Introduction
Due to the poor survival rate of patients with
glioma, particularly those with high-grade glioma (HGG), a number
of studies have investigated the different groups of the B7 family
(1). The B7 family belongs to the
immunoglobulin superfamily, which consists of B7-1, -2, -H1, -H2,
-DC, -H3 and -H4 (2). The B7 family
has important implications for the treatment of cancer,
transplantation and autoimmune diseases (3). B7-H3 is reported to be released by
monocytes, dendritic cells and activated T cells (4). It is a tumor-associated antigen, which
regulates important cellular responses, including proliferation,
apoptosis, adhesion, tumor metastasis and immunity (5–9), thus
demonstrating its novel biological role in tumor progression and
metastasis (9). B7-H3 protein
expression has been detected in various types of tumor, including
ovarian, lung, stomach, prostate and pancreatic tumors, as well as
clear cell renal and colorectal carcinoma (10–15).
Lemke et al (16) reported
that B7-H3 expression is present in human glioma tissues (16). Although its function remains
uncertain, its expression has been associated with disease
progression. B7-H1 is an important regulator of antitumor immunity
(2). B7-H1 is expressed in
hematopoietic malignancies, including leukemia, thymic neoplasms
and multiple myeloma, as well as in the majority of types of solid
human cancer, including breast, colon, esophageal, gastric, head
and neck squamous cell, kidney, liver, lung, ovarian, pancreatic,
salivary gland and urothelial carcinomas, as well as glioblastoma
(tumor tissues only), Wilms’ tumor and melanoma (17–22).
Furthermore, soluble B7-H1 (sB7-H1) expression has been detected in
the blood serum of patients with renal cell carcinoma (23).
B7-H3 and B7-H1 are significant in the interaction
between tumors and the immune system. This has been observed in
numerous different tumor types (1,2,5,10–15,25,26),
including glioma (16,27). The expression of sB7-H3 and sB7-H1
has previously been analyzed in the CSF and blood serum of patients
with various types of tumor, however, not in those with glioma
(10,23,24).
Sun et al (5), Chen et al (28) and Frigola et al (23) reported the expression of sB7-H3 and
sB7-H1 in the blood serum of patients with colorectal carcinoma,
the CSF and plasma of patients with bacterial meningitis and the
blood serum of patients with renal cell carcinoma, respectively.
However, these studies did not investigate the expression of sB7-H3
and sB7-H1 in the CSF and blood serum of patients with glioma. The
aim of the present study was to evaluate whether patients with
glioma have increased levels of B7-H3 and B7-H1 in their CSF, tumor
tissue and blood serum and whether this correlates with the glioma
grading.
Patients and methods
Patients and sample collection
A total of 78 patients (40 males and 38 females)
with a median age of 45 years (age range, 18–70 years) who had been
diagnosed with brain glioma and treated surgically at the
Department of Neurosurgery, Huashan Hospital, Fudan University
(Shanghai, China), between January 2012 and August 2012 were
selected for inclusion in the present study, subsequent to
obtaining Institutional Review Board approval. Patients provided
written informed consent. The study was approved by the ethics
committee of the Department of Neurosurgery, Huashan Hospital of
Fudan University.
The preoperative collection of CSF and blood serum
from the consenting patients was initiated at the Huashan Hospital
of Fudan University in January 2012. CSF was obtained via lumbar
puncture, and arterial and venous blood samples were obtained
preoperatively in the operating theater of Huashan Hospital of
Fudan University. Patients, who exhibited symptoms that indicated
increasing intracranial pressure, including a severe headache,
nausea, vomiting and papilloedema, were excluded due to the high
risk associated with performing a CSF aspiration in these patients.
Patients with a history of meningitis or a particularly large tumor
were also excluded. Each blood sample was centrifuged (Sigma 3K30;
Sigma-Aldrich Chemie Gmbh, Munich, Germany) at 20°C at 13,400 × g
for 15 min and the blood serum was isolated. CSF and blood serum
samples were stored at −80°C for the detection of B7-H3 and B7-H1
using enzyme-linked immunosorbent assay (ELISA); the patient health
data and pathological features were undisclosed. CSF from four
patients with a moderate traumatic brain injury [Glasgow Coma Scale
(GCS) score, 9–12], as well as 40 healthy donors with no evidence
of a history of brain tumor, served as controls and were analyzed.
Tumor samples were collected from patients at the time of surgical
resection at Huashan Hospital of Fudan University. Four brain
tissue samples from patients with a moderate traumatic brain injury
(controls) were obtained from the operating theatre at the
Department of Neurosurgery at the Huashan Hospital of Fudan
University. Samples were snap frozen in liquid nitrogen and stored
at −80°C until use. Among the 78 cases, 55 CSF samples, 60 tumor
samples and 78 blood serum samples were obtained for B7-H3
analysis, while 40 CSF samples, 43 tumor samples and 60 blood serum
samples were collected for B7-H1 analysis (Table I).
 | Table ISummary of the patient morphology from
which the B7-H3 and B7-H1 samples were obtained. |
Table I
Summary of the patient morphology from
which the B7-H3 and B7-H1 samples were obtained.
| B7-H3 sample
type | B7-H1 sample
type |
|---|
|
|
|
|---|
| Patient feature | CSF | Blood serum | Tumor tissue | CSF | Blood serum | Tumor tissue |
|---|
| Age (years) |
| Median | 44.5 | 45.0 | 44.5 | 44.0 | 45.0 | 44.0 |
| Range | 18–70 | 18–70 | 18–70 | 18–70 | 18–70 | 18–69 |
| Gender (n) |
| Male | 33 | 40 | 36 | 22 | 34 | 20 |
| Female | 22 | 38 | 24 | 18 | 26 | 23 |
| Total | 55 | 78 | 60 | 40 | 60 | 43 |
| Glioma (n) |
| Classification |
| LGG | 21 | 30 | 24 | 20 | 30 | 20 |
| HGG | 34 | 48 | 36 | 20 | 30 | 23 |
|
Primary/Recurrent |
| Primary glioma | 51 | 74 | 56 | 36 | 56 | 39 |
| Recurrent glioma
and RN | 4 | 4 | 4 | 4 | 4 | 4 |
| Position of tumor
(n) |
| Frontal lobe | 20 | 27 | 22 | 16 | 26 | 25 |
| Parietal lobe | 6 | 15 | 10 | 9 | 5 | 4 |
| Temporal lobe | 21 | 22 | 18 | 10 | 20 | 7 |
| Occipital lobe | 2 | 6 | 2 | 3 | 2 | 2 |
| Other | 6 | 8 | 8 | 2 | 7 | 5 |
| Insular | 1 | 2 | 2 | 1 | 2 | 2 |
| Cerebellum | 2 | 2 | 3 | 1 | 2 | 1 |
| Putamen | 2 | 2 | 2 | 0 | 1 | 1 |
| Hippocampal | 1 | 1 | 1 | 0 | 1 | 1 |
| Thalamus | 0 | 1 | 0 | 0 | 1 | 0 |
Quantitative sandwich ELISA of the CSF
and blood serum
CSF and blood serum samples were allowed to clot for
30 min, prior to centrifugation (Sigma 3K30; Sigma-Aldrich Chemie
Gmbh) for 15 min at 13,400 × g. The samples were aliquoted and
stored at ≤20°C. Standards were reconstituted with 1 ml deionized
water. For the investigation of B7-H3 (catalog no. DB7H30; R&D
Systems, Minneapolis, MN, USA), 900 μl calibrator diluent RD6–41
was pipetted into 50 ng/ml tubes and 500 μl calibrator diluent
RD6–41 was pipetted into the remaining tubes, and a dilution series
was generated as follows: 50, 25, 12.5, 6.25, 3.12, 1.56, 0.781 and
0.00 ng/ml, which served as a standard. A total of 100 μl assay
diluent RD1–109 was added to each well, and 50 μl B7-H3 standard
plus the samples was added to each well. For the investigation of
B7-H1 (catalog no. E0513; Wuhan EIAab Science Co., Ltd., Wuhan,
China), 900 μl sample diluent was pipetted into 10 ng/ml tubes and
500 μl sample diluent was pipetted into the remaining tubes and a
dilution series was generated as follows: 10, 5, 2.50, 1.25, 0.62,
0.31, 0.15 and 0.00 ng/ml, which served as a standard. A total of
100 μl B7-H1 standard plus the samples was added to each well.
After a 2-h incubation period at room temperature on a horizontal
orbital microplate shaker (0.12 orbit; mini size microplate
shaker-2AD, Maple Lab Scientific, Dalang, China) set at 500 ± rpm,
the plates were washed three times using wash buffer. A total of
200 μl B7-H3 or B7-H1 conjugate was added and incubated for 2 h at
room temperature on the shaker. The plates were washed three times
using wash buffer, decanted, inverted and blotted using clean paper
towels. Subsequently, 200 μl substrate solution was added to each
well and incubated for 30 min at room temperature in the dark. Stop
solution [50 μl; B7-H3 (cat no. DB7H30; R&D Systems); B7-H1
(cat no. E0513; Wuhan EIAab Science Co., Ltd.)] was added to each
well and the plate was gently tapped to ensure thorough mixing. The
plates were read at an absorbance of 405 and 570 nm using a
SpectraMax® M2 fluorescence absorbance cuvette
(Molecular Devices, LLC., Sunnyvale, CA, USA). The experiments were
performed in duplicate and obtained similar results.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis of brain tumor
samples
For tissue preparation for the RNA and protein
extraction, the tissue was homogenized using a pestle and mortar in
liquid nitrogen. Total RNA was extracted using an RNA purification
system (Qiagen, Valencia, CA, USA) and treated with RNase-free
DNase I (Roche, Mannheim, Germany) to remove any genomic DNA. The
complementary DNA was prepared from 5–6 mg total RNA using
SuperScript® RNase H-Reverse Transcriptase (Invitrogen
Life Technologies, Carlsbad, CA, USA) and random hexamers
(Sigma-Aldrich, St. Louis, MO, USA). For the qPCR analysis, gene
expression was measured using an ABI Prism® 7500
sequence detection system (Applied Biosystems, Foster City, CA,
USA) with SYBR® Green Master Mix (Eurogentec, Seraing,
Belgium) and primers at optimized concentrations. The primers
(Sigma-Aldrich) were selected to span exon-exon junctions. The
sequences for human B7-H3 and human B7-H1 were as follows: Forward,
5′-CCTGCTGCCTTATTATTTCACA-3′ and reverse,
5′-CACTGCAAGAAGAGGGTGGT-3′ for 4IgB7-H3; and forward,
5′-GGACAAGCAGTGACCATCAAG-3′ and reverse,
5′-CCCAGAATTACCAAGTGAGTCCT-3′ for h-B7-H1. The DNA was amplified
under the following conditions: 95°C for 1 min, followed by 35
cycles of 95°C for 30 sec and 72°C for 1 min. All results were
normalized using glyceraldehyde 3-phosphate dehydrogenase (GAPDH).
The primer sequences for the housekeeping gene, GAPDH were as
follows: Forward, 5′-AGAAGGCTGGGGCTCATTTG-3′ and reverse,
5′-AGGGGCCATCCACAGTCTTC-3′. The PCR products were separated on a
1.2% agarose gel and visualized using ethidium bromide staining.
Standard curves were generated for each gene and the amplification
was 90–100% efficient. Relative quantification of gene expression
was determined via comparison of the threshold and raw quantitative
(RQ) values (the average RQ value) of each tumor sample. The
experiments were performed in triplicate and obtained similar
results.
Immunohistochemistry (IHC)
Tumor tissues were collected at the time of surgical
resection from the Huashan Hospital of Fudan University and were
fixed using 4% paraformaldehyde. The tumor tissues were
subsequently incubated in blocking buffer (2% horse serum, 0.2%
Triton X-100 and 0.1% bovine serum albumin in phosphate-buffered
saline; Sigma-Aldrich) for 1 h at room temperature. Sections were
cut to 3 mm and were processed using a Ventana BenchMark XT
immunostainer (Ventana Medical Systems, Inc., Tucson, AZ, USA). The
staining procedure included a pre-treatment with Cell Conditioner 1
(pH 8; Sigma-Aldrich) for 60 min. For the detection of B7-H3,
sections were incubated with goat anti-human B7-H3 antibodies
(dilution, 1:200; R&D Systems) at 37°C for 32 min followed by
rabbit anti-goat immunoglobulins (P0446; Dako, Glostrup, Denmark)
for 32 min at room temperature. For the detection of B7-H1,
sections were incubated with anti-B7-H1 antibodies (dilution,
1:200; MIH1; ebioscience Inc., San Diego, CA, USA) overnight at 4°C
followed by incubation with fluorescein isothiocyanate-conjugated
goat anti-mouse (dilution, 1:50; Kirkegaard & Perry Lab Inc.,
Gaithersburg, MD, USA) or rhodamine-conjugated goat anti-mouse
(dilution, 1:150, Kirkegaard & Perry Lab Inc.) antibodies for
60 min. Nuclei were counterstained using
4′,6-diamidino-2-phenylindole. Incubation was followed by Ventana
standard signal amplification, UltraWash to remove excess antibody,
counterstaining with one drop of hematoxylin for 4 min and one drop
of bluing reagent (Shandon Inc., Swickley, PA, USA) for 4 min. For
visualization, an ultraView Universal DAB Detection kit (Ventana
Medical Systems, Inc.) was used.
Statistical analysis
Experiments were performed in duplicate or
triplicate. Results are presented as the mean ± standard deviation
and the χ2 test was conducted. Non-parametric tests,
including the Kolmogorov-Smirnov and Shapiro-Wilk tests were also
performed to compare data between the different groups. P<0.05
was considered to indicate a statistically significant difference.
All statistical calculations were performed using SPSS for Windows
(version 13. 0; SPSS Inc., Chicago, IL, USA).
Results
B7-H3 and B7-H1 expression in the CSF and
blood serum
ELISA revealed that the concentrations of sB7-H3 and
sB7-H1 in the CSF were significantly lower in the patients with
low-grade glioma (LGG; B7-H3, 1.129±1.256 ng/ml and B7-H1,
0.099±0.133 ng/ml) compared with the concentrations in patients
with HGG (B7-H3, 7.228±6.063 ng/ml and B7-H1, 1.557±1.200 ng/ml).
The CSF from four patients with moderate traumatic brain injury
(GCS score, 9–12) served as a control and demonstrated that the
levels of sB7-H3 and sB7-H1 were 0.306±0.218 and 0.019±0.003 ng/ml,
respectively (Fig. 1A and B and
Table II). This shows that sB7-H3
and sB7-H1 are expressed at greater levels in the CSF of the
patients with glioma compared with the patients with a traumatic
brain injury. The expression of sB7-H3 in the blood serum was
9.323±1.569 ng/ml in the patients with LGG, 17.090±4.278 ng/ml in
the patients with HGG and 17.020±5.466 ng/ml in the healthy control
subjects. Furthermore, the expression of sB7-H1 in the blood serum
was 0.003±0.003 ng/ml in the patients with LGG, 1.489±1.008 ng/ml
in the patients with HGG and 0.916±0.575 ng/ml in the healthy
control subjects (Fig. 1A and B and
Table II). These findings
demonstrate that sB7-H3 and sB7-H1 were expressed at greater levels
in the blood serum of patients with HGG compared with the patients
with LGG, however, there was no significant difference identified
in the expression of sB7-H3 and sB7-H1 in the blood serum of the
patients with glioma compared with that of the healthy control
subjects. This indicates that the expression of sB7-H3 and sB7-H1
may be significant in the CSF, but not in the blood serum in
patients with glioma.
 | Table IIB7-H3 and B7-H1 in the CSF, blood
serum and tumor tissues of the cases and control subjects. |
Table II
B7-H3 and B7-H1 in the CSF, blood
serum and tumor tissues of the cases and control subjects.
| Biomarker | CSF (ng/ml) | Blood serum
(ng/ml) | Tumor (RQ
value) |
|---|
| B7-H3 |
| LGG | 1.129±1.256 | 9.323±1.569 | 0.610±0.583 |
| HGG | 7.228±6.063 | 17.090±4.278 | 7.287±5.207 |
| Control | 0.306±0.218 | 17.020±5.466 | 0.173±0.064 |
| B7-H1 |
| LGG | 0.099±0.133 | 0. 003±0.003 | 0.849±0.397 |
| HGG | 1.557±1.200 | 1.489±1.008 | 3.813±3.350 |
| Control | 0.019±0.003 | 0.916±0.575 | 0.273±0.116 |
B7-H3 and B7-H1 expression in glioma
tissue
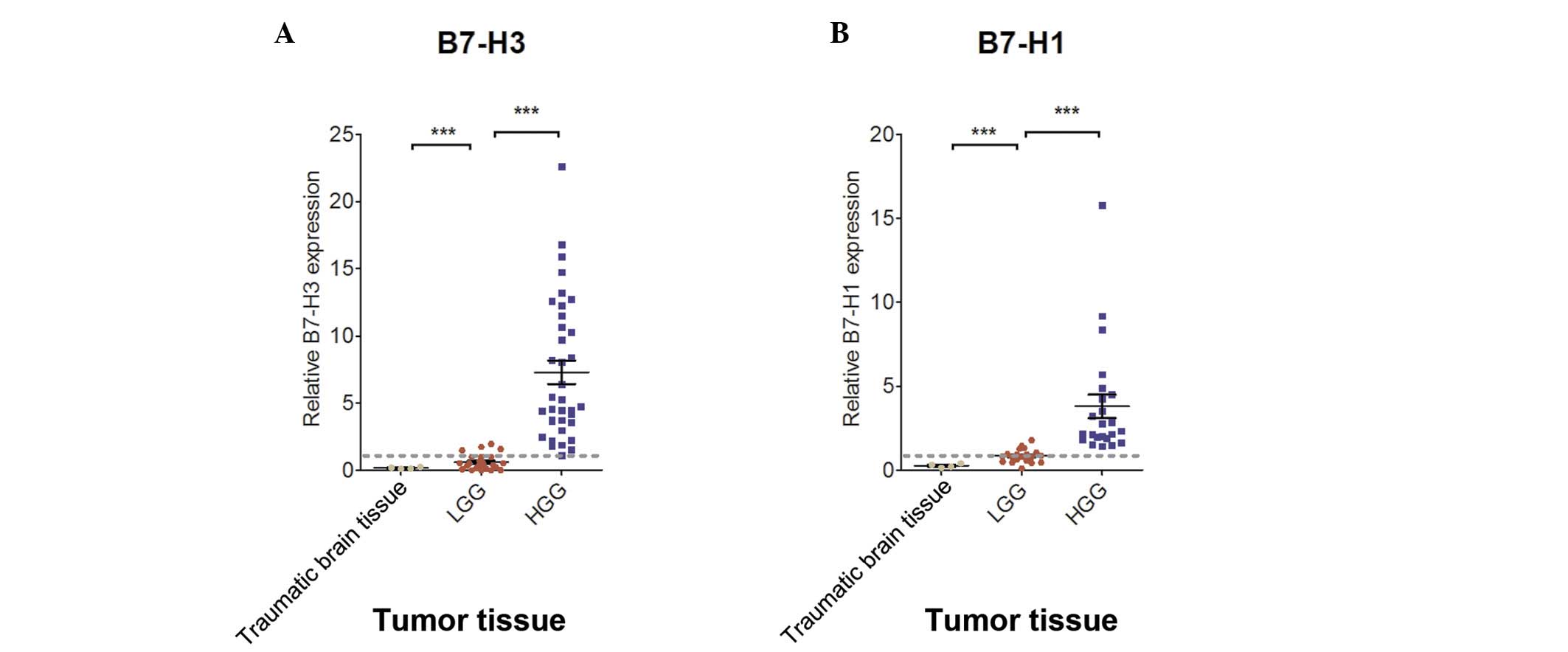
RT-qPCR analysis revealed that the mean RQ values of
B7-H3 and B7-H1 in the glioma tissue of the patients with LGG were
0.610±0.583 and 0.849±0.397, respectively, and in the patients with
HGG were 7.287±5.207 and 3.813±3.350, respectively (Fig. 2A and B and Table II). The expression of B7-H3 and
B7-H1 in the glioma tissue was found to be significantly correlated
with LGG and HGG (P<0.001). Furthermore, the average RQ values
of B7-H3 and B7-H1 in the moderate traumatic brain injury tissues
were 0.173±0.064 and 0.273±0.116, respectively. These findings show
that B7-H3 and B7-H1 were expressed at greater levels in the brain
glioma tissue compared with the traumatic brain injury tissue.
B7-H3 and B7-H1 expression is correlated
with the glioma grade
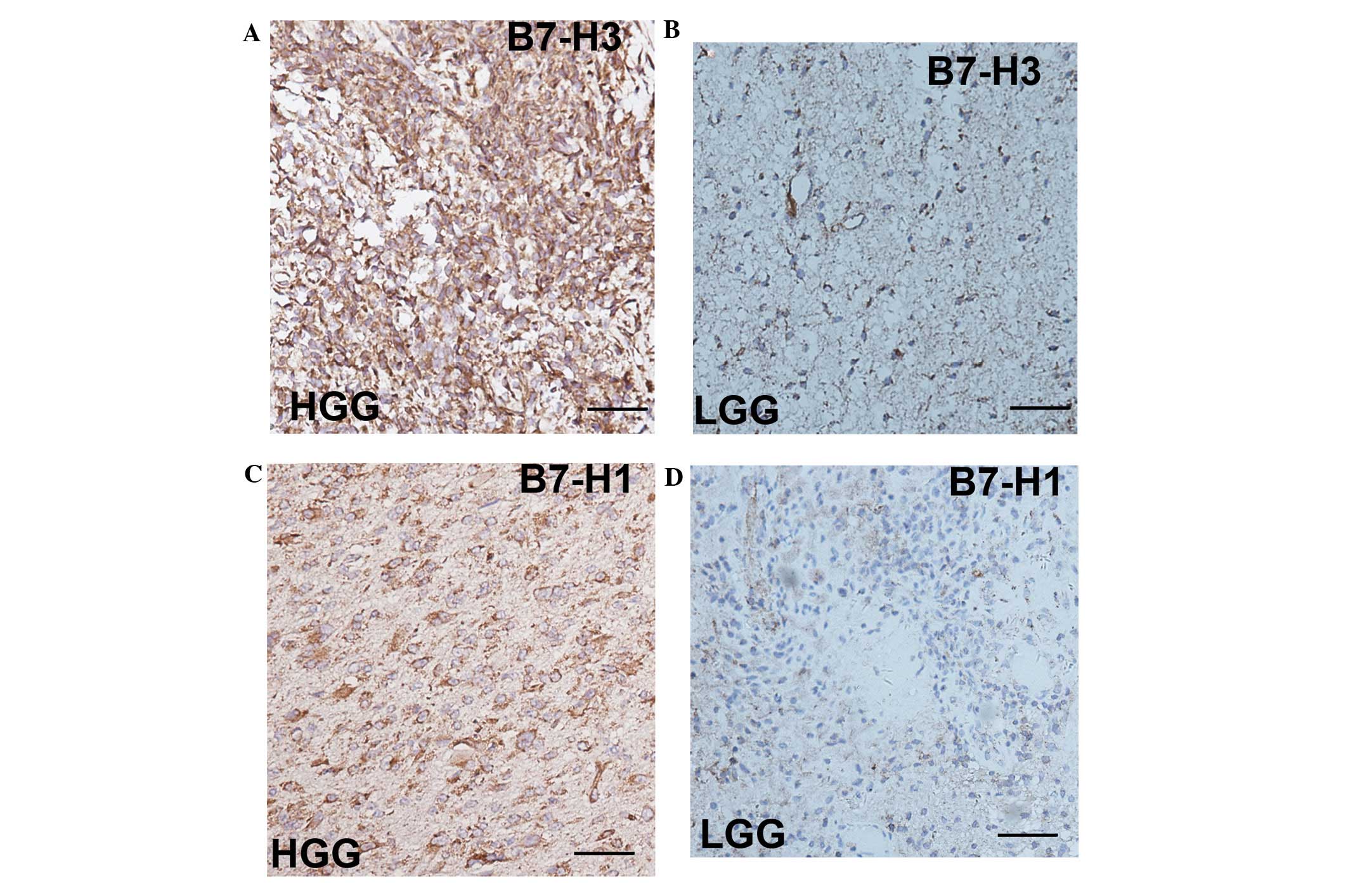
IHC analysis revealed that the expression of B7-H3
and B7-H1 was found to be correlated with the glioma grade in
freshly dissected human glioma tissue, which is consistent with the
study by Lemke et al (16). The
expression of B7-H3 and B7-H1 is demonstrated in Fig. 3 and was found to be increased in the
HGG tissue (Fig. 3A and C) compared
with the LGG tissue (Fig. 3B and
D).
Discussion
Previous studies have investigated B7-H3 and B7-H1
in different types of tumors (1,2,5,10–15,25,26),
including in glioma tissues (16,27).
Furthermore, recent studies have investigated the expression of
B7-H3 and B7-H1 in the blood serum of patients with colorectal
carcinoma and renal cell carcinoma (5,23), as
well as in the CSF of patients with bacterial meningitis (28). However, to the best of our
knowledge, no studies have investigated the expression of these
proteins in the CSF and blood serum of patients with brain glioma.
In the present study, significant sB7-H3 and sB7-H1 expression was
observed in the CSF, although not in the blood serum, of patients
with glioma. Table II shows the
mean concentrations of sB7-H3 and sB7-H1 in CSF and blood serum
samples and the RQ values of B7-H3 and B7-H1 expression in tumor
tissue samples. In the present study, the expression of sB7-H3 and
sB7-H1 was found to be higher in the CSF of patients with LGG
compared with that in the patients with a moderate traumatic brain
injury. In addition, the expression of sB7-H3 and sB7-H1 in the
blood serum of the healthy individual was observed to be as high as
that in the patients with glioma. Furthermore, in the brain tissues
of the patients with a moderate brain injury, the expression of
sB7-H3 and sB7-H1 was found to be lower than that in the brain
tumor tissue of the patients with glioma and was also found to
correlate with the glioma grade.
Sun et al (5)
and Frigola et al (23)
showed that sB7-H3 and sB7-H1 are expressed in the blood serum of
patients with colorectal carcinoma and renal cell carcinoma.
However, the present study identified no significant difference in
the concentrations of sB7-H3 and sB7-H1 in the blood serum of the
patients with brain glioma. This may be due to the different
characteristic features and sizes of the proteins that pass through
the blood-brain barrier and the receptor-mediated transcytosis to
the central nervous system (24).
This may explain why the expression of B7-H3 and B7-H1 may not be
as significant in the blood serum of patients with brain glioma
compared with that in patients with other tumors. In addition, with
regard to the expression of B7-H3 and B7-H1 in the tumor tissue,
the results of the present study were similar to those reported by
Lemke et al (16) and our
previous study (27), which showed
that the expression of B7-H3 and B7-H1 in glioma tissue was
correlated with the glioma grade. In the present study, a
functional assay of the samples was not performed; however, such
investigations will be performed in the future.
In conclusion, the present study demonstrates that
in patients with glioma, the expression of B7-H3 and B7-H1 in CSF
and tumor tissues, although not in blood serum, correlates with the
glioma grade.
Acknowledgements
The present study was supported by a grant from the
National Science Foundation for Distinguished Young Scholars of
China (grant no. 81272797) that was awarded to Professor Ying Mao
and a grant from the Innovation Program of Shanghai Municipal
Education Commission (grant no. 13ZZ010), which was awarded to
Professor Yu Yao.
Abbreviations:
|
CSF
|
cerebral spinal fluid
|
|
LGG
|
low-grade glioma
|
|
HGG
|
high-grade glioma
|
|
PCR
|
polymerase chain reaction
|
|
ELISA
|
enzyme-linked immunosorbent assay
|
|
GAPDH
|
glyceraldehyde 3-phosphate
dehydrogenase
|
|
IHC
|
immunohistochemistry
|
References
|
1
|
Chapoval AI, Ni J, Lau JS, et al: B7-H3: a
costimulatory molecule for T cell activation and IFN-gamma
production. Nat Immunol. 2:2692001.
|
|
2
|
Dong H, Strome SE, Salomao DR, et al:
Tumor-associated B7-H1 promotes T-cell apoptosis: a potential
mechanism of immune evasion. Nat Med. 8:793–800. 2002.
|
|
3
|
Hashiguchi M, Kobori H, Ritprajak P,
Kamimura Y, Kozono H and Azuma M: Triggering receptor expressed on
myeloid cell-like transcript 2 (TLT-2) is a counter-receptor for
B7-H3 and enhances T cell responses. Proc Natl Acad Sci USA.
105:10495–10500. 2008.
|
|
4
|
Zhang G, Hou J, Shi J, Yu G, Lu B and
Zhang X: Soluble CD276 (B7-H3) is released from monocytes,
dendritic cells and activated T cells and is detectable in normal
human serum. Immunology. 123:538–546. 2008.
|
|
5
|
Sun J, Chen LJ, Zhang GB, et al: Clinical
significance and regulation of the costimulatory molecule B7-H3 in
human colorectal carcinoma. Cancer Immunol Immunother.
59:1163–1171. 2010.
|
|
6
|
Merimsky O, Shoenfeld Y, Chaitchik S,
Yecheskel G and Fishman P: Antigens and antibodies in malignant
melanoma. Tumor Biol. 15:188–202. 1994.
|
|
7
|
Streit M and Detmar M: Angiogenesis,
lymphangiogenesis, and melanoma metastasis. Oncogene. 22:3172–3179.
2003.
|
|
8
|
Miller AJ and Mihm MC Jr: Melanoma. N Engl
J Med. 355:51–65. 2006.
|
|
9
|
Chen YW, Tekle C and Fodstad O: The
immunoregulatory protein human B7H3 is a tumor-associated antigen
that regulates tumor cell migration and invasion. Curr Cancer Drug
Targets. 8:404–413. 2008.
|
|
10
|
Zang X, Sullivan PS, Soslow RA, et al:
Tumor associated endothel expression of B7-H3 predicts survival in
ovarian carcinomas. Mod Pathol. 23:1104–1112. 2010.
|
|
11
|
Sun Y, Wang Y, Zhao J, et al: B7-H3 and
B7-H4 expression in non-small-cell lung cancer. Lung Cancer.
53:143–151. 2006.
|
|
12
|
Wu CP, Jiang JT, Tan M, et al:
Relationship between co-stimulatory molecule B7-H3 expression and
gastric carcinoma histology and prognosis. World J Gastroenterol.
12:457–459. 2006.
|
|
13
|
Roth TJ, Sheinin Y, Lohse CM, et al: B7-H3
ligand expression by prostate cancer: a novel marker of prognosis
and potential target for therapy. Cancer Res. 67:7893–7900.
2007.
|
|
14
|
Yamato I, Sho M, Nomi T, et al: Clinical
importance of B7-H3 expression in human pancreatic cancer. Br J
Cancer. 101:1709–1716. 2009.
|
|
15
|
Loos M, Hedderich DM, Ottenhausen M, et
al: Expression of the costimulatory molecule B7-H3 is associated
with prolonged survival in human pancreatic cancer. BMC Cancer.
9:4632009.
|
|
16
|
Lemke D, Pfenning PN, Sahm F, et al:
Costimulatory protein 4IgB7H3 drives the malignant phenotype of
glioblastoma mediating immune escape and invasiveness. Clin Cancer
Res. 18:105–117. 2012.
|
|
17
|
Suh WK, Gajewska BU, Okada H, et al: The
B7 family member B7-H3 preferentially down-regulates T helper type
1-mediated immune responses. Nat Immunol. 4:899–906. 2003.
|
|
18
|
Luo L, Chapoval AI, Flies DB, et al: B7-H3
enhances tumor immunity in vivo by costimulating rapid clonal
expansion of antigen-specific CD8+ cytolytic T cells. J
Immunol. 173:5445–5450. 2004.
|
|
19
|
Ma L, Luo L, Qiao H, et al: Complete
eradication of hepatocellular carcinomas by combined vasostatin
gene therapy and B7H3-mediated immunotherapy. J Hepatol. 46:98–106.
2007.
|
|
20
|
Geng L, Huang D, Liu J, et al: B7-H1
up-regulated expression in human pancreatic carcinoma tissue
associates with tumor progression. J Cancer Res Clin Oncol.
134:1021–1027. 2008.
|
|
21
|
Loos M, Giese NA, Kleeff J, et al:
Clinical significance and regulation of the costimulatory molecule
B7-H1 in pancreatic cancer. Cancer Lett. 268:98–109. 2008.
|
|
22
|
Boorjian SA, Sheinin Y, Crispen PL, et al:
T-cell coregulatory molecule expression in urothelial cell
carcinoma: clinicopathologic correlations and association with
survival. Clin Cancer Res. 14:4800–4808. 2008.
|
|
23
|
Frigola X, Inman BA, Lohse CM, et al:
Identification of a soluble form of B7-H1 that retains
immunosuppressive activity and is associated with aggressive renal
cell carcinoma. Clin Cancer Res. 17:1915–1923. 2011.
|
|
24
|
Rubin LL and Staddon JM: The cell biology
of the blood-brain barrier. Annu Rev Neurosci. 22:11–28. 1999.
|
|
25
|
Tseng SY, Otsuji M, Gorski K, et al:
B7-DC, a new dendritic cell molecule with potent costimulatory
properties for T cells. J Exp Med. 193:839–846. 2001.
|
|
26
|
Latchman Y, Wood CR, Chernova T, et al:
PD-L2 is a second ligand for PD-1 and inhibits T cell activation.
Nat Immunol. 2:261–268. 2001.
|
|
27
|
Yao Y, Tao R, Wang X, Wang Y, Mao Y and
Zhou LF: B7-H1 is correlated with malignancy-grade gliomas but is
not expressed exclusively on tumor stem-like cells. Neuro Oncol.
11:757–766. 2009.
|
|
28
|
Chen X, Zhang G, Li Y, et al: Circulating
B7-H3(CD276) elevations in cerebrospinal fluid and plasma of
children with bacterial meningitis. J Mol Neurosci. 37:86–94.
2009.
|