Introduction
Tracheal neoplasms occur rarely, with an incidence
rate of ~0.1 individuals per 100,000 population worldwide. Although
they account for <1% of all reported malignancies, >80% are
malignant. The majority of tracheal neoplasms are squamous cell
carcinomas (SCCs). Only 10% are adenoid cystic carcinomas (ACCs;
cylindromas). Furthermore, the majority of ACCs are diagnosed in
middle-aged individuals, with no gender predilection, and not in
children and juveniles (1). ACCs
arise from the mixed seromucinous glands present in the
tracheobronchial submucosa. ACC is a rare tumor which predominantly
affects the major and accessory salivary glands (2). An ACC is a locally invasive tumor,
which usually spreads via direct extension, submucosal or
perineural invasion. It may also give rise to distant hematogenous
metastases, with >50% of patients exhibiting metastases as the
primary diagnosis. Among these, pulmonary metastases are the most
common; however, metastases to the brain, bone, liver, kidneys,
skin, abdomen and heart have also been reported (3–6). There
have been few reports of direct extension of an ACC of the
laryngotracheal complex to the thyroid, with clinical manifestation
as a thyroid tumor; thus far, only eight cases have been reported
(7). In those patients, the tumor
most frequently involved the cricoid ring, the larynx and the
subglottic area. In patients with tumors in the lower area of the
trachea, the tumor was found to primarily invade the lungs, and
laryngeal involvement was rare (8).
Previously, basaloid squamous cell carcinomas have
frequently been confused with ACCs and mucoepidermoid carcinomas of
the upper aerodigestive tract in pathological investigations;
however, different genetic abnormalities are now known to be
responsible for the former. The defining molecular feature of ACCs
is the presence of a recurrent chromosomal translocation,
[t(6;9);(q22–23; p23–24)], with the fusion transcript involving the
MYB genes (transcriptional activator Myb) and nuclear factor 1
B-type (9). ACC is positive for
cytokeratins (CKs), CK8, CK14 and CK17, and mucoepidermoid
carcinoma is immunopositive for CK8, CK14, CK17 and CK19.
Carcinoembryonic antigen immunoreactivity and carbohydrate antigen
19-9 has been detected in 100 and 50% of adenocarcinomas,
respectively (10). The expression
of c-kit may not serve as a useful marker for predicting outcomes
in ACC patients (11). Furthermore,
elevated carcinoembryonic antigen serum levels have been found to
decline following surgical resection and correlate with disease
recurrence (12,13). The biological behavior of the ACC of
the tracheobronchial tree differs from that of other tracheal
neoplasms; however, little is known with regards to the molecular
biology of the disease. A series of case studies in Taiwan
(14) revealed that the
overexpression of human epidermal growth factor receptor 2, tumor
suppressor protein p53 and prostaglandin-endoperoxide synthase 2
(cyclooxygenase-2, COX-2) affects the prognosis of ACC patients.
The increased expression of p53 and B-cell lymphoma 2, which
regulate cell death (apoptosis), was noted in 90% of ACCs, whereby
the majority of tumor cells (66–99%) were found to be
immunopositive. However, an association between the overexpression
of these factors and the histological types, clinical staging and
survival was not identified until recently (15). Metastatic ACC cells demonstrate
epithelial-mesenchymal transition (EMT) and sphere-forming
abilities. These cells exhibit a high expression of EMT-related
genes, including Snail, Twist1, Twist2, Slug, zinc finger
E-box binding homeobox 1 and 2 (Zeb1 and Zeb2),
glycogen synthase kinase 3β and transforming growth factor β2 and
stem cell markers (Nodal, Lefty, Oct-4, Pax6, Rex1, and
Nanog), as well as differentiation markers (sex-determining
region Y), Brachyury and α-fetoprotein (16). The identification of genomic,
proteomic and metabolomic abnormalities that promote the
development and progression of ACC is critical to the development
of specific targeted therapies. Recently, the role of molecular
analysis-based treatment was analyzed in a single case (17). As a result of immunohistochemical
analysis the mammalian target of rapamycin (mTOR) pathway was found
to be significant in the pathobiology of ACCs. It has already been
confirmed that mTOR is a central protein involved in carcinogenesis
in other tumors and thus a reasonable drug target (18). Administration of its inhibitors,
such as everolimus, may prolong survival in certain types of
carcinoma and has already been approved by Food and Drug
Administration. mTOR inhibitors, including temsirolimus and
everolimus (19) may also present a
potential drug to be tested for the treatment of ACC (17). At present, the design of robust
clinical trials based on translational studies is critical for
determining novel treatment moieties with optimal dosing schedules,
and combinations of such therapies with classical treatment.
Further study in this challenging field requires multicenter
cooperation to compile molecular data and to initiate prospective
trials to determine the roles of promising novel agents (9). Patient provided written informed
consent.
Case report
A 17-year-old female non-smoker with no significant
past medical history presented with a non-productive cough,
hemoptysis, dyspnea and breathlessness associated with wheezing for
three months. The patient was diagnosed clinically with a
pathological mass in the thyroid gland. In August 2007,
ultrasonography was performed which revealed a 37×26 mm
hypoechogenic lesion between the left lobe and the trachea, with
enlarged hypoechogenic cervical nodes. Supra and infraclavicular
lymph nodes were within normal size. Fine-needle aspiration biopsy
was performed and indicated an ACC. Bronchoscopic examination
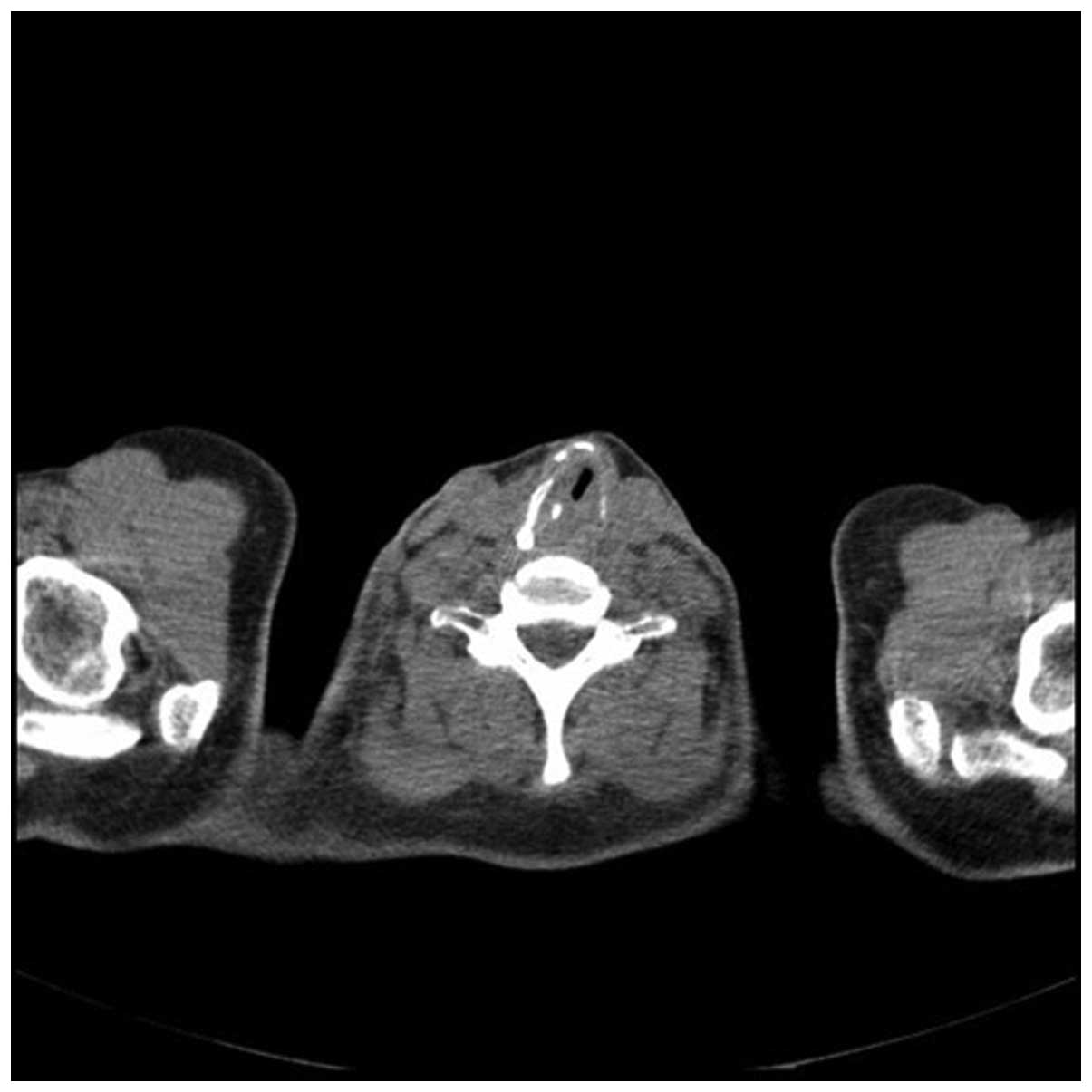
revealed stenosis 1 cm in length under the patient’s vocal cords,
with regular mucosa. A computed tomography (CT) scan confirmed a
34×37×50-mm tumor surrounding the trachea, infiltrating the two
thyroid lobes and causing esophageal constriction. The cricoid and
left arytenoid cartilage could not be assessed due to infiltration
of the cancer. In November 2007, based on the results of imaging
and pathological studies, the patient was referred to the
Department of Otolaryngology at the Czerniakowski Hospital (Warsaw,
Poland). The surgery plan included limited resection of the
trachea, possibly extended with laryngectomy, and followed by
thyroidectomy. In case of local invasion, segmental esophageal
resection, with subsequent jejunal reconstruction was planned. The
segmental tracheal resection was performed and completed with
partial cricoid cartilage removal (Fig.
1). The procedure was completed with end-to-end anastomosis. In
addition, thyroidectomy and tracheostomy finalized the surgical
treatment. The postoperative histopathological examination
confirmed the previous observations and revealed infiltration of
the cricoid cartilage, muscles and fibrous sheath of the thyroid.
No visible cancer cells were identified in the glandular tissue.
Due to the microscopically positive surgical margin of the tumor,
postoperative radiotherapy was performed with a fractional dose of
2 Gy and a total dose of 70 Gy. In August 2008, following
postsurgical bilateral vocal fold paralysis, the patient underwent
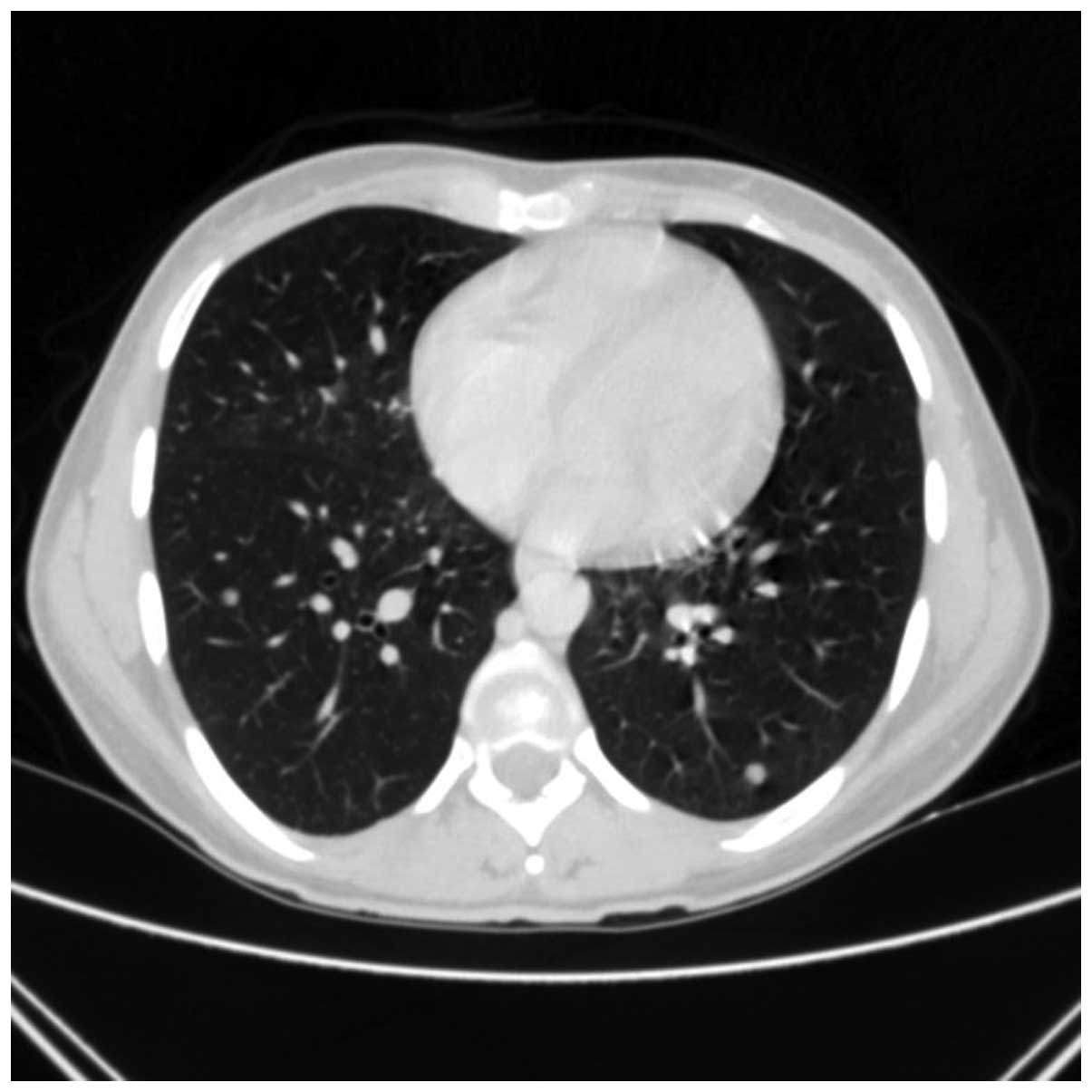
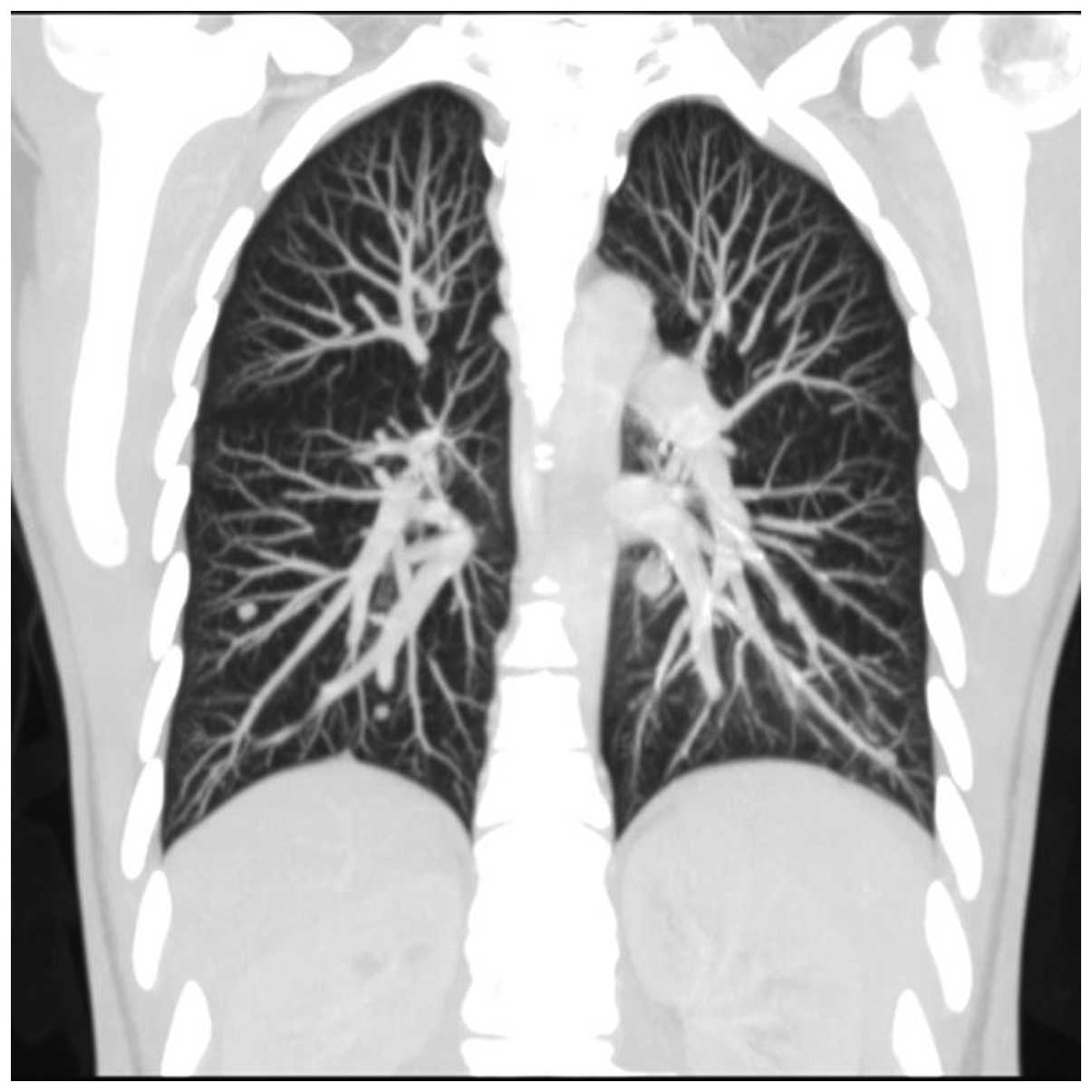
arytenoidectomy. In May 2010, a follow-up chest contrast-enhanced
CT scan (Figs. 2 and 3) revealed multiple small metastatic-like
lesions up to 13 mm in the lungs. The bronchofiberoscopy performed
simultaneously ruled out neoplasm recurrence in the trachea.
Therefore, in June and July 2010, the patient underwent bilateral
thoracotomy, with marginal resection of the two lungs.
Histopathological examination of the lung tissue confirmed
metastatic disease. The patient was scheduled for routine
follow-up. In April 2012, scheduled CT scans of the patient’s chest
and neck revealed complete pathological remission. The patient has
remained in a good condition since the removal of the tracheostomy
tube in November 2012, and is able to eat without aspiration and
does not require a gastric tube. Since the surgery, the patient has
been treated with high-dose thyroid hormones and Ca2+
supplementation, and undergoes regular endocrinological
evaluations. The patient has shown no signs of tetany. It is now
more than five years since the diagnosis and more than a year since
the pulmonary metastasectomy. The patient remains in a follow-up
program and is in good health with a high quality of life. The
patient undergoes scheduled oncological, laryngological and
endocrinological consultations, and contrast-enhanced CT is used
for routine imaging.
Discussion
Rare types of cancer always pose a novel set of
challenges. There are insufficient data to determine the best
treatment approach for such cancers, including ACCs. Therapeutic
decisions are based on small retrospective studies and case reports
with limited periods of patient observation (20). In contrast to ACC of the salivary
glands, the biological features of the tumors that develop from the
tracheobronchial tree remain poorly understood. ACCs exhibit a
predilection for perineural invasion and local and distant
recurrences. The cancer cells of ACCs exhibit a high synthetic
phase fraction, mitotic activity, vascular and lymphatic invasion,
and often advanced tumor grading.
Generally, surgery must be considered as first-line
therapy for patients with local disease, as it may be curative.
Recently, it was suggested that surgical resection and primary
reconstruction is the best curative treatment for primary tracheal
cancer; however, in population-based studies this treatment is only
applied in 10–25% of patients (21). Determination of the type of
resection depends on the location of the tumor and its infiltration
of other tissues. It may involve tracheal, laryngotracheal or
carinal resection, with primary reconstruction or end-to-end
anastomosis. The surgical approach includes transverse cervical
incision for tumors restricted to the cervical trachea. Right
posterolateral thorocotomy is reserved for lower parts of the
airway tree; however, a combination of these methods may be used
(22). For benign tumors,
endoscopic resection and laser and electrocautery fulguration may
be used as treatment options. The median survival time of patients
with surgically resected tumors (68.8±1 months) is higher than that
of patients with unresectable tumors (21.2±20.8 months).
Furthermore, the five- and 10-year survival following resection
varies from 59–79% and 29–51%, respectively (23). In addition, ≥27% of patients present
with local recurrence 155±30 months following surgery. Distant
metastases may occur in 55% of patients following a median time
interval of 96 months (range, 24–180 months) from the initial
surgery (24). The occurrence of
adverse effects in the postoperative period is associated with the
length of the resection, tension on the suture line, histological
type of the tumor and the requirement for laryngeal release. The
identification of surgery-associated risk factors is important due
to a high mortality rate (≤10.8%) soon after surgery (22).
The function of radiotherapy in patients with ACC
has been evaluated in several studies (25–27),
with varying results, and its role remains uncertain. Neutron beam
radiotherapy may be used for adjuvant therapy (28). Furthermore, ACC cells are less
sensitive to ionizing radiation than SCC cells; however, this
treatment must be recommended for all patients with unresectable
disease or as adjuvant therapy. Patients with unresectable disease
must be considered as candidates for definitive radiotherapy with a
conventional photon dose of 80 Gy (26). In selected cases, the administered
dose must be provided as five 2-Gy fractions per week over six
weeks, with a total dose of 60–70 Gy. Intensity-modulated radiation
therapy (IMRT) (29) or positron
emission tomography CT-directed IMRT (27) may be employed rather than standard
procedures. However, in contrast to SCC patients, studies have
shown that ACC patients with node involvement who underwent
complete resection may not receive any benefits from this treatment
modality (23,30). Although radiotherapy alone is not
recommended in the treatment of tracheal ACC, it may provide local
disease control, and the majority of tumors respond to
radiotherapy, which often results in long periods of remission
(8).
If distant metastases are diagnosed, palliative
treatment, including endobronchial treatment, low dose irradiation
and best supportive care, are advisable (21). Surgery must be selected first
whenever possible; however, for palliative treatment, radiotherapy,
chemotherapy or chemoradiotherapy based on cisplatin may also be
effective (23). Chemoradiation is
a feasible treatment option and may lead to sustained locoregional
tumor control in patients with non-resected ACCs. Surgical
metastasectomy has also been reported as a useful treatment method
(3–5). In a single case report, liver
metastasectomy showed clinical efficacy (3). Pulmonary resections may result in
locoregional control of primary disease and may extend disease-free
survival (4,31). Endotracheal procedures, including
forceps biopsy and suction, electrocoagulation, cryotherapy, laser,
photodynamic therapy or argon-beam coagulation and stents, are used
in palliative treatment to maintain upper airways patency (21).
Overall, ACC patients exhibit improved treatment
outcomes when compared with SCC patients, with a five-year survival
rate of 84 and 34%, respectively (31). However, it must be considered that
although the five- and 10-year survival rates for ACC patients may
be notable (79 and 57%, respectively), the long-term outcome is
poor due to late local recurrences and late metastatic spread
(24).
In conclusion, a tracheal ACC may present as a
thyroid tumor. The present case report highlights the importance of
detailed diagnosis of the tumors of the thyroid gland and detailed
pathological evaluation of thyroid tumor specimens. In the current
case, the patient’s disease course further clarifies the
understanding of the metastatic course of ACC and confirms the
importance of rapid metastasectomy. Finally, the analysis of this
case contributes important information to the knowledge base
regarding ACC treatment outcomes in young individuals. In general,
it must be acknowledged that patients with tracheal ACCs may
present with a midline swelling on their neck, without any
respiratory complaints, hoarseness of voice or dysphagia, and a
large, firm, non-tender, multilobular mass in the thyroid gland on
physical examination. The differential diagnosis of primary
tracheal ACCs includes basal cell adenomas, pleomorphic adenomas
and basal cell adenocarcinomas. Complete surgical resection may
lead to prolonged survival or complete remission. In young
patients, anastomosis is feasible even when 6–7 cm of the trachea
must be resected. Furthermore, subsequent oncological follow-up
must be conducted for much longer than five years. No molecular
markers have been found to be useful in predicting the progression
of the disease or patient prognosis. Additional study of the
molecular oncology of ACC is required in order to identify novel
drug targets and develop effective treatment strategies.
Acknowledgements
This study was supported by grants awarded to
Professor Cezary Szczylik and Dr Anna M. Czarnecka by the Military
Institute of Medicine statutory founding [grant no. 1/1744 (101)],
the National Science Centre projects (grant nos.
2011/01/B/NZ5/02822 and 2011/01/B/NZ4/01602) and the Foundation for
Polish Science TEAM project (grant no. TEAM/2010-6/8). The study
was also supported by a grant awarded to Dr Anna M. Czarnecka by
the Ministry of Science and Higher Education ‘Juventus’ (grant no.
CRU/WIM/275/2012), and a grant awarded to Dr Wojciech Kukwa by the
Medical University of Warsaw (grant no. 2012/13-2013/14).
References
|
1
|
Gaissert HA and Mark EJ: Tracheobronchial
gland tumors. Cancer Control. 13:286–294. 2006.
|
|
2
|
Delbouck C, Roper N, Aubert C, Souchay C,
Choufani G and Hassid S: Unusual presentation of adenoid cystic
carcinoma of the maxillary antrum. B-ENT. 5:265–268. 2009.
|
|
3
|
Park I, Lim SN, Yoon DH, et al:
Metastasectomy for hepatic metastases from adenoid cystic carcinoma
of the trachea. Gut Liver. 3:127–129. 2009.
|
|
4
|
Takahashi H, Kubota M, Nakata T, Nagai I,
Kimura S and Noguchi S: A case of pulmonary metastasis from adenoid
cystic carcinoma of the trachea. Nihon Kyobu Geka Gakkai Zasshi.
38:1063–1067. 1990.(In Japanese).
|
|
5
|
Bruzgielewicz A, Osuch-Wójcikiewicz E,
Majszyk D, et al: Adenoid cystic carcinoma of the head and neck - a
10 years experience. Otolaryngol Pol. 65(Suppl 5): 6–11. 2011.(In
Polish).
|
|
6
|
Selcuk A, Dere H, Bahar S, Sarikaya Y and
Ozcan M: Adenoid cystic carcinoma of the parotid gland presenting
as temporal bone neoplasm: a case report. B-ENT. 3:153–156.
2007.
|
|
7
|
Nuwal P, Dixit R and Singhal AK: Primary
adenoid cystic carcinoma of trachea presenting as midline neck
swelling and mimicking thyroid tumor: A case report and review of
literature. Lung India. 27:167–169. 2010.
|
|
8
|
Yang PY, Liu MS, Chen CH, Lin CM and Tsao
TC: Adenoid cystic carcinoma of the trachea: a report of seven
cases and literature review. Chang Gung Med J. 28:357–363.
2005.
|
|
9
|
Bell D and Hanna EY: Head and neck adenoid
cystic carcinoma: what is new in biological markers and treatment?
Curr Opin Otolaryngol Head Neck Surg. 21:124–129. 2013.
|
|
10
|
Tsubochi H, Suzuki T, Suzuki S, et al:
Immunohistochemical study of basaloid squamous cell carcinoma,
adenoid cystic and mucoepidermoid carcinoma in the upper
aerodigestive tract. Anticancer Res. 20:1205–1211. 2000.
|
|
11
|
Aslan DL, Oprea GM, Jagush SM, et al:
c-kit expression in adenoid cystic carcinoma does not have an
impact on local or distant tumor recurrence. Head Neck.
27:1028–1034. 2005.
|
|
12
|
Kuhel WI, Chow H, Godwin TA, Minick CR and
Libby DM: Elevated carcinoembryonic antigen levels correlating with
disease recurrence in a patient with adenoid cystic carcinoma. Head
Neck. 17:431–436. 1995.
|
|
13
|
Tamura S, Yamaguchi K, Terada M, et al:
Immunohistochemical analysis of CA19–9, SLX, and CA125 in adenoid
cystic carcinoma of trachea and bronchus. Nihon Kyobu Shikkan
Gakkai Zasshi. 30:407–411. 1992.(In Japanese).
|
|
14
|
Lin CM, Li AF, Wu LH, Wu YC, Lin FC and
Wang LS: Adenoid cystic carcinoma of the trachea and bronchus - a
clinicopathologic study with DNA flow cytometric analysis and
oncogene expression. Eur J Cardiothorac Surg. 22:621–625. 2002.
|
|
15
|
Carlinfante G, Lazzaretti M, Ferrari S,
Bianchi B and Crafa P: P53, bcl-2 and Ki-67 expression in adenoid
cystic carcinoma of the palate. A clinico-pathologic study of 21
cases with long-term follow-up. Pathol Res Pract. 200:791–799.
2005.
|
|
16
|
Shimoda M, Sugiura T, Imajyo I, et al: The
T-box transcription factor Brachyury regulates
epithelial-mesenchymal transition in association with cancer
stem-like cells in adenoid cystic carcinoma cells. BMC Cancer.
12:3772012.
|
|
17
|
Ishida M and Okabe H: Dedifferentiated
adenoid cystic carcinoma of the trachea: a case report with respect
to the immunohistochemical analyses of mammalian target of
rapamycin pathway proteins. Hum Pathol. 44:1700–1703. 2013.
|
|
18
|
Porta C, Szczylik C and Escudier B:
Combination or sequencing strategies to improve the outcome of
metastatic renal cell carcinoma patients: a critical review. Crit
Rev Oncol Hematol. 82:323–337. 2012.
|
|
19
|
Bellmunt J, Eisen T, Szczylik C, Mulders P
and Porta C: A new patient-focused approach to the treatment of
metastatic renal cell carcinoma: establishing customized treatment
options. BJU Int. 107:1190–1199. 2011.
|
|
20
|
Suzuki T: What is the best management
strategy for adenoid cystic carcinoma of the trachea? Ann Thorac
Cardiovasc Surg. 17:535–538. 2011.
|
|
21
|
Li W, Hua W, Yan FG, Shen HH and Xu H:
Adenoid cystic carcinoma of trachea: a case report and review of
literature. Chin Med J (Engl). 125:2238–2239. 2012.
|
|
22
|
Refaely Y and Weissberg D: Surgical
management of tracheal tumors. Ann Thorac Surg. 64:1429–1432;
discussion 1432–1423. 1997.
|
|
23
|
Macchiarini P: Primary tracheal tumours.
Lancet Oncol. 7:83–91. 2006.
|
|
24
|
Prommegger R and Salzer GM: Long-term
results of surgery for adenoid cystic carcinoma of the trachea and
bronchi. Eur J Surg Oncol. 24:440–444. 1998.
|
|
25
|
Allen AM, Rabin MS, Reilly JJ and Mentzer
SJ: Unresectable adenoid cystic carcinoma of the trachea treated
with chemoradiation. J Clin Oncol. 25:5521–5523. 2007.
|
|
26
|
Bonner Millar LP, Stripp D, Cooper JD,
Both S, James P and Rengan R: Definitive radiotherapy for
unresected adenoid cystic carcinoma of the trachea. Chest.
141:1323–1326. 2012.
|
|
27
|
Haresh KP, Prabhakar R, Rath GK, Sharma
DN, Julka PK and Subramani V: Adenoid cystic carcinoma of the
trachea treated with PET-CT based intensity modulated radiotherapy.
J Thorac Oncol. 3:793–795. 2008.
|
|
28
|
Azar T, Abdul-Karim FW and Tucker HM:
Adenoid cystic carcinoma of the trachea. Laryngoscope.
108:1297–1300. 1998.
|
|
29
|
Chang CY, Cheng SL and Chang SC: Adenoid
cystic carcinoma of trachea treated with tumor curettage and
adjuvant intensity modulated radiation therapy. South Med J.
104:68–70. 2011.
|
|
30
|
Shadmehr MB, Farzanegan R, Graili P, et
al: Primary major airway tumors; management and results. Eur J
Cardiothorac Surg. 39:749–754. 2011.
|
|
31
|
Liu D, Labow DM, Dang N, et al: Pulmonary
metastasectomy for head and neck cancers. Ann Surg Oncol.
6:572–578. 1999.
|