Introduction
T-cell acute lymphoblastic leukemia (T-ALL), an
aggressive malignancy arising from T-cell progenitors, accounts for
~15% of ALL cases in children and ~25% in adults. The long-term
survival rate for children and adolescents with this disease is
70–75%, while for adults the rate is only 35–40% in Western
populations (1). Over 50% of
patients with T-ALL harbor activation mutations of Notch1 (2), thus, γ-secretase inhibitors, which
block Notch signaling, were a promising target for therapy.
However, associated problems, such as limited therapeutic benefit
and intestinal toxicity have since been reported (3). Therefore, there is a requirement for
the development of novel and effective treatment strategies for
adult T-ALL.
Notch1 mutations mainly involve the
heterodimerization domain (HD) and the proline, glutamic acid,
serine, threonine-rich domain (PEST) (2). The HD mutation results in
ligand-independent proteolytic cleavage of Notch1, while the PEST
mutation blocks Fbw7 interaction with Notch1 and, thereby, prevents
its polyubiquitination and degradation (3,4). The
mutations of these two domains result in constitutive activation of
the Notch signaling pathway (1).
Moreover, ~30% of T-ALL patients harbor inactivating mutations of
the Fbw7 gene, which also activate Notch1 signaling (1). It has been demonstrated that c-Myc is
a direct and important target gene of Notch1, and its expression
levels have been observed to increase along with activation of
Notch1 signaling in T-ALL (5). In
addition, a previous study showed that the half-life of c-Myc in
B-cell or T-cell ALL was markedly prolonged (6). Therefore, c-Myc may be a target for
therapy in T-ALL.
Valproic acid (VPA), a histone deacetylase inhibitor
(HDACI), has been reported to treat various hematological
malignancies (7–9). Previous studies revealed that VPA was
more efficacious in combination with other agents, such as
idarubicin, 5-azacitidina and all-trans retinoic acid (10–12).
In acute myeloid leukemia (AML), VPA inhibited cell growth, mainly
by downregulation of c-Myc expression (13). However, the role of c-Myc in the
growth inhibition of T-ALL cells induced by VPA remains unclear. We
hypothesized that its role in T-ALL was the same as that in AML,
and that a c-Myc inhibitor would be able to augment the
anti-leukemic effect of VPA. In the present study, the effect of
VPA combined with a c-Myc inhibitor (10058-F4) on T-ALL cell lines
(Jurkat and CCRF-CEM cells) was investigated.
Materials and methods
Cell lines and reagents
T-ALL cell lines, Jurkat and CCRF-CEM cells, were
purchased from the American Type Culture Collection (Manassas, VA,
USA). These cells were maintained in RPMI-1640 (Gibco-BRL, Grand
Island, NY, USA) supplemented with heat-inactivated fetal bovine
serum (Gibco-BRL) at 37°C in a 5% CO2 humidified
incubator. VPA and 10058-F4 (Sigma-Aldrich, St. Louis, MO, USA)
were dissolved in dimethylsulfoxide (DMSO) at 1 M and 20 mM and
then stored at −20°C in small aliquots. Z-VAD-FMK (Sigma-Aldrich),
a pan-caspase inhibitor (14), was
also dissolved in DMSO and its final concentration was 20 μM.
Cell viability assay
Cell viability was measured by
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT;
Sigma-Aldrich) assay. Briefly, cells were seeded in 96-well plates
and treated with VPA (0–3.2 mM), alone or in combination with 60 μM
10058-F4, for 24 h. In each well, MTT solution (final
concentration, 0.5 mg/ml) was added and the cells were then
incubated at 37°C for 4 h. The absorbance value of each well was
measured by a spectrophotometry at 570 nm.
Flow cytometry
Cell death was detected by Annexin V-fluorescein
isothiocyanate (FITC) and propidium iodide (PI) (BD Pharmingen, San
Diego, CA, USA) staining. Cells were treated with VPA (0–2.4 mM),
with or without 60 μM 10058-F4, for 24 h. Cells were collected in a
tube using pipet tips, washed twice in 4°C PBS and then resuspended
in 50 μl Annexin V binding buffer (BD Pharmingen). Subsequently, 5
μl Annexin V-FITC and 5 μl PI were added, and these samples were
incubated in the dark at 25°C for 15 min. Following the addition of
450 μl Annexin V binding buffer, cell death in the samples was
measured on a FACScanto™ II flow cytometer (Becton Dickinson,
Franklin Lakes, NJ, USA).
Quantitative polymerase chain reaction
(qPCR)
The expression level of the c-MYC gene was
determined using reverse transcription-qPCR with SYBR Green I
[Takara Biotechnology (Dalian) Co., Ltd., Dalian, China], and GAPDH
was used as a reference gene. Total RNA was extracted using TRIzol
(Invitrogen Life Technologies, Carlsbad, CA, USA) and reverse
transcribed into cDNA by a SuperScript II first-strand cDNA
synthesis kit (Invitrogen Life Technologies). The reaction system
contained 12.5 μl 2X SYBR Premix Ex Taq [Takara Biotechnology
(Dalian) Co., Ltd.], 1 μl cDNAs and 10 pmol of each primer. The
primer sequences were as follows: Forward,
5′-ATGGGGAAGGTGAAGGTCG-3′ and reverse,
5′-GGGTCATTGATGGCAACAATATC-3′ for GAPDH; and forward,
5′-CGTCTCCACACATCAGCACAA-3′ and reverse,
5′-CACTGTCCAACTTGACCCTCTTG-3′ for c-MYC. The reaction was performed
at 95°C for 1 min, followed by 40 cycles of denaturation at 95°C
for 15 sec and annealing/extension at 60°C for 60 sec, on an iQ5
Real-Time PCR instrument (Bio-Rad Laboratories, Inc., Hercules, CA,
USA).
Western blot
Jurkat and CCRF-CEM cells were lysed at 0°C in lysis
buffer for 30 min, and their protein concentrations were measured
by the Bradford protein assay method. The samples were separated by
12% sodium dodecyl sulfatepolyacrylamide gel electrophoresis and
then transferred onto polyvinylidene fluoride (PVDF) membranes.
PVDF membranes were blocked with Tris-buffered saline containing
0.1% Tween (TBST; prepared in the laboratory, Department of
Hematology, Institute of Hematology, The First Affiliated Hospital,
Zhejiang University School of Medicine, Hangzhou, China) and 5%
non-fat milk for 2 h at room temperature. The membranes were then
incubated with the primary antibodies, rabbit anti-human monoclonal
c-Myc (D84C12 XP®), rabbit anti-human monoclonal
caspase-3 (8G10) and mouse anti-human monoclonal β-actin (8H10D10)
(dilution, 1:1000–5000; Cell Signaling Technology, Beverly, MA,
USA), overnight at 4°C. Following washing four times for 10 min
each time with TBST, these PVDF membranes were incubated with the
goat anti-rabbit (catalogue number, 7074) or horse anti-mouse
(catalogue number, 7076) IgG horseradish peroxidase-conjugated
secondary antibodies (dilution, 1:2000; Cell Signaling Technology)
for 2 h at room temperature. The membranes were again washed four
times with TBST, and protein bands were then visualized with the
enhanced chemiluminescence detecting kit (EZ-ECL chemiluminescence
detection kit; Biological Industries, Beit-Haemek, Israel) and
exposed to X-ray films.
Statistical analysis
The significant differences between experimental and
control groups were compared by Student’s t-test. P<0.05 was
considered to indicate a statistically significant difference. All
statistical analyses were performed using SPSS 16.0 software (SPSS
Inc., Chicago, IL, USA).
Results
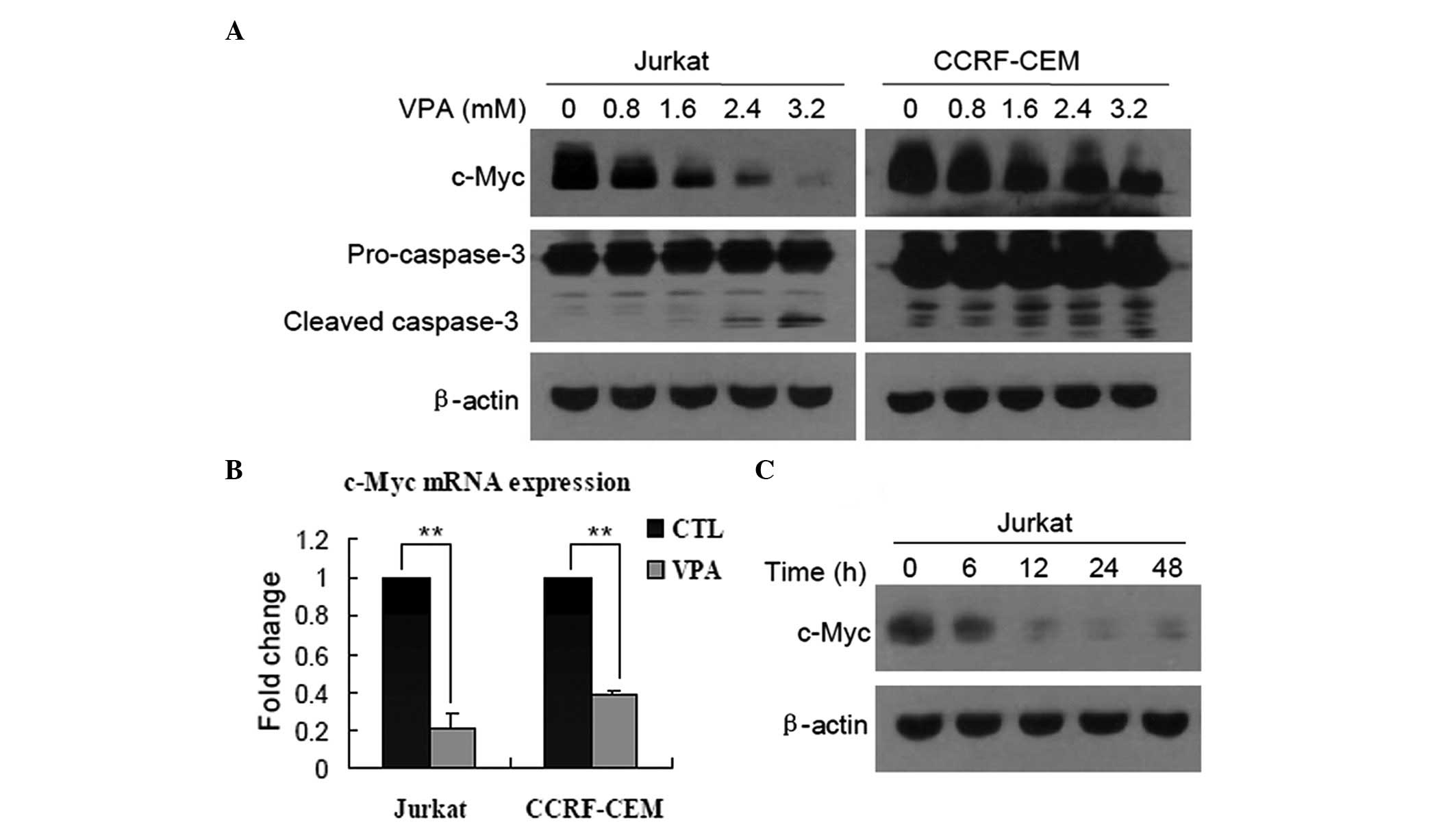
VPA downregulates the expression of c-Myc
in Jurkat and CCRF-CEM cells
First, the role of c-Myc in the growth inhibition of
T-ALL cells induced by VPA was identified. Western blots revealed
that after Jurkat and CCRF-CEM cells were treated with VPA at
various concentrations for 24 h, the expression of c-Myc protein
was markedly downregulated in a dose-dependent manner, and was
accompanied by cleavage of caspase-3 (Fig. 1A). qPCR was then performed to
measure the c-Myc mRNA levels, and it was found that the expression
of c-Myc was also decreased in Jurkat and CCRF-CEM cells treated
with 0.8 mM VPA for 24 h (Fig. 1B;
P<0.01), indicating that c-Myc was downregulated at a
transcriptional level. In addition, decreased expression of c-Myc
protein in a time-dependent manner was observed in Jurkat cells
(Fig. 1C), but not examined in
CCRF-CEM cells.
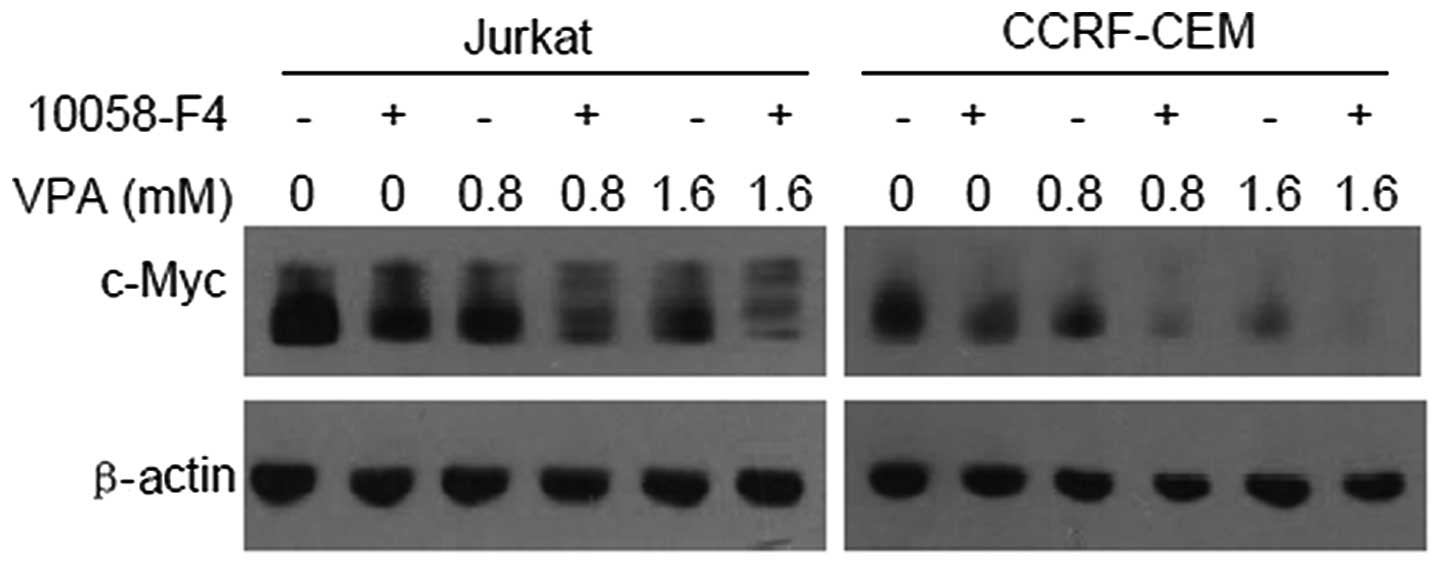
10058-F4 further promotes downregulation
of c-Myc expression by VPA
It has been demonstrated that 10058-F4 can block the
dimerization of c-Myc and Max, and then inhibit c-Myc
transactivating activity (15).
Thus, we hypothesized that 10058-F4 promotes the downregulation of
c-Myc expression induced by VPA. The western blotting results
showed that c-Myc expression levels in Jurkat and CCRF-CEM cells
treated with VPA (0, 0.8 and 1.6 mM) combined with 60 μM 10058-F4
decreased further compared with the corresponding controls
(Fig. 2).
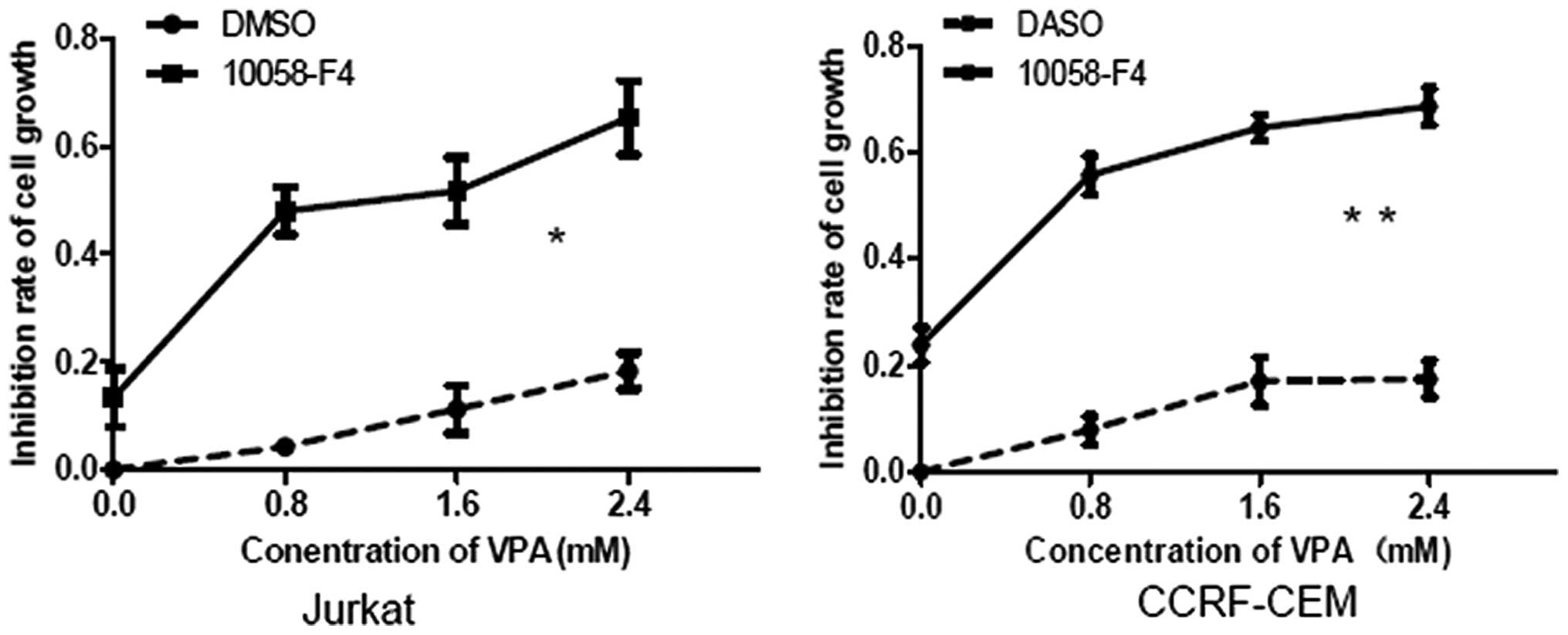
10058-F4 increases the growth inhibition
of Jurkat and CCRF-CEM cells induced by VPA
c-Myc is an important oncogene, which contributes to
the growth of T-ALL cells, particularly T-ALL cells with Notch1
mutations (5). The present study
results showed that 10058-F4 and VPA synergistically downregulated
c-Myc expression in Jurkat and CCRF-CEM cells. It was next
investigated whether 10058-F4 could increase cell growth inhibition
induced by VPA in Jurkat and CCRF-CEM cells, by MTT assay. The
growth inhibition rates of Jurkat cells treated with VPA (0, 0.8,
1.6 and 2.4 mM) combined with 10058-F4 for 24 h were 17.06±1.03,
47.99±4.35, 51.70±6.19 and 65.27±6.86% respectively, which were
significantly higher than those treated with corresponding
concentrations of VPA (0, 4.28±0.13, 11.21±4.46 and 17.86±2.60%)
(P=0.012). In CCRF-CEM cells, the growth inhibition rates for VPA
(0, 0.8, 1.6 and 2.4 mM) plus 10058-F4 were 23.80±3.37, 55.76±3.72,
64.65±2.48 and 68.60±3.48%, respectively, which were also
significantly higher than those for the corresponding
concentrations of VPA alone (0, 7.92±2.62, 17.03±4.54 and
17.42±3.42%) (P=0.007) (Fig.
3).
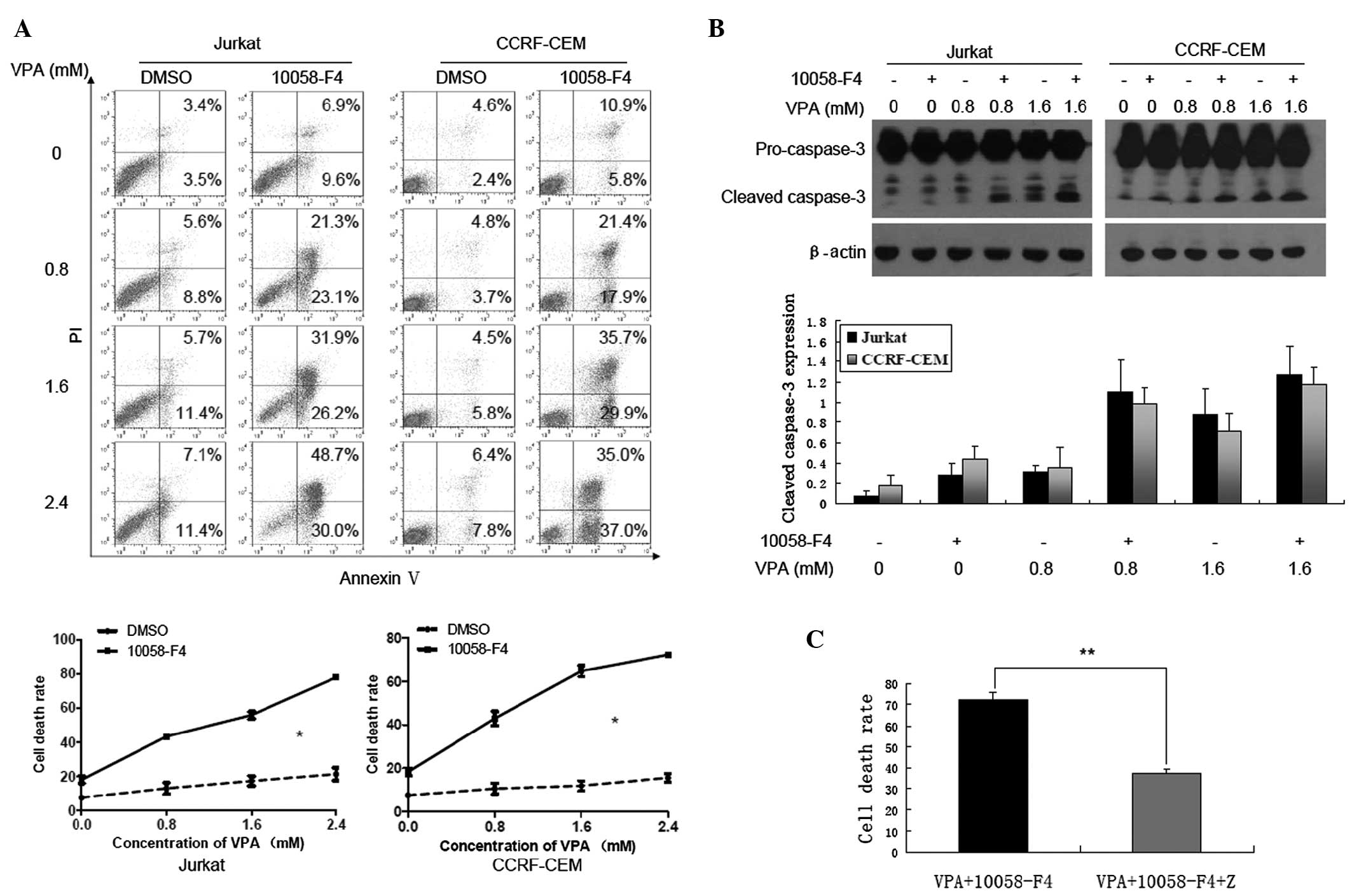
10058-F4 markedly increases the cell
death induced by VPA in a caspase-dependent and -independent
manner
Previous studies have demonstrated that 10058-F4
efficiently induced cell death in myeloma and AML cells (15,16).
Concordant with this, cell death of Jurkat and CCRF-CEM cells
treated with VPA (0, 0.8, 1.6 and 2.4 mM) was increased when
combined with 10058-F4. As shown in Fig. 4A, compared with treatment with
corresponding concentrations of VPA alone, cell death rates
(Annexin V+/PI+ and Annexin
V+/PI-) of Jurkat and CCRF-CEM cells treated
with 10058-F4 combined with VPA increased significantly (P=0.038
and P=0.037, respectively). In addition, western blot analysis
revealed that 10058-F4 could promote the cleavage of caspase-3
induced by VPA (Fig. 4B). The
results also demonstrated that Z-VAD-FMK, a pan-caspase inhibitor,
could partially inhibit cell death of Jurkat cells induced by
10058-F4 combined with VPA (72.33±3.35 vs. 37.4±1.87%, P<0.001)
(Fig. 4C). These findings indicate
that 10058-F4 dramatically increases cell death induced by VPA
through caspase-dependent and -independent pathways.
Discussion
It has been demonstrated that VPA could prevent
growth of cancer cells by inducing apoptosis and cell cycle arrest,
and by promoting cellular differentiation (17). HDACIs, including VPA, can
selectively alter the expression levels of a relatively small
proportion of genes by recovering the deacetylation of histones
(18). Regarding these genes in
which the expression levels are altered by HDACIs, upregulation of
the surface TRAIL death receptors (DR4 and DR5) in multiple myeloma
(19), upregulation of p21 and p27
in mantle cell lymphoma (20), and
downregulation of c-Myc in AML and endometrial cancer cells
(13,21) played a key role in the induction of
apoptosis and cell cycle arrest or the promotion of cellular
differentiation.
Since activating mutations of Notch1 were observed
in >50% T-ALL patients (3) and
c-Myc is an important direct target of Notch1 (5), the present study examined whether
c-Myc was downregulated in the growth inhibition of T-ALL cells
induced by VPA. The results showed that, as in AML (13), c-Myc expression was markedly
downregulated in Jurkat and CCRF-CEM cells in which activation of
Notch1 signaling occurred (22).
Further investigation revealed that 10058-F4 could reinforce the
downregulation of c-Myc induced by VPA, indicating that the former
may increase the sensitivity to the latter.
Previous studies have demonstrated that VPA can
induce apoptosis in leukemic cells (7,23).
Notably, VPA also augmented apoptosis of leukemic cells induced by
other agents, such as cytarabine (24), etoposide (25) and bortezomib (26). Thus, the present study next examined
the cell death of Jurkat and CCRF-CEM cells induced by VPA combined
with 10058-F4. The results showed that 10058-F4 could markedly
increase the cell death of Jurkat and CCRF-CEM cells induced by VPA
(0, 0.8, 1.6 and 2.4 mM). Activation of caspase-3 also increased
correspondingly. However, Z-VAD-FMK partially inhibited the cell
death induced by 10058-F4 combined with VPA, indicating that their
apoptotic effects involved in both caspase-dependent and
-independent pathways. Previous studies have also demonstrated that
not only 10058-F4, but also VPA, induced apoptosis through both
caspase-dependent and -independent pathways (15,23,27).
Although there have been arguments for and against
the therapeutic targeting of Myc (28), recent experiments in vivo and
in vitro have demonstrated that it is a promising
therapeutic strategy in high-risk hematologic malignancies
(29,30). The present study showed that
downregulation of c-Myc by 10058-F4 markedly increased VPA-induced
apoptosis. These findings suggest that VPA combined with c-Myc
inhibitors may be a novel potent therapeutic strategy for adult
T-ALL patients. However, further investigation with regard to the
clinical effect of their combination is required in the future.
Acknowledgements
This study was supported by the Foundation of
Innovation Team for Basic and Clinical Research of Zhejiang
Province (grant no. 2011R50015), the National Public Health Grand
Research Foundation (grant no. 201202017) and the Foundation of
Ningbo Medical Science and Technology Project (grant no.
2011A02).
References
|
1
|
Demarest RM, Ratti F and Capobianco AJ:
It’s T-ALL about Notch. Oncogene. 27:5082–5091. 2008.
|
|
2
|
Weng AP, Ferrando AA, Lee W, et al:
Activating mutations of NOTCH1 in human T cell acute lymphoblastic
leukemia. Science. 306:269–271. 2004.
|
|
3
|
Sarmento LM and Barata JT: Therapeutic
potential of Notch inhibition in T-cell acute lymphoblastic
leukemia: rationale, caveats and promises. Expert Rev Anticancer
Ther. 11:1403–1415. 2011.
|
|
4
|
Thompson BJ, Jankovic V, Gao J, et al:
Control of hematopoietic stem cell quiescence by the E3 ubiquitin
ligase Fbw7. J Exp Med. 205:1395–1408. 2008.
|
|
5
|
Weng AP, Millholland JM, Yashiro-Ohtani Y,
et al: c-Myc is an important direct target of Notch1 in T-cell
acute lymphoblastic leukemia/lymphoma. Genes Dev. 20:2096–2109.
2006.
|
|
6
|
Malempati S, Tibbitts D, Cunningham M, et
al: Aberrant stabilization of c-Myc protein in some lymphoblastic
leukemias. Leukemia. 20:1572–1581. 2006.
|
|
7
|
Shao N, Ma D, Wang J, Lu T, Guo Y and Ji
C: Notch1 signaling is irresponsible to the anti-leukemic effect of
HDACis in B-ALL Nalm-6 cells. Ann Hematol. 92:33–39. 2013.
|
|
8
|
Nie D, Huang K, Yin S, et al:
Synergistic/additive interaction of valproic acid with bortezomib
on proliferation and apoptosis of acute myeloid leukemia cells.
Leuk Lymphoma. 53:2487–2495. 2012.
|
|
9
|
Kuendgen A, Knipp S, Fox F, et al: Results
of a phase 2 study of valproic acid alone or in combination with
all-trans retinoic acid in 75 patients with myelodysplastic
syndrome and relapsed or refractory acute myeloid leukemia. Ann
Hematol. 84:61–66. 2005.
|
|
10
|
Sanchez-Gonzalez B, Yang H, Bueso-Ramos C,
et al: Antileukemia activity of the combination of an anthracycline
with a histone deacetylase inhibitor. Blood. 108:1174–1182.
2006.
|
|
11
|
Yang H, Hoshino K, Sanchez-Gonzalez B,
Kantarjian H and Garcia-Manero G: Antileukemia activity of the
combination of 5-aza-2′-deoxycytidine with valproic acid. Leuk Res.
29:739–748. 2005.
|
|
12
|
Leiva M, Moretti S, Soilihi H, et al:
Valproic acid induces differentiation and transient tumor
regression, but spares leukemia-initiating activity in mouse models
of APL. Leukemia. 26:1630–1637. 2012.
|
|
13
|
Cheng YC, Lin H, Huang MJ, Chow JM, Lin S
and Liu HE: Downregulation of c-Myc is critical for valproic
acid-induced growth arrest and myeloid differentiation of acute
myeloid leukemia. Leuk Res. 31:1403–1411. 2007.
|
|
14
|
Kutuk O, Pedrech A, Harrison P and Basaga
H: Pramanicin induces apoptosis in Jurkat leukemia cells: a role
for JNK, p38 and caspase activation. Apoptosis. 10:597–609.
2005.
|
|
15
|
Huang MJ, Cheng YC, Liu CR, Lin S and Liu
HE: A small-molecule c-Myc inhibitor, 10058-F4, induces cell-cycle
arrest, apoptosis, and myeloid differentiation of human acute
myeloid leukemia. Exp Hematol. 34:1480–1489. 2006.
|
|
16
|
Holien T, Vatsveen TK, Hella H, et al:
Bone morphogenetic proteins induce apoptosis in multiple myeloma
cells by Smad-dependent repression of MYC. Leukemia. 26:1073–1080.
2012.
|
|
17
|
Masetti R, Serravalle S, Biagi C and
Pession A: The role of HDACs inhibitors in childhood and
adolescence acute leukemias. J Biomed Biotechnol.
2011:1480462011.
|
|
18
|
Dokmanovic M, Clarke C and Marks PA:
Histone deacetylase inhibitors: overview and perspectives. Mol
Cancer Res. 5:981–989. 2007.
|
|
19
|
Fandy TE, Shankar S, Ross DD, Sausville E
and Srivastava RK: Interactive effects of HDAC inhibitors and TRAIL
on apoptosis are associated with changes in mitochondrial functions
and expressions of cell cycle regulatory genes in multiple myeloma.
Neoplasia. 7:646–657. 2005.
|
|
20
|
Heider U, Kaiser M, Sterz J, et al:
Histone deacetylase inhibitors reduce VEGF production and induce
growth suppression and apoptosis in human mantle cell lymphoma. Eur
J Haematol. 76:42–50. 2006.
|
|
21
|
Zhao ZN, Bai JX, Zhou Q, et al: TSA
suppresses miR-106b-93-25 cluster expression through downregulation
of MYC and inhibits proliferation and induces apoptosis in human
EMC. PLoS One. 7:e451332012.
|
|
22
|
O’Neil J, Grim J, Strack P, et al: FBW7
mutations in leukemic cells mediate NOTCH pathway activation and
resistance to gamma-secretase inhibitors. J Exp Med. 204:1813–1824.
2007.
|
|
23
|
Kawagoe R, Kawagoe H and Sano K: Valproic
acid induces apoptosis in human leukemia cells by stimulating both
caspase-dependent and -independent apoptotic signaling pathways.
Leuk Res. 26:495–502. 2002.
|
|
24
|
Xie C, Edwards H, Xu X, et al: Mechanisms
of synergistic antileukemic interactions between valproic acid and
cytarabine in pediatric acute myeloid leukemia. Clin Cancer Res.
16:5499–5510. 2010.
|
|
25
|
Jasek E, Lis GJ, Jasinska M, Jurkowska H
and Litwin JA: Effect of histone deacetylase inhibitors
trichostatin A and valproic acid on etoposide-induced apoptosis in
leukemia cells. Anticancer Res. 32:2791–2799. 2012.
|
|
26
|
Wang AH, Wei L, Chen L, et al: Synergistic
effect of bortezomib and valproic acid treatment on the
proliferation and apoptosis of acute myeloid leukemia and
myelodysplastic syndrome cells. Ann Hematol. 90:917–931. 2011.
|
|
27
|
Schwartz C, Palissot V, Aouali N, et al:
Valproic acid induces non-apoptotic cell death mechanisms in
multiple myeloma cell lines. Int J Oncol. 30:573–582. 2007.
|
|
28
|
Prochownik EV and Vogt PK: Therapeutic
Targeting of Myc. Genes Cancer. 1:650–659. 2010.
|
|
29
|
Ott CJ, Kopp N, Bird L, et al: BET
bromodomain inhibition targets both c-Myc and IL7R in high-risk
acute lymphoblastic leukemia. Blood. 120:2843–2852. 2012.
|
|
30
|
Filippakopoulos P, Qi J, Picaud S, et al:
Selective inhibition of BET bromodomains. Nature. 468:1067–1073.
2010.
|