Introduction
Glioblastomas are a particularly devastating form of
primary brain tumor. Due to the highly infiltrative and invasive
nature of such tumors, it remains clinically intractable. Patient
survival time is generally only 12–18 months even following
surgical resection with subsequent radiation and chemotherapy
(1–3). Recent studies have indicated that the
development of a tumor is often associated with the regulation of a
variety of transcription factors (1,4,5).
Activating transcription factor 5 (ATF5) is a novel factor that is
closely associated with tumor cell differentiation, proliferation
and apoptosis. ATF5 is a member of the ATF/cAMP responsive
element-binding (CREB) family of transcription factors, which
includes a large group of basic leucine zipper proteins that
mediate diverse transcriptional regulatory functions (6–9). ATF5,
an anti-apoptotic factor, is highly expressed in malignant glioma
and is important in the promotion of cell survival (5). ATF5 loss of function induces apoptosis
in a number of glioma and breast cancer cell lines (10,11),
however, interfering with ATF5 function in non-tumor brain cells
has not been found to affect their survival (10). Dluzen et al (12) demonstrated that B-cell
lymphoma/leukemia-2 (Bcl-2) is a downstream target of ATF5 in
gliomas and breast cancer. The Bcl-2 family of proteins includes
anti-apoptotic proteins, such as Bcl-2, Bcl-Xl and induced myeloid
leukemia cell differentiation protein, and apoptotic proteins, such
as Bcl-2 homologous antagonist/killer, Bcl-2 associated X protein
(BAX), BH3 interacting-domain and B-cell lymphoma 2 interacting
mediator of cell death. The regulation and balance of the Bcl-2
family proteins in a particular cell results in the inhibition or
induction of apoptotic signaling pathways (12–14).
The human cytomegalovirus (HCMV) infection has been
detected in malignant gliomas in a high percentage of cases,
although not in the adjacent healthy brain tissues (15). Growing evidence indicates that HCMV
infection may increase the malignancy of infected cells by
disrupting cellular pathways, such as apoptosis (16–18).
Apoptosis is detrimental to HCMV, as it functions as a cellular
antivirus response to eliminate infected cells (by activating the
immune response) or is deleterious (an inevitable consequence of
the stress that is inflicted by viruses on host cells). To survive,
these viruses have developed numerous strategies to prevent the
premature cell death of host cells (19,20).
HCMV infection in glial cells that does not lead to cell apoptosis
may promote clonal expansion without producing a productive or
cytopathic virus infection. Long-term persistence of HCMV in
malignant glioma cells may result in the occurrence of variant
strains, which exhibit a minimal cytopathic effect, and therefore,
HCMV may be reactivated in latently infected glioma cells when
cells are exposed to inflammatory stimuli or superinfected with
other HCMV strains (21,22). The sustained expression of specific
HCMV gene products may promote the overall glioma phenotype, as
HCMV encodes for gene products that regulate cellular pathways
involved in mutagenesis and apoptosis, and host antitumor immune
responses (23). HCMV
immediate-early (IE) genes 1 and 2 are the first set of viral genes
that are activated within HCMV-infected cells (24). IE1 and IE2 proteins regulate
transcription of viral and cellular genes within HCMV-infected
cells (25). In addition, the IE
protein has a binding site for the ATF/CREB family of transcription
factors, which upon binding forms a complex to activate downstream
elements (26).
Due to the high prevalence of HCMV and ATF5
expression observed in vivo in human malignant glioma, the
aim of the present study was to investigate the role of the ATF5
signaling pathway in HCMV-infected glioblastoma cells.
Materials and methods
Cell lines and viruses
Human glioblastoma U87 cell lines were purchased
from the Shanghai Cell Resource Center of the Chinese Academy of
Sciences (Shanghai, China). U87 cells were propagated in
HyCloneTM Minimum Essential Medium with 10% fetal bovine
serum (Thermo Fisher Scientific Inc., Rockford, IL, USA) and
maintained at 37°C in a humidified atmosphere containing 5% (v/v)
CO2. HCMV AD169 (France Pasteur Laboratory, Paris,
France) was tittered by plaque titration in human embryonic lung
fibroblast cells and expressed as the number of plaque-forming
units per milliliter. The HCMV was propagated in human embryonic
lung fibroblast cells with serum-free medium and the cell
supernatant was harvested and stored at −80°C.
Loss of ATF5 expression or function
achieved using small interfering (si) RNA or a dominant-negative
(dn) form of ATF5
To establish U87 cell lines with a stable knockdown
of ATF5, a GV113 control plasmid and three GV113 plasmids
containing ATF5 lentivirus short hairpin RNAs, LV-ATF5-RNAi
(8842-1; GCGAGATCCAGTACGTCAA), LV-ATF5-RNAi (8843-1;
TCTTGGATACTCTGGACTT) and LV-ATF5-RNAi (8844-1;TGGAACAGATGGAAGACTT)
(Shanghai GeneChem Co., Ltd., Shanghai, China), targeting the ATF5
coding sequence were separately transduced into U87 cells.
The pLeGFP-C1-NTAzip-ATF5 plasmid was transfected to
block the function of ATF5 simultaneously. Cells were transfected
with pLeGFP-C1-NTAzip-ATF5 and the pLeGFP-C1 plasmid using
Lipofectamine 2000 (Invitrogen Life Technologies, Carlsbad, CA,
USA). To analyze proliferation and apoptosis and to perform western
blot assays, cells were collected at 0, 12, 24 and 48 h following
transfection.
3-(4,5-Dimethylthiazol-2-yl)-2,
5-diphenyltetrazolium bromide (MTT) assay
Cell proliferation was determined using the MMT
assay (Sigma-Aldrich, St. Louis, MO, USA). Glioblastoma U87 cells
were plated in 96-well microplate formats according to the
manufacturer’s instructions. Cell lines were seeded in five
replication wells at 5,000 cells/well and cultured for 0, 12, 24 or
48 h. Following MTT uptake for a duration of 4 h, cells were lysed
in 150 μl dimethyl sulfoxide and absorbance was measured at 475 nm
using a fluorescence microplate reader (Sunrise Remote, Tecan
Austria GmbH, Grödig, Austria).
RNA extraction, and quantitative
polymerase chain reaction (qPCR)
RNA was extracted using TRIzol reagent [Takara
Biotechnology (Dalian) Co., Ltd., Dalian, China] according to the
manufacturer’s instructions. To produce cDNAs, 1 μg RNA was
reverse-transcribed using the PrimeScript RT reagent kit with gDNA
eraser (Perfect Real Time; Takara Biotechnology [Dalian] Co., Ltd.)
according to the manufacturer’s instructions. qPCR analyses were
performed using the GoTaq qPCR Master Mix (Promega Corporation,
Madison, WI, USA). The following primers were used: Forward,
5′-AGTGGGCTGGGATGGCTCGTAGAC-3′ and reverse,
5′-CTCGGGTGGTGGCAGGATGTGG-3′ for ATF5; forward,
5′-GCGCAATATCATGAAAGATAAGAACA-3′ and reverse,
5′-GATTGGTGTTGCGGAACATG-3′ for IE2; forward,
5′-CTGCACCTGACGCCCTTCACC-3′ and reverse,
5′-CACATGACCCCACCGAACTCAAAGA-3′ for Bcl-2; forward,
5′-TGGAGCTGCAGAGGATGATTG-3′ and reverse,
5′-GAAGTTGCCGTCAGAAAACATG-3′ for BAX; and forward,
5′-TGGAACGGTGAAGGTGACAG-3′ and reverse, 5′-GGCTTTTAGGATGGCAAGGG-3′
for β-actin.
Western blot analysis
Cells were washed three times with ice-cold
phosphate-buffered saline (PBS). Next, cells were dissolved in 500
μl lysis buffer and 5 μl phenylmethylsulfonyl fluoride (Beyotime
Institute of Biotechnology, Shanghai, China). Samples were
centrifuged (Eppendorf 5804R, Eppendorf Corporation, Hamburg,
Germany) at 20,100 × g for 5 min at 4°C to separate the membrane
fraction from the cytosolic fraction. After boiling for 5 min,
equivalent amounts of protein (30 μg) were resolved by 12%
SDS-PAGE, electroblotted onto polyvinylidene fluoride membrane
(Millipore, Billerica, MA, USA) and immunoreacted overnight with IE
genes (Virostat, Inc., Westbrook, ME, USA), ATF5 (Abcam, Cambridge,
UK), Bcl-2 and Bax (Bioss, Inc., Woburn, MA, USA), followed by a
2-h incubation with the horseradish peroxidase-conjugated secondary
antibody (Bioss, Inc.). Chemiluminescent signals were generated by
the SuperSignal West Pico Trial Kit (Thermo Fisher Scientific Inc.)
and detected using the Vilber Lourmat imaging system (Vilber
Lourmat Corporation, Torcy, France).
Terminal deoxynucleotidyl
transferase-mediated dUTP nick end labeling (TUNEL) assay
To detect the apoptosis rate of HCMV-infected
glioblastoma cells, an in situ cell death detection kit
(Roche Diagnostics Corporation, Indianapolis, IN, USA) was used.
Cells grown in 6-well culture clusters were treated with HCMV for
48 h. Treated cells were fixed onto poly-(L-lysine) coated slides
with 4% paraformaldehyde. The slides were rinsed with PBS and the
cells were permeabilized with 0.1% Triton X-100 [Sangon Biotech
(Shanghai) Co., Ltd., Shanghai, China]. Then, the slides were
washed with PBS and the cells were incubated in 50 μl TUNEL
reaction mixture for 60 min at 37°C in the dark. Next, 50 μl
converter-POD (Roche, Basel, Switzerland) was added and incubated
for 30 min at 37°C in a humidified chamber. Slides were rinsed with
PBS. Next, 50 μl 3,3′-diaminobenzidine substrate was added and
incubated for 10 min at 20°C. A total of 50 μl hematoxylin was then
added and incubated for 3 min at room temperature. The slides were
mounted under a glass coverslip with PBS and analyzed under a light
microscope (Motic B1-223A, Motic Deutschland GmbH, Wetzlar,
Germany).
Statistical analysis
Data are presented as the mean ± standard deviation.
Statistical analysis was performed using the Student’s t-test and
P<0.05 was considered to indicate a statistically significant
difference.
Results
Effect of HCMV infection on cell
proliferation and expression of ATF5, Bcl-2 and BAX
To determine the role of ATF5 expression in the
HCMV-infected U87 glioblastoma cells, the proliferation ratio of
U87 cells was examined in response to HCMV infection. Compared with
normal U87 cells, proliferation was enhanced by HCMV infection
(Fig. 1A). This observation
indicated that HCMV infection promotes growth proliferation in U87
glioblastoma cells. To elucidate the underlying mechanisms, the
expression of ATF5 in HCMV-infected U87 cells was examined. As
shown in Fig. 1B, ATF5 mRNA was
upregulated 12 h following HCMV infection and continued to increase
until 48 h following infection. Similarly, ATF5 protein levels were
upregulated (Fig. 1C and D;
P<0.05). These results demonstrated that HCMV infection
upregulates ATF5 expression in U87 cells. Studies have shown that
anti-apoptotic Bcl-2 is regulated by ATF5 in glioblastoma cells and
BAX is an apoptosis member of the Bcl-2 family. To examine the
expression of Bcl-2 and BAX in HCMV-infected U87 cells, qPCR and
western blot analysis were performed. As shown in Fig. 1C–E, the ratio of Bcl-2 to BAX
increased in U87 cells treated with HCMV (P<0.05). In addition,
Bcl-2 protein levels were upregulated in U87 cells treated with
HCMV (Fig. 1C and D; P<0.05).
These results indicated that HCMV infection enhances the
anti-apoptotic ability of U87 cells.
 | Figure 1Effect of HCMV infection on cell
proliferation and expression of ATF5, Bcl-2 and BAX in U87 cells.
(A) Cell proliferation was measured using a
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay.
Data are presented as the mean ± SD of three independent
experiments (*P<0.05 and **P<0.01 vs.
U87 + HCMV). (B) qPCR analyses showing the expression levels of IE2
and ATF5 mRNA in U87 cells following HCMV infection for 0, 12, 24
and 48 h. (C) Western blot analysis of IE genes, ATF5, Bcl-2 and
BAX protein in HCMV-infected U87 cells. (D) Relative expression of
IE, ATF5, Bcl-2 and BAX, all vs. β-actin in U87 cells infected with
HCMV for 0, 12, 24 and 48 h, according to the results of figure 1C.
Data are presented as the mean ± SD (*P<0.01 vs. 0 h
ATF5 expression). (E) qPCR analyses of Bcl-2 and BAX mRNA
expression in U87 cells following HCMV infection, where the ratio
of Bcl-2/BAX was calculated. HMCV, human cytomegalovirus; ATF5,
activating transcription factor 5; Bcl-2, B-cell
lymphoma/leukmia-2; BAX, Bcl-2-associated X protein; SD, standard
deviation; qPCR, quantitative polymerase chain reaction; IE,
immediate-early. |
Interfering with ATF5 affects
HCMV-infected U87 cell proliferation
To investigate the function of ATF5 in HCMV-infected
U87 cells, the effect of silencing ATF5 on the proliferation of
HMCV-infected U87 cells was examined. U87 cells were infected with
lentiviral RNAi to interfere with ATF5. Western blot analysis
revealed that ATF5 protein levels were markedly decreased (data not
shown). The cells were subsequently infected with HCMV at various
time points and cell proliferation was determined by MTT assay. As
shown in Fig. 2A, compared with U87
cells, cell proliferation was reduced following ATF5 interference
in U87 cells (P<0.05; between12 and 48 h). Cell viability
following ATF5 interference in U87 cells was marginally increased
following HCMV infection when compared with siATF5 U87 cells. To
further validate this observation, U87 cells were transfected with
dnATF5 plasmids to interfere with ATF5. As shown in Fig. 2B, the results were consistent with
Fig. 2A. These results indicated
that ATF5 may be involved in the regulation of cell proliferation
of HCMV-infected U87 cells in vitro.
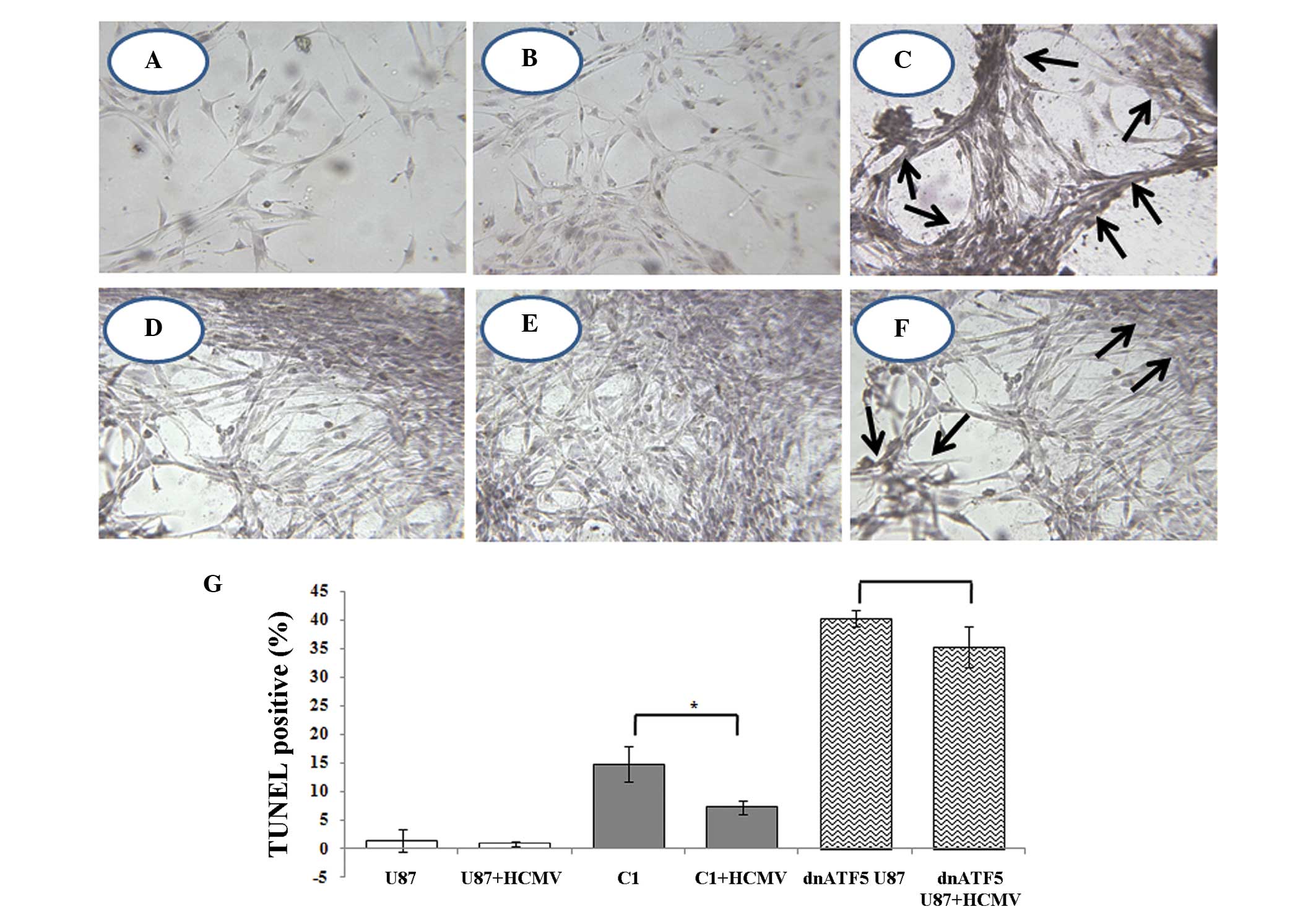
Analysis of apoptosis in dnATF5 U87
treated with HCMV using TUNEL
Previous studies have demonstrated that HCMV may
increase the malignancy of glioma by blocking apoptosis. ATF5 is
closely associated with tumor cell apoptosis. In addition, the
present study found that HCMV infection regulates the expression of
ATF5 in glioma. It is hypothesized that anti-apoptosis of HCMV
infection may be associated with ATF5 pathways. In siATF5 U87
cells, almost no apoptotic cells were identified. However,
apoptosis occurred in U87 cells, which were transfected with dnATF5
plasmids. Furthermore, apoptotic cell death was detected in ~40% of
dnATF5 U87 cells. The number of TUNEL-positive cells (dead cells)
decreased by 4–5% following HCMV infection. As shown in Fig. 3, TUNEL-positive cells were not
observed in normal or U87 cells (Fig.
3A and B), however, TUNEL-positive cells were detected in
dnATF5 U87 cells (Fig. 3C).
Following HCMV infection for 48 h, 40% of TUNEL-positive cells were
detected in dnATF5 U87 cells whereas 35% of TUNEL-positive cells
were detected in HCMV-infected dnATF5 cells (Fig. 3G). Compared with the control, in U87
cells, which had lost ATF5 function, no significant difference was
identified in the decline of TUNEL-positive cells (Fig. 3G). These results support the
hypothesis that the anti-apoptotic effect of HCMV infection is
associated with ATF5 expression.
Bcl-2 and BAX expression following
interference with ATF5 in HMCV-treated U87 cells
Bcl-2 is a downstream target of ATF5. Interaction of
BAX and Bcl-2 is important in the regulation of apoptosis. In the
present study, it was found that HCMV infection in U87 cells
regulated the expression of ATF5. Furthermore, following
interference with ATF5 in U87 cells, the anti-apoptotic ability was
decreased following HCMV infection. The expression of
anti-apoptotic Bcl-2 and apoptotic BAX protein was further detected
in HCMV-infected dnATF5 U87 cells by western blot analysis. In
dnATF5 U87 cells, changes in Bcl-2 and BAX protein expression
following HCMV infection are shown in Fig. 4. Compared with the control group, in
HCMV-infected U87 cells, which had lost ATF5 expression, no
significant differences in Bcl-2 and BAX protein levels between 0
and 48 h were identified.
Discussion
Increasing evidence implicates infectious agents as
causal factors in the development of human cancers. Infectious
agents may be promoters of neoplastic transformation. For example,
HCMV may modify host cell transcription to prolong its replication
by establishing a latent infection. However, the modification may
cause significant morbidity of cell life. Glioblastoma multiforme
(GBM) is a malignant and lethal brain cancer of unknown origin, and
the majority of cases are resistant to radiotherapy and
chemotherapy (27). Over the past
decade, various studies have searched for the presence of HCMV in
GBM samples (23) and Cobbs et
al (15) initially reported the
expression of HCMV proteins and oligonucleotides in a high
percentage of gliomas. Growing evidence that HCMV is specifically
detected in a variety of human malignancies at low levels of
expression indicates that the virus may facilitate the neoplastic
process in malignancy via oncomodulation. In addition,
oncomodulation implies that HCMV infects established tumor cells
and increases their malignant potential without necessarily being
oncogenic (16). Therefore, it is
important to understand how this virus modifies the host.
Viruses have acquired the capacity to modify the
host environment to enable the successful completion of their life
cycle. The use and control of the cellular transcriptional
machinery are among the most important functions of viruses. For
example, apoptosis of infected cells may limit viral replication
and thus serves as an innate defense mechanism against viral
infection. Consequently, viruses delay apoptosis by affecting
anti-apoptotic proteins. Recent studies have confirmed that HCMV
establishes a replication-favorable environment to avoid
prematurely compromising the cell’s ability to produce viral
progeny (28). In addition, our
previous studies have also confirmed that HCMV infection inhibits
tumor necrosis factor-α induced apoptosis (29).
The present study revealed that HCMV infection
blocks apoptosis in glioblastoma U87 cells and increases the
expression levels of the ATF5 and Bcl-2 to BAX ratio. ATF5, a
member of the ATF/CREB family of basic leucine zipper proteins, is
an anti-apoptotic protein, which is highly expressed in malignant
glioma, but not in normal brain tissues, and is essential for the
survival of glioma cells. Our previous studies demonstrated that
ATF5 is highly expressed in epithelial ovarian carcinomas and human
pancreatic carcinomas, compared with healthy ovarian and pancreatic
tissues (30,31). Although ATF5 is critically involved
in cell survival, cell proliferation and differentiation, the
upstream mechanism that regulates ATF5 function remains
unclear.
The results of the current study revealed that the
ATF5 signaling pathway is involved in the anti-apoptotic effects
that are induced by HCMV infection in U87 cells. Previous studies
have shown that ATF5 is an important anti-apoptotic protein in
malignant glioma. However, to the best of our knowledge, no studies
have shown that the anti-apoptotic effect of HCMV infection in
malignant glioma is associated with ATF5. The results of the
present study demonstrated that HCMV infection in U87 cells
promotes cell proliferation and upregulates the expression of ATF5.
In addition, when interfering with ATF5 in U87 cells, the
anti-apoptotic ability was decreased following HCMV infection
(Fig. 3). In revealing the
anti-apoptotic mechanism of ATF5 in HCMV-infected glioma, it was
found that Bcl-2 was downregulated in the U87 cells in which ATF5
was inhibited, when compared with the control (Fig. 4). Furthermore, previous studies have
shown that Bcl-2 is a downstream target of ATF5 that mediates the
pro-survival function of ATF5 in C6 glioma and MCF-7 breast cancer
cells. ATF5 binds to an ATF5-specific regulatory element that is
downstream of and adjacent to the negative regulatory element in
the Bcl-2 P2 promoter, stimulating Bcl-2 expression (12) and the results of the present study
were consistent with this. These results provide a novel insight
into the influence of HCMV infection on tumor cell apoptosis.
In conclusion, the present study found that ATF5 is
involved in the anti-apoptotic effect induced in HCMV-infected U87
cells. HCMV infection promotes the expression of ATF5 and elevates
the Bcl-2 to BAX ratio and enhances anti-apoptosis in U87 cells.
Furthermore, ATF5 knockdown may reduce the anti-apoptotic ability
of HCMV-infected U87 cells. These results facilitate the
understanding regarding the influence of HCMV infection on glioma
cell apoptosis and thus, HCMV may present a potential therapeutic
target.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81070501 and
30770105), the Natural Science Foundation of Shandong Province
(grant no. J122211) and the American Cancer Society and the U.S.
Department of Defense (D.X.L.).
Abbreviations:
|
ATF5
|
activating transcription factor 5
|
|
Bcl-2
|
B-cell lymphoma/leukmia-2
|
|
BAX
|
Bcl-2-associated X protein
|
|
CREB
|
cAMP response element-binding
protein
|
|
HCMV
|
human cytomegalovirus
|
|
IE
|
immediate early
|
|
MTT
|
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
|
|
PBS
|
phosphate-buffered saline
|
|
TUNEL
|
terminal deoxynucleotidyl
transferase-mediated dUTP nick end labeling
|
References
|
1
|
Arias A, Lamé MW, Santarelli L, Hen R,
Greene LA and Angelastro JM: Regulated ATF5 loss-of-function in
adult mice blocks formation and causes regression/eradication of
gliomas. Oncogene. 31:739–751. 2012.
|
|
2
|
Stupp R, Mason WP, van den Bent MJ, et al:
European Organisation for Research and Treatment of Cancer Brain
Tumor and Radiotherapy Groups; National Cancer Institute of Canada
Clinical Trials Group: Radiotherapy plus concomitant and adjuvant
temozolomide for glioblastoma. N Engl J Med. 352:987–996. 2005.
|
|
3
|
Vescovi AL, Galli R and Reynolds BA: Brain
tumour stem cells. Nat Rev Cancer. 6:425–436. 2006.
|
|
4
|
Michaelis M, Doerr HW and Cinatl J: The
story of human cytomegalovirus and cancer: increasing evidence and
open questions. Neoplasia. 11:1–9. 2009.
|
|
5
|
Sheng Z, Li L, Zhu LJ, et al: A
genome-wide RNA interference screen reveals an essential
CREB3L2-ATF5-MCL1 survival pathway in malignant glioma with
therapeutic implications. Nat Med. 16:671–677. 2010.
|
|
6
|
Persengiev SP and Green MR: The role of
ATF/CREB family members in cell growth, survival and apoptosis.
Apoptosis. 8:225–228. 2003.
|
|
7
|
Watatani Y, Ichikawa K, Nakanishi N, et
al: Stress-induced translation of ATF5 mRNA is regulated by the
5′-untranslated region. J Biol Chem. 283:2543–2553. 2008.
|
|
8
|
Angelastro JM, Ignatova TN, Kukekov VG, et
al: Regulated expression of ATF5 is required for the progression of
neural progenitor cells to neurons. J Neurosci. 23:4590–4600.
2003.
|
|
9
|
Persengiev SP, Devireddy LR and Green MR:
Inhibition of apoptosis by ATFx: a novel role for a member of the
ATF/CREB family of mammalian bZIP transcription factors. Genes Dev.
16:1806–1814. 2002.
|
|
10
|
Angelastro JM, Canoll PD, Kuo J, et al:
Selective destruction of glioblastoma cells by interference with
the activity or expression of ATF5. Oncogene. 25:907–916. 2006.
|
|
11
|
Monaco SE, Angelastro JM, Szabolcs M and
Greene LA: The transcription factor ATF5 is widely expressed in
carcinomas, and interference with its function selectively kills
neoplastic, but not nontransformed, breast cell lines. Int J
Cancer. 120:1883–1890. 2007.
|
|
12
|
Dluzen D, Li G, Tacelosky D, Moreau M and
Liu DX: BCL-2 is a downstream target of ATF5 that mediates the
prosurvival function of ATF5 in a cell type-dependent manner. J
Biol Chem. 286:7705–7713. 2011.
|
|
13
|
Chipuk JE, Moldoveanu T, Llambi F, Parsons
MJ and Green DR: The BCL-2 family reunion. Mol Cell. 37:299–310.
2010.
|
|
14
|
Harris MH and Thompson CB: The role of the
Bcl-2 family in the regulation of outer mitochondrial membrane
permeability. Cell Death Differ. 7:1182–1191. 2000.
|
|
15
|
Cobbs CS, Soroceanu L, Denham S, Zhang W
and Kraus MH: Modulation of oncogenic phenotype in human glioma
cells by cytomegalovirus IE1-mediated mitogenicity. Cancer Res.
68:724–730. 2008.
|
|
16
|
Barami K: Oncomodulatory mechanisms of
human cytomegalovirus in gliomas. J Clin Neurosci. 17:819–823.
2010.
|
|
17
|
Michaelis M, Kotchetkov R, Vogel JU, Doerr
HW and Cinatl J Jr: Cytomegalovirus infection blocks apoptosis in
cancer cells. Cell Mol Life Sci. 61:1307–1316. 2004.
|
|
18
|
Ranganathan P, Clark PA, Kuo JS, Salamat
MS and Kalejta RF: Significant association of multiple human
cytomegalovirus genomic Loci with glioblastoma multiforme samples.
J Virol. 86:854–864. 2012.
|
|
19
|
Xuan B, Qian Z, Torigoi E and Yu D: Human
cytomegalovirus protein pUL38 induces ATF4 expression, inhibits
persistent JNK phosphorylation, and suppresses endoplasmic
reticulum stress-induced cell death. J Virol. 83:3463–3474.
2009.
|
|
20
|
Benedict CA, Norris PS and Ware CF: To
kill or be killed: viral evasion of apoptosis. Nat Immunol.
3:1013–1018. 2002.
|
|
21
|
Poland SD, Costello P, Dekaban GA and Rice
GP: Cytomegalovirus in the brain: in vitro infection of human
brain-derived cells. J Infect Dis. 162:1252–1262. 1990.
|
|
22
|
Ogura T, Tanaka J, Kamiya S, Sato H, Ogura
H and Hatano M: Human cytomegalovirus persistent infection in a
human central nervous system cell line: production of a variant
virus with different growth characteristics. J Gen Virol.
67:2605–2616. 1986.
|
|
23
|
Cobbs CS, Harkins L, Samanta M, et al:
Human cytomegalovirus infection and expression in human malignant
glioma. Cancer Res. 62:3347–3350. 2002.
|
|
24
|
Sadanari H, Yamada R, Ohnishi K, Matsubara
K and Tanaka J: SUMO-1 modification of the major immediate-early
(IE) 1 and 2 proteins of human cytomegalovirus is regulated by
different mechanisms and modulates the intracellular localization
of the IE1, but not IE2, protein. Arch Virol. 150:1763–1782.
2005.
|
|
25
|
Zhu H, Shen Y and Shenk T: Human
cytomegalovirus IE1 and IE2 proteins block apoptosis. J Virol.
69:7960–7970. 1995.
|
|
26
|
Schwartz R, Helmich B and Spector DH: CREB
and CREB-binding proteins play an important role in the IE2
86-kilodalton protein-mediated transactivation of the human
cytomegalovirus 2.2-kilobase RNA promoter. J Virol. 70:6955–6966.
1996.
|
|
27
|
Pu P, Kang C, Zhang Z, Liu X and Jiang H:
Downregulation of PIK3CB by siRNA suppresses malignant glioma cell
growth in vitro and in vivo. Technol Cancer Res Treat. 5:271–280.
2006.
|
|
28
|
Teng MW, Bolovan-Fritts C, Dar RD, et al:
An endogenous accelerator for viral gene expression confers a
fitness advantage. Cell. 151:1569–1580. 2012.
|
|
29
|
Bai Z, Li L, Wang B, et al: Effect of
inducible expressed human cytomegalovirus immediate early 86
protein on cell apoptosis. Biosci Biotechnol Biochem. 73:1268–1273.
2009.
|
|
30
|
Hu M, Wang B, Qian D, et al: Interference
with ATF5 function enhances the sensitivity of human pancreatic
cancer cells to paclitaxel-induced apoptosis. Anticancer Res.
32:4385–4394. 2012.
|
|
31
|
Chen A, Qian D, Wang B, et al: ATF5 is
overexpressed in epithelial ovarian carcinomas and interference
with its function increases apoptosis through the downregulation of
Bcl-2 in SKOV-3 cells. Int J Gynecol Pathol. 31:532–537. 2012.
|
|
32
|
Augelastro JM, Canoll PD, Kuo J, et al:
Selective destruction of glioblastoma cells by interference with
the activityor expression of ATF5. Oncogene. 25:907–916. 2006.
|