG: Undifferentiated
(embryonal) sarcoma of the liver: report of 31 cases. Cancer.
42:336–348. 1978.
|
|
7
|
Lenze F, Birkfellner T, Lenz P, et al:
Undifferentiated embryonal sarcoma of the liver in adults. Cancer.
112:2274–2282. 2008.
|
|
8
|
O’Sullivan MJ, Swanson PE, Knoll J,
Taboada EM and Dehner LP: Undifferentiated embryonal sarcoma with
unusual features arising within mesenchymal hamartoma of the liver:
report of a case and review of the literature. Pediatr Dev Pathol.
4:482–489. 2001.
|
|
9
|
Kim DY, Kim KH, Jung SE, Lee SC, Park KW
and Kim WK: Undifferentiated (embryonal) sarcoma of the liver:
combination treatment by surgery and chemotherapy. J Pediatr Surg.
37:1419–1423. 2002.
|
|
10
|
Shehata BM, Gupta NA, Katzenstein HM, et
al: Undifferentiated embryonal sarcoma of the liver is associated
with mesenchymal hamartoma and multiple chromosomal abnormalities:
a review of eleven cases. Pediatr Dev Pathol. 14:111–116. 2011.
|
|
11
|
Zaman S, Hanif G, Hussain M, et al:
Hepatic tumours in childhood: an experience at the Children
Hospital and Institute of Child Health, Lahore. J Pak Med Assoc.
61:1079–1082. 2011.
|
|
12
|
Kelly MJ, Martin L, Alonso M and Altura
RA: Liver transplant for relapsed undifferentiated embryonal
sarcoma in a young child. J Pediatr Surg. 44:e1–e3. 2009.
|
|
13
|
Uchiyama M, Iwafuchi M, Yagi M, et al:
Treatment of ruptured undifferentiated sarcoma of the liver in
children: a report of two cases and review of the literature. J
Hepatobiliary Pancreat Surg. 8:87–91. 2001.
|
|
14
|
Shattaf A, Jamil A, Khanani MF, et al:
Undifferentiated sarcoma of the liver: a rare pediatric tumor. Ann
Saudi Med. 32:203–205. 2012.
|
|
15
|
Yu RS, Chen Y, Jiang B, Wang LH and Xu XF:
Primary hepatic sarcomas: CT findings. Eur Radiol. 18:2196–2205.
2008.
|
|
16
|
Sakellaridis T, Panagiotou I, Georgantas
T, Micros G, Rontogianni D and Antiochos C: Undifferentiated
embryonal sarcoma of the liver mimicking acute appendicitis. Case
report and review of the literature. World J Surg Oncol.
4:92006.
|
|
17
|
Joshi SW, Merchant NH and Jambhekar NA:
Primary multilocular cystic undifferentiated (embryonal) sarcoma of
the liver in childhood resembling hydatid cyst of the liver. Br J
Radiol. 70:314–316. 1997.
|
|
18
|
Chowdhary SK, Trehan A, Das A, Marwaha RK
and Rao KL: Undifferentiated embryonal sarcoma in children: beware
of the solitary liver cyst. J Pediatr Surg. 39:E9–E12. 2004.
|
|
19
|
Gao J, Fei L, Li S, et al:
Undifferentiated embryonal sarcoma of the liver in a child: A case
report and review of the literature. Oncol Lett. 5:739–742.
2013.
|
|
20
|
Lee MK, Kwon CG, Hwang KH, et al: F-18 FDG
PET/CT findings in a case of undifferentiated embryonal sarcoma of
the liver with lung and adrenal gland metastasis in a child. Clin
Nucl Med. 34:107–108. 2009.
|
|
21
|
Nishio J, Iwasaki H, Sakashita N, et al:
Undifferentiated (embryonal) sarcoma of the liver in middle-aged
adults: smooth muscle differentiation determined by
immunohistochemistry and electron microscopy. Hum Pathol.
34:246–252. 2003.
|
|
22
|
Scudiere JR and Jakate S: A 51-year-old
woman with a liver mass. Undifferentiated embryonal sarcoma of the
liver. Arch Pathol Lab Med. 130:e24–e26. 2006.
|
|
23
|
Zheng JM, Tao X, Xu AM, Chen XF, Wu MC and
Zhang SH: Primary and recurrent embryonal sarcoma of the liver:
clinicopathological and immunohistochemical analysis.
Histopathology. 51:195–203. 2007.
|
|
24
|
Watanabe K, Tajino T, Sekiguchi M and
Suzuki T: h-Caldesmon as a specific marker for smooth muscle
tumors. Comparison with other smooth muscle markers in bone tumors.
Am J Clin Pathol. 113:663–668. 2000.
|
|
25
|
Nakayama H, Kamiji I, Naruse K, et al:
Well differentiated adult-type fibrosarcoma arising from the
occipital subcutaneous tissue in a 17-year-old man: case report
with immunohistochemical study. Jpn J Clin Oncol. 28:511–516.
1998.
|
|
26
|
Nucci MR, O’Connell JT, Huettner PC, Cviko
A, Sun D and Quade BJ: h-Caldesmon expression effectively
distinguishes endometrial stromal tumors from uterine smooth muscle
tumors. Am J Surg Pathol. 25:455–463. 2001.
|
|
27
|
Settakorn J, Kaewpila N, Burns GF and
Leong AS: FAT, E-cadherin, beta catenin, HER 2/neu, Ki67
immuno-expression, and histological grade in intrahepatic
cholangiocarcinoma. J Clin Pathol. 58:1249–1254. 2005.
|
|
28
|
Stroescu C, Dragnea A, Ivanov B, et al:
Expression of p53, Bcl-2, VEGF, Ki67 and PCNA and prognostic
significance in hepatocellular carcinoma. J Gastrointestin Liver
Dis. 17:411–417. 2008.
|
|
29
|
Weitz J, Klimstra DS, Cymes K, et al:
Management of primary liver sarcomas. Cancer. 109:1391–1396.
2007.
|
|
30
|
Dower NA and Smith LJ: Liver
transplantation for malignant liver tumors in children. Med Pediatr
Oncol. 34:136–140. 2000.
|
|
31
|
Okajima H, Ohya Y, Lee KJ, et al:
Management of undifferentiated sarcoma of the liver including
living donor liver transplantation as a backup procedure. J Pediatr
Surg. 44:e33–e38. 2009.
|