Introduction
Prostate cancer (PCa) is the second most commonly
diagnosed form of cancer and the sixth leading cause of
cancer-related mortality among males worldwide (1). For localized cancer, the five-year
survival rate is ~100%, however, in patients exhibiting distant
metastases, the five-year survival rate is 31% (2). For metastatic PCa, androgen
deprivation therapy (ADT) remains the most important and primary
treatment (3), which leads to
symptomatic improvement and a reduction in the serum levels of
prostate-specific antigen (PSA) in the majority of patients.
However, the majority of these patients progress to hormone
independence, the predominant obstacle that currently stops
improvements in patient survival and quality of life (4,5).
Therefore, it is important to determine markers that may identify
patients expected to undergo disease progression, in order to
optimize therapeutic decisions for individual patients. At present,
the serum PSA level is the best prostate-specific tumor marker
available and has also been demonstrated to be a useful prognostic
indicator for survival in different clinical settings (6). However, in human PCa, serum PSA is not
independently diagnostic or prognostic due to significant
specificity and sensitivity limitations (7,8).
Therefore, the identification of additional molecular markers is
required to improve the screening criteria, diagnosis and prognosis
of PCa.
Nicotinamide N-methyltransferase
(NNMT) is an S-adenosyl-L-methionine (SAM)-dependent
cytoplasmic enzyme, which exhibits a critical role in the
biotransformation and detoxification of numerous drugs and
xenobiotic compounds (9,10). Previous studies have revealed the
abnormal expression of NNMT in a number of cancers, and this may
contribute to tumor development and progression, as well as
resistance to radiotherapy and chemotherapy. In addition, NNMT has
potential as a non-invasive biomarker of cancer in body fluids,
including serum (11,12), saliva (13) and urine (14). To the best of our knowledge, the
role of NNMT in PCa has not been identified thus far.
In the present study, immunohistochemistry was used
to analyze the expression profile of NNMT in benign and malignant
prostate specimens, as well as the correlation between NNMT
expression and clinicopathological parameters. In addition, the
prognostic significance of NNMT expression in advanced PCa was
analyzed.
Materials and methods
Patient characteristics
This study was approved by the Research Ethics
Committee of the Third Xiangya Hospital, Central South University
(Changsha, China) and written informed consent was obtained from
all patients. Primary PCa tissues were obtained by biopsy (84
cases) or radical prostatectomy (36 cases), high-grade prostatic
intraepithelial neoplasia (HGPIN) tissues were obtained by biopsy
(18 cases) and benign prostate hyperplasia (BPH) tissues were
obtained from suprapubic prostatectomy (26 cases). All tissues were
obtained between 2001 and 2005. The age ranges of the patients with
PCa, HGPIN and BPH were 45–86 years (mean, 69.08±8.21), 59–84 years
(mean, 71.50±6.55) and 55–85 years (mean, 70.00±8.16),
respectively, and no significant differences were identified
between the three groups. The pre-treatment PSA levels in the serum
of the PCa, HGPIN and BPH patients were 0.16–216.0 ng/ml (mean,
48.46±40.92), 7.69–100.0 ng/ml (mean, 34.10±25.72) and 0.02–8.71
ng/ml (mean, 1.61±2.13), respectively. No patients with PCa
received adjuvant hormonal therapy or chemotherapy prior to
surgery.
The cancerous lesions were evaluated by two
pathologists and consisted of tumors with a Gleason score of 5
(n=1), 6 (n=30), 7 (n =21), 8 (n=40), 9 (n=24), and 10 (n=4). The
primary pathological tumor stages were pT1 in two cases, pT2a in
four cases, pT2b in six cases, pT2c in 13 cases, pT3a in 15 cases,
pT3b in 23 cases and pT4 in 57 cases. Furthermore, the regional
lymph node stages were pN0 in 92 cases and pN1 in 28 cases, and the
distant metastasis stages were pM0 in 64 cases and pM1 in 56 cases.
Patients with advanced PCa at pT4 or (and) pN1 or (and) pM1 in this
study were treated with maximal androgen blockade, and appropriate
chemotherapy and radiotherapy were administered as required
following the failure of hormone therapy.
Patient follow-up
The survival rates of all the advanced PCa patients
were analyzed, whereby the two end-points of progression-free
survival (PFS) time and overall survival (OS) time were evaluated.
The follow-up time ranged between seven and 96 months (median, 52
months). Disease progression during initial hormone therapy was
defined by any of the following criteria: The identification of ≥1
novel bone metastases, attributable to metastatic disease; evidence
of worsening of any existing bone metastases, attributable to
metastatic disease; the identification of ≥1 novel extra-skeletal
metastases or an increase in diameter of ≥20% (compared with the
maximal diameter recorded prior to hormone administration) of any
existing extra-skeletal metastases; and an increased serum PSA
level (identified by three consecutive tests revealing increased
serum PSA or a post-treatment PSA level of >4 ng/ml). Patients
lost to follow-up and mortalities caused by diseases independent of
PCa were regarded as censored data in the survival analysis.
Immunohistochemistry
All samples were fixed with formalin and
subsequently embedded in paraffin. Slices were cut at a 4-μm
thickness and mounted on polylysine-coated glass slides. Sections
were heated for 25 min at 56°C and then deparaffinized in xylene
followed by rehydration using a graded series of ethanol
concentrations. Immunohistochemistry was then performed following
incubation for 20 min at 80°C with an antigen unmasking solution
(Vector Laboratories, Burlingame, CA, USA). Endogenous peroxidase
activity in the deparaffinized tissue was blocked by treatment with
3% hydrogen peroxide in phosphate-buffered solution (PBS) for 15
min at 25°C. Next, non-specific background staining was blocked by
a 2-h incubation in 2% bovine serum albumin (Sigma-Aldrich,
Oakville, ON, Canada) with 0.3% Triton X-100. The sections were
then incubated with mouse monoclonal antibody against NNMT (1:150;
Abcam, Cambridge, UK) overnight at 4°C. The sections were then
rinsed in PBS with 0.1% Triton X-100 and incubated with
biotinylated secondary antibody (Vector Laboratories) and
streptavidin-horseradish peroxidase (Jackson ImmunoResearch, West
Grove, PA, USA) for 1 h at 25°C. Following slide incubation for 2–5
min with 3,3′-diaminobenzidine (Vector Laboratories) to allow color
development, all slides were counterstained with Mayer’s
hematoxylin blue (Sigma-Aldrich) in 0.3% ammonia. For the positive
controls of NNMT, normal liver tissues were used. For the negative
controls, the sections were treated in an identical manner, with
the exception of using primary antibody in order to confirm that
these sections failed to develop specific staining.
Immunohistochemical analysis
Immunostaining was scored by two independent
pathologists who were blinded to the clinicopathological data and
clinical outcomes of the patients. The scores of the two
pathologists were compared and any discrepancies were solved by
reexamination of the staining to achieve a consensus score. The
number of positively stained cells in 10 representative microscopic
fields was counted and the percentage of positive cells was
calculated. The percentage scoring of immunoreactive tumor cells
was as follows: 0, 0%; 1, <1%; 2, 1–10%; 3, 11–33%; 4, 34–67%;
and 5, >67%. The staining intensity was visually scored and
stratified as follows: 0, none; 1, weak; 2, moderate; and 3,
strong. The final NNMT immunohistochemical score was presented as a
composite (intensity + extent) (15). This overall score was then averaged
with the number of 10 fields that were performed for each patient.
An immunoreactivity (IR) score of 4 was defined as the cut-off for
high NNMT IR to identify a potential correlation between NNMT
expression in the malignant epithelium and the clinicopathological
characteristics, as almost all benign foci exhibited IR scores of
≤3 for NNMT.
Statistical analysis
Data are presented as the mean ± standard deviation.
All statistical calculations were performed using SPSS version 11.0
software (SPSS, Inc., Chicago, IL, USA). Differences in the NNMT
expression of three different types of prostate tissue were
assessed using the Mann Whitney U test. Pearson’s χ2
test and Spearman’s correlation analysis were applied to
investigate the association between NNMT expression and patient
characteristics. Survival curves for patients exhibiting high and
low NNMT expression were plotted using the Kaplan-Meier method, and
statistical differences were compared using a log-rank test.
Univariable and multivariable survival analyses were performed
using the Cox regression analysis. P<0.05 was considered to
indicate a statistically significant difference.
Results
Upregulated NNMT expression in HGPIN and
PCa tissues
A total of 26 BPH, 18 HGPIN and 120 PCa tissues were
evaluated using immunohistochemistry. While the majority of BPH
cases (18/26; 69.2%) exhibited absent or extremely weak NNMT
expression, NNMT was significantly upregulated in the HGPIN (15/18;
83.3%) and PCa (77/120; 64.2%) tissues, when using an IR score of 4
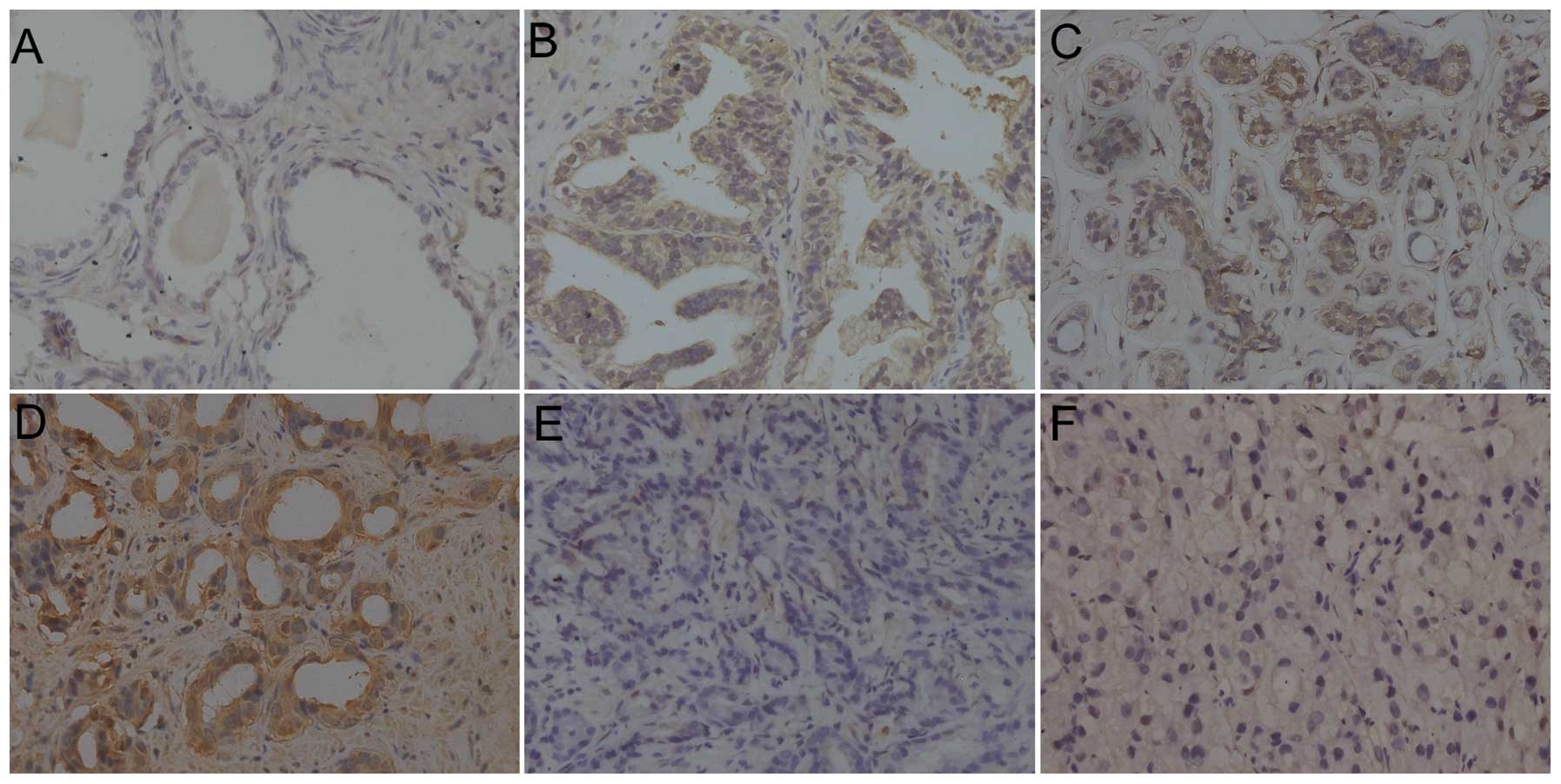
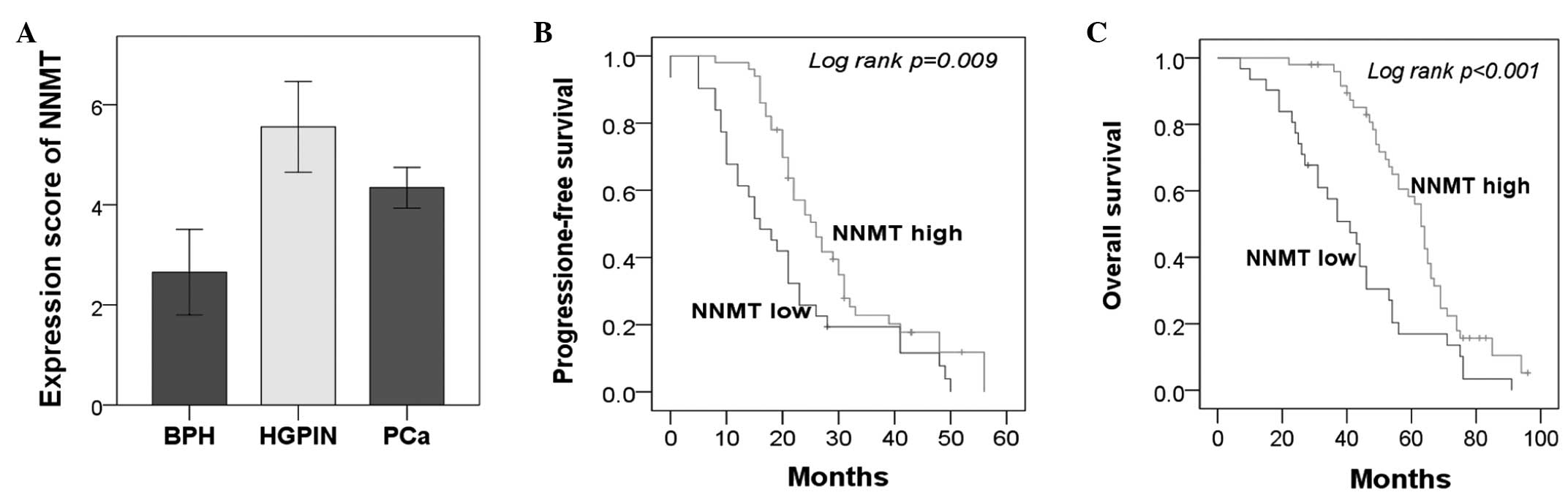
as a cutoff (Figs. 1 and 2A). The IR scores for the BPH, HGPIN and
PCa tissues were 2.65±2.12, 5.56±1.82 and 4.34±2.26, respectively.
The Mann Whitney U test revealed that NNMT expression in the HGPIN
and PCa tissues was significantly higher than in the BPH tissues
(P<0.001 and P=0.001, respectively). In carcinoma, NNMT was
expressed at various intensities (Fig.
1C–F). The IR of NNMT was predominantly observed in the
cytoplasm, however, it was also present in the nuclei of epithelial
cells. No evident heterogeneity was identified in the staining
pattern between duplicate tissue cores.
Correlation between NNMT expression and
clinicopathological variables in PCa
The association between NNMT expression and the
clinicopathological factors of PCa, including patient age, serum
PSA level, tumor stage, Gleason score, distant metastasis and lymph
node metastasis, was investigated. Gleason score was found to
negatively correlate with NNMT expression (P<0.001) (Table I). Spearman’s correlation analysis
identified a correlation coefficient of −0.268 (P=0.003) between
the IR and Gleason scores. However, no correlation was identified
between other clinicopathological factors and NNMT expression.
 | Table IAssociation between NNMT expression
and the clinicopathological parameters of PCa. |
Table I
Association between NNMT expression
and the clinicopathological parameters of PCa.
| | NNMT expression, n
(%) | |
|---|
| |
| |
|---|
| Characteristics | Patients, n | High | Low | P-value |
|---|
| Age, years | | | | 0.219 |
| <69 | 61 | 40 (65.6) | 21 (34.4) | |
| ≥69 | 59 | 33 (55.9) | 26 (44.1) | |
| PSA, ng/ml | | | | 0.808 |
| ≤10 | 21 | 14 (66.7) | 7 (33.3) | |
| >10 | 99 | 59 (59.6) | 40 (40.4) | |
| Distant metastasis
(pM1) | | | | 0.452 |
| Yes | 56 | 32 (57.1) | 24 (42.9) | |
| No | 64 | 41 (64.1) | 23 (35.9) | |
| Lymph metastasis
(pN1) | | | | 0.385 |
| Yes | 28 | 19 (67.9) | 9 (32.1) | |
| No | 92 | 54 (58.7) | 38 (41.3) | |
| pT-status | | | | 0.442 |
| pT1/2 | 25 | 17 (68.0) | 8 (32.0) | |
| pT3/4 | 95 | 56 (58.9) | 39 (41.1) | |
| Gleason score | | | | <0.001a |
| 5–6 | 31 | 28 (90.3) | 3 (9.7) | |
| 7 | 21 | 15 (71.4) | 6 (28.6) | |
| 8–10 | 68 | 30 (44.1) | 38 (55.9) | |
NNMT expression in advanced PCa
correlates with prolonged PFS and OS time
Since the majority of specimens were obtained from
patients with advanced PCa (81/120 cases; 67.5%), the prognosis of
the patients with NNMT overexpression in this group was
investigated. Kaplan-Meier analysis demonstrated that high NNMT
expression was significantly associated with significantly
prolonged PFS (P=0.009) and OS (P<0.001) times (Fig. 2B and C). In addition, the five-year
OS rate was significantly higher in the NNMT high expression group
compared with the NNMT low expression group [5/31 (16.1%) vs. 26/50
(52%); P=0.026]. Furthermore, these associations were confirmed by
Cox univariate analysis [PFS: hazard ratio (HR), 0.54; 95%
confidence interval (CI), 0.33–0.87; P=0.012; and OS: HR, 0.44; 95%
CI, 0.27–0.71; P=0.001] and multivariate analysis, with adjustments
for age, Gleason score, serum PSA level, distant metastasis, and
lymph node metastasis (PFS: HR, 0.37; 95% CI, 0.20–0.67; P=0.001;
and OS: HR, 0.29; 95% CI, 0.15–0.57; P<0.001) (Table II).
 | Table IICox univariate and multivariate
analysis of PFS and OS according to NNMT expression. |
Table II
Cox univariate and multivariate
analysis of PFS and OS according to NNMT expression.
| Univariate (PFS) | Multivariate
(PFS) | Univariate (OS) | Multivariate
(OS) |
|---|
|
|
|
|
|
|---|
| Variables | HR | P-value | HR | P-value | HR | P-value | HR | P-value |
|---|
| Age, years (<60
vs. ≥60) | 1.27 (0.79–2.05) | 0.322 | 1.21 (0.75–1.97) | 0.435 | 1.50 (0.93–2.42) | 0.099 | 1.81 (1.08–3.03) | 0.024a |
| Gleason score (5–6
vs. 7 or 8–10) | 1.54 (1.07–2.22) | 0.021a | 1.59 (1.05–2.41) | 0.028a | 1.71 (1.17–2.49) | 0.005a | 1.78 (1.07–2.62) | 0.023a |
| PSA, ng/ml (≤10 vs.
>10) | 2.70 (1.32–5.53) | 0.007a | 5.91
(2.52–13.85) | <0.001a | 2.20 (1.12–4.29) | 0.021a | 6.09
(2.66–18.96) | <0.001a |
| Distant metastasis
(yes vs. no) | 2.24 (1.30–3.86) | 0.004a | 2.53 (1.44–4.45) | 0.001a | 2.01 (1.20–3.35) | 0.008a | 2.29 (1.34–3.90) | 0.002a |
| Lymph node metastasis
(yes vs. no) | 1.82 (1.08–3.06) | 0.024a | 2.03 (1.19–3.45) | 0.010a | 2.19 (1.29–3.72) | 0.004a | 2.59 (1.51–4.47) | 0.001a |
| NNMT expression (high
vs. low) | 0.54 (0.33–0.87) | 0.012a | 0.37 (0.20–0.67) | 0.001a | 0.44
(0.27–0.71) | 0.001a | 0.29
(0.15–0.57) | <0.001a |
Discussion
To the best of our knowledge, this study was the
first to identify, using immunohistochemistry, that NNMT expression
was significantly increased in HGPIN and PCa tissues compared with
BPH tissues. Notably, NNMT expression was found to negatively
correlate with the Gleason score. Furthermore, in advanced PCa,
patients with high NNMT expression had significantly prolonged PFS
and OS times compared with those exhibiting low NNMT
expression.
NNMT catalyzes the transmethylation from
S-adenosylmethionine to nicotinamide and to certain other
azaheterocycles, playing a critical role in the biotransformation
and detoxification of numerous xenobiotics. Usually, NNMT is highly
expressed in the liver, whereas low expression has been identified
in the kidneys, lungs, skeletal muscle, placenta, heart and brain.
However, abnormal NNMT expression has been identified in several
types of tumors, including glioblastoma (16), stomach adenocarcinoma (17), papillary thyroid cancers (18), colorectal cancer (12), hepatocellular carcinoma (19), lung cancer (11) and renal cancer (20), suggesting that NNMT may have a
significant role in cancer. Among non-neoplastic disorders, the
enhanced expression of NNMT has been detected in chronic
obstructive pulmonary disease (21), atherosclerosis (22), and Parkinson’s disease (23).
In the present study, the expression of the NNMT
enzyme was investigated in different prostatic tissues; those of
BPH, HGPIN and PCa. While absent or weak in the majority of the BPH
tissues, NNMT expression was significantly increased in the HGPIN
and PCa tissues. HGPIN is the only premalignant precursor to PCa
that has been identified. Similar genetic and molecular changes in
HGPIN and PCa have been found (24). Notably, in the cancer tissues, this
overexpression was more evident in the well-differentiated cases
than in the undifferentiated cases. Furthermore,
clinicopathological correlation analysis indicated that NNMT
expression level was significantly associated with the Gleason
score. Thus, these results suggested that the enzyme may be
important in an initial step of the malignant conversion of PCa.
These results were consistent with previous studies on oral
squamous cell carcinoma (25).
The majority of advanced-stage PCa cases progress to
castration-resistant prostate cancers. The treatment of advanced
PCa remains a significant challenge. Establishing effective
prognostic biomarkers may provide more information for the
treatment of advanced PCa. The present study revealed that patients
with tumors expressing high levels of NNMT exhibited a
significantly prolonged PFS and OS times, which was also confirmed
by multivariate analysis following adjustments for conventional
prognostic factors. Therefore, aggressive treatment may be useful
for advanced PCa patients that express low levels of NNMT.
Considering the observation that NNMT expression is increased in
HGPIN and well-differentiated PCa compared with BPH, we hypothesize
that NNMT may exhibit different roles in the early stages of
prostate cancer and during its progression toward metastatic or
castrate-resistant states. Thus, we hypothesize that the underlying
mechanisms for NNMT involvement in primary tumors may be distinct
from metastatic tumors, and may function via different signaling
pathways.
Due to its catalytic activity, NNMT may be involved
in the regulation of intracellular levels of nicotinamide,
1-methylnicotinamide and S-adenosyl methionine. Nicotinamide
is involved in the production of the coenzymes NAD(H) and NADP(H),
which are essential for cellular functions. Several enzymes, which
use NAD as substrate, including ADP-ribosyltransferases, CD38 and
sirtuins, contain a nicotinamide-product site and may be inhibited
by nicotinamide (26). In PCa
cells, Sirtuin type 1 inhibition by nicotinamide has been shown to
result in the significant inhibition of growth and viability, while
exhibiting no effect on normal prostate epithelial cells (27). As a result of this inhibition, the
salvage and/or elimination of nicotinamide is crucial for NAD
metabolism, and thus, the NNMT enzyme may exhibit a crucial role in
the regulation of these cellular events. NNMT activity may also be
significant for the regulation of biological processes associated
with 1-methylnicotinamide. In a previous study, overexpressing NNMT
in SH-SY5Y neuroblastoma cells or incubating the cells with
1-methylnicotinamide significantly decreased the cell death rate,
which was found to correlate with increased intracellular ATP
content, ATP/ADP ratio and complex I activity, and reduced the
degradation of complex I inhibitors (28), thus suggesting that
1-methylnicotinamide may mediate the cellular effects of NNMT.
Recently, Ulanovskaya et al (29) showed that in cancer cells with high
NNMT expression, NNMT impairs the methylation potential by
consuming methyl units from S-adenosylmethionine, in order
to generate the stable metabolic product, 1-methylnicotinamide.
Consequently, NNMT-expressing cancer cells exhibit an altered
epigenetic state, including hypomethylated histones and additional
cancer-associated proteins combined with the increased expression
of protumorigenic gene products. Therefore, inhibition of NNMT is
considered to enhance the methylation potential and thereby may
exhibit therapeutic benefits in cancers associated with
insufficient histone methylation.
Based on previous studies, it appears that, via
abnormal enzyme expression, the tumor cells regulate cellular
processes associated with nicotinamide or 1-methylnicotinamide
concentration or control transmethylation reactions using
S-adenosylmethionine (25).
In the present study on advanced PCa, improved PFS and OS times
were revealed for patients bearing tumors with higher NNMT
expression levels compared with patients with tumors with lower
NNMT expression. Thus, the identification of the NNMT expression
status is likely to aid the prediction of the prognosis for each
patient.
In conclusion, the present study suggests that,
although a preliminary result, NNMT expression has significant
potential for the development of PCa treatment. In addition, high
NNMT expression levels are considered to result in increased PFS
and OS times for patients with advanced PCa. Further studies are
required to assess its promising role as a novel therapeutic drug
target or prognosis biomarker for PCa.
Acknowledgements
This study was supported by the Fundamental Research
Funds for the Central Universities of Central South University
(grant no. 72150050368).
References
|
1
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010.
|
|
2
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010.
|
|
3
|
Ross RW, Oh WK, Xie W, et al: Inherited
variation in the androgen pathway is associated with the efficacy
of androgen-deprivation therapy in men with prostate cancer. J Clin
Oncol. 26:842–847. 2008.
|
|
4
|
Debes JD and Tindall DJ: Mechanisms of
androgen-refractory prostate cancer. N Engl J Med. 351:1488–1490.
2004.
|
|
5
|
Chen CD, Welsbie DS, Tran C, et al:
Molecular determinants of resistance to antiandrogen therapy. Nat
Med. 10:33–39. 2004.
|
|
6
|
Choueiri TK, Xie W, D’Amico AV, et al:
Time to prostate-specific antigen nadir independently predicts
overall survival in patients who have metastatic hormone-sensitive
prostate cancer treated with androgen-deprivation therapy. Cancer.
115:981–987. 2009.
|
|
7
|
Bickers B and Aukim-Hastie C: New
molecular biomarkers for the prognosis and management of prostate
cancer - the post PSA era. Anticancer Res. 29:3289–3298. 2009.
|
|
8
|
Shariat SF, Karam JA and Roehrborn CG:
Blood biomarkers for prostate cancer detection and prognosis.
Future Oncol. 3:449–461. 2007.
|
|
9
|
Aksoy S, Szumlanski CL and Weinshilboum
RM: Human liver nicotinamide N-methyltransferase. cDNA cloning,
expression, and biochemical characterization. J Biol Chem.
269:14835–14840. 1994.
|
|
10
|
Rini J, Szumlanski C, Guerciolini R and
Weinshilboum RM: Human liver nicotinamide N-methyltransferase:
ion-pairing radiochemical assay, biochemical properties and
individual variation. Clin Chim Acta. 186:359–374. 1990.
|
|
11
|
Tomida M, Mikami I, Takeuchi S, Nishimura
H and Akiyama H: Serum levels of nicotinamide N-methyltransferase
in patients with lung cancer. J Cancer Res Clin Oncol.
135:1223–1229. 2009.
|
|
12
|
Roessler M, Rollinger W, Palme S, et al:
Identification of nicotinamide N-methyltransferase as a novel serum
tumor marker for colorectal cancer. Clin Cancer Res. 11:6550–6557.
2005.
|
|
13
|
Sartini D, Pozzi V, Renzi E, et al:
Analysis of tissue and salivary nicotinamide N-methyltransferase in
oral squamous cell carcinoma: basis for the development of a
noninvasive diagnostic test for early-stage disease. Biol Chem.
393:505–511. 2012.
|
|
14
|
Sartini D, Muzzonigro G, Milanese G, et
al: Upregulation of tissue and urinary nicotinamide
N-methyltransferase in bladder cancer: potential for the
development of a urine-based diagnostic test. Cell Biochem Biophys.
65:473–483. 2013.
|
|
15
|
Allred DC, Clark GM, Elledge R, et al:
Association of p53 protein expression with tumor cell proliferation
rate and clinical outcome in node-negative breast cancer. J Natl
Cancer Inst. 85:200–206. 1993.
|
|
16
|
Markert JM, Fuller CM, Gillespie GY, et
al: Differential gene expression profiling in human brain tumors.
Physiol Genomics. 5:21–33. 2001.
|
|
17
|
Jang JS, Cho HY, Lee YJ, Ha WS and Kim HW:
The differential proteome profile of stomach cancer: identification
of the biomarker candidates. Oncol Res. 14:491–499. 2004.
|
|
18
|
Xu J, Moatamed F, Caldwell JS, et al:
Enhanced expression of nicotinamide N-methyltransferase in human
papillary thyroid carcinoma cells. J Clin Endocrinol Metab.
88:4990–4996. 2003.
|
|
19
|
Kim J, Hong SJ, Lim EK, et al: Expression
of nicotinamide N-methyltransferase in hepatocellular carcinoma is
associated with poor prognosis. J Exp Clin Cancer Res.
28:202009.
|
|
20
|
Tang SW, Yang TC, Lin WC, et al:
Nicotinamide N-methyltransferase induces cellular invasion through
activating matrix metalloproteinase-2 expression in clear cell
renal cell carcinoma cells. Carcinogenesis. 32:138–145. 2011.
|
|
21
|
Kim HC, Mofarrahi M, Vassilakopoulos T, et
al: Expression and functional significance of nicotinamide N-methyl
transferase in skeletal muscles of patients with chronic
obstructive pulmonary disease. Am J Respir Crit Care Med.
181:797–805. 2010.
|
|
22
|
Mateuszuk Ł, Khomich TI, Słomińska E, et
al: Activation of nicotinamide N-methyltrasferase and increased
formation of 1-methylnicotinamide (MNA) in atherosclerosis.
Pharmacol Rep. 61:76–85. 2009.
|
|
23
|
Williams AC and Ramsden DB: Autotoxicity,
methylation and a road to the prevention of Parkinson’s disease. J
Clin Neurosci. 12:6–11. 2005.
|
|
24
|
Sakr WA and Partin AW: Histological
markers of risk and the role of high-grade prostatic
intraepithelial neoplasia. Urology. 57:115–120. 2001.
|
|
25
|
Emanuelli M, Santarelli A, Sartini D, et
al: Nicotinamide N-Methyltransferase upregulation correlates with
tumour differentiation in oral squamous cell carcinoma. Histol
Histopathol. 25:15–20. 2010.
|
|
26
|
Zhang J: Are poly(ADP-ribosyl)ation by
PARP-1 and deacetylation by Sir2 linked? Bioessays. 25:808–814.
2003.
|
|
27
|
Jung-Hynes B, Nihal M, Zhong W and Ahmad
N: Role of sirtuin histone deacetylase SIRT1 in prostate cancer. A
target for prostate cancer management via its inhibition? J Biol
Chem. 284:3823–3832. 2009.
|
|
28
|
Parsons RB, Aravindan S, Kadampeswaran A,
et al: The expression of nicotinamide N-methyltransferase increases
ATP synthesis and protects SH-SY5Y neuroblastoma cells against the
toxicity of Complex I inhibitors. Biochem J. 436:145–155. 2011.
|
|
29
|
Ulanovskaya OA, Zuhl AM and Cravatt BF:
NNMT promotes epigenetic remodeling in cancer by creating a
metabolic methylation sink. Nat Chem Biol. 9:300–306. 2013.
|