Introduction
Periductal stromal sarcoma (PSS) is an extremely
rare neoplasm arising in the connective tissue of the breast,
specifically from the periductal stroma. PSS is a distinct,
low-grade breast sarcoma with no clinical or radiological
specificity. It has a biphasic morphology with benign ductal
elements and a sarcomatous stroma lacking phyllodes tumor
architecture, and thus is difficult to diagnose. The clinical
presentation of a breast mass is non-specific and does not aid in
the differentiation of this tumor from benign or malignant lesions.
Similar to phyllodes tumors, PSS has a tendency for local
recurrence when incompletely excised, and a potential to develop
specific soft tissue sarcomas, as well as metastasis in cases
harboring more aggressive sarcomatous patterns (1–5).
Surgery with safe margins is the preferred therapeutic method for
treating PSS, but the efficacy of adjuvant treatment (such as
chemotherapy or radiotherapy) is yet to be clearly determined
(1,2). Patient provided written informed
consent.
Case report
A 43-year-old woman, with no history of disease,
presented to The Affiliated Cancer Hospital of Guangxi Medical
University (Nanning, China) with a recurrence of nodules in the
left breast. A pathological diagnosis of phyllodes tumor had been
confirmed following lumpectomy at the People’s Hospital of Wuming
(Nanning, China) 5 months previously. Upon presentation to our
hospital, clinical examination identified a small mass measuring
~2.5 cm in diameter (ellipse in shape) in the upper outer quadrant
of the left breast, and a large mass measuring ~5 cm in diameter
(irregular shape) in the upper quadrant of the left breast adjacent
to the areola with no signs of inflammation or association with
axillary lymph nodes. Magnetic resonance imaging and molybdenum
target computed radiography showed that the nodules of the left
breast exhibited characteristics of phyllodes tumor or breast
cancer; therefore, the patient underwent a core needle biopsy.
Microscopic examination revealed mammary gland, acini and
interstitial fibrous tissue hyperplasia, mild atypia and no obvious
mitosis. Immunohistochemical analysis showed that the ductal
epithelial cells were cytokeratin (CK)5/6-positive and less
reactive for Ki-67 (~1% positive), while the stromal cells were
~20% positive for Ki-67. Comprehensive analysis of the patient’s
medical history and immunohistochemical results suggested recurrent
phyllodes tumor. Subsequently, the patient underwent lumpectomy of
the left breast.
Grossly, the tumor of the upper quadrant of the left
breast was a gray, solid and tenacious mass measuring 5.5×2.5×2 cm
in size, with no significant hemorrhage or necrosis. The mass
located in the upper quadrant of the left breast adjacent to the
areola was part of the breast envelope, gray, solid and tenacious
with no significant hemorrhage or necrosis, measuring 6.5×3.5×3 cm
in size.
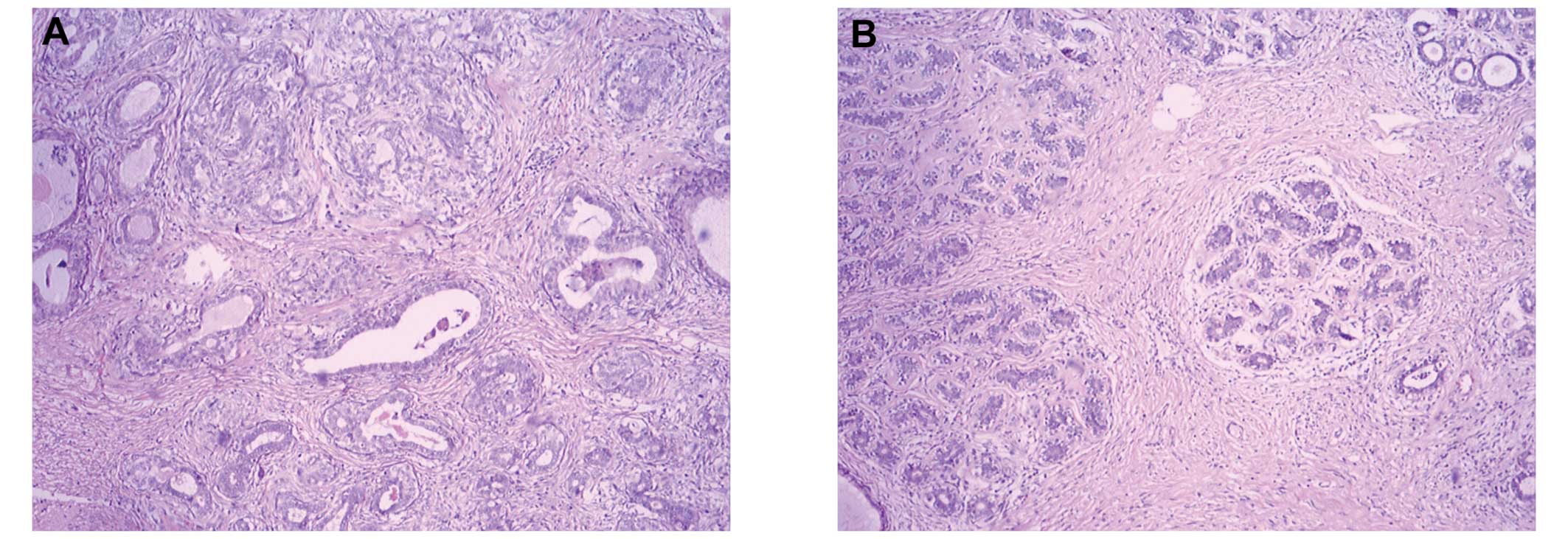
Microscopic examination revealed no leafy
architecture, however, biphasic proliferation composed of
epithelial and mesenchymal components, predominantly polygonal
(epithelioid) cells and spindle cell stromal proliferation around
the epithelial (myoepithelial layer of ducts or ductules devoid of
a phyllodes architecture) were observed, determining a diagnosis of
PSS (low-grade, spindle and epithelioid types). The tissue sections
were morphologically similar to those obtained during lumpectomy at
the People’s Hospital of Wuming 5 months previously. More than 10
stromal mitotic figures per 10 high power fields were observed in
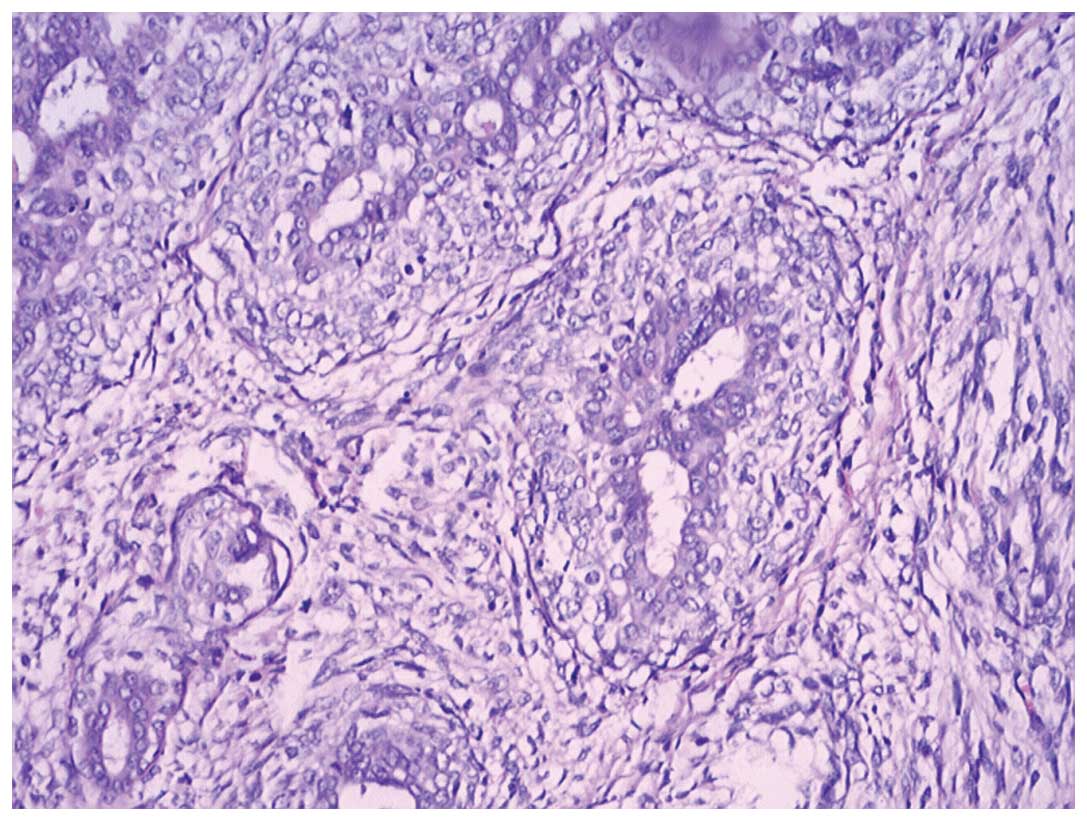
the tissue from the second lumpectomy only (Figs. 1 and 2). Immunohistochemical analysis revealed
that the glandular epithelial cells were CK19- and CK5/6-positive,
the myoepithelial cells were smooth muscle antibody-, smooth muscle
myosin heavy chain- and S-100-positive and glial fibrillary acidic
protein-negative, and the tumor cells were cluster of
differentiation (CD)34-positive and estrogen receptor (ER)- and
progesterone receptor (PR)-negative (Fig. 3). Whether the resection margins were
negative was not determined. The patient underwent a simple
mastectomy of the left breast.
Microscopic examination revealed mesenchymal cell
proliferation and no adjuvant treatment was administered. In a
follow-up period of 9 months, the patient did not show any symptoms
or signs of local or distant recurrence.
Discussion
PSS was previously considered to be a variant of
cystosarcoma with adipose metaplasia (1,3–5);
however, currently, PSS is recognized as a separate entity and was
classified by the World Health Organization in 2002 (6).
PSS occurs in perimenopausal and postmenopausal
women (median age, 55.3 years) who are a decade older compared with
those presenting with phyllodes tumor (median age, 45 years)
(1). The common symptoms of PSS are
similar to other benign and malignant breast tumors and have no
radiological specificity (1). In
the present case, the progress of PSS was similar to that of a
malignant breast tumor and radiological examination led to a
diagnosis of phyllodes tumor or breast cancer. A core needle biopsy
and pathological examination were unable to conclude a
diagnosis.
For middle-aged women presenting with breast lumps,
clinicians must eliminate phyllodes tumor, which is an uncommon
biphasic breast tumor with the ability to recur and metastasize and
is biologically comparable to a stromal neoplasm. Similar to
phyllodes tumor, PSS has a tendency for local recurrence when
incompletely excised as well as a potential to develop specific
soft tissue sarcomas and metastasize (1). Histologically, PSS is a biphasic
breast tumor with benign ductal elements and a sarcomatous stroma
lacking phyllodes tumor architecture (6). Thus, in this case, the patient was
diagnosed with phyllodes tumor, following initial lumpectomy, which
was then considered to have recurred after 5 months.
The histological characteristics of PSS were defined
by the Armed Forces Institute of Pathology (AFIP) (1) as follows: i) A predominant spindle
cell proliferation of variable cellularity and atypia around open
tubules and ducts devoid of a phyllodes tumor pattern; ii) one or
multiple nodules separated by adipose tissue; iii) stromal mitotic
activity of ≥3/10 high-power fields; and iv) stromal infiltration
into the surrounding breast tissue.
The histological grading depends on atypia and
mitotic activity and ranges from low- to high-grade PSS (1). Immunohistochemically, PSS is
CD34-positive and lacks S-100, ER and PR expression (6–9).
PSS is a tumor of intermediate behavior, resection
with significant margins is generally considered sufficient and
axillary lymphadenectomy is not required. With regard to adjuvant
therapy, the currently available literature does not suggest any
benefit of radiotherapy or chemotherapy. In the present case, the
recurred nodules of the left breast following a lumpectomy 5 months
before may be a result of resection performed without adequate
margins. The patient underwent lumpectomy at our hospital, but as
it was unclear whether the resection was performed with sufficient
margins, the patient underwent further surgery. Microscopic
examination of the breast tissue revealed mesenchymal cell
proliferation; thus, the simple mastectomy of the left breast may
have prevented future recurrence.
The tendency of PSS to recur and progress into
phyllodes tumor or soft tissue sarcomas, as well as the occasional
appearance of intraepithelial changes ranging from ordinary
hyperplasia to intraductal carcinoma (1,2,9),
indicate that close follow-up is required. The patient in the
present case is currently recurrence-free 9 months after
treatment.
In conclusion, the number of available studies on
PSS is currently limited and a therapeutic strategy for PSS has yet
to be determined. Histological diagnosis of PSS is based on the
criteria established by the AFIP, and surgery with significant
margins is the cornerstone of treatment. The prognosis of patients
with PSS remains unclear; thus, increased experience of such cases
and a longer follow-up period are required to investigate the
optimal management and clinical behavior of this neoplasm.
Abbreviations:
|
PSS
|
periductal stromal sarcoma
|
References
|
1
|
Burga AM and Tavassoli FA: Periductal
stromal tumor: a rare lesion with low-grade sarcomatous behavior.
Am J Surg Pathol. 27:343–348. 2003.
|
|
2
|
Rao AC, Geetha V and Khurana A: Periductal
stromal sarcoma of breast with lipoblast-like cells: a case report
with review of literature. Indian J Pathol Microbiol. 51:252–254.
2008.
|
|
3
|
Powell CM and Rosen PP: Adipose
differentiation in cystosarcoma phyllodes. A study of 14 cases. Am
J Surg Pathol. 18:720–727. 1994.
|
|
4
|
Oberman HA, Nosanchuk JS and Finger JE:
Periductal stromal tumors of breast with adipose metaplasia. Arch
Surg. 98:384–387. 1969.
|
|
5
|
Callery CD, Rosen PP and Kinne DW: Sarcoma
of the breast. A study of 32 patients with reappraisal of
classification and therapy. Ann Surg. 201:527–532. 1985.
|
|
6
|
Tavassoli FA and Devilee P: World Health
Classification of Tumours Pathology and Genetics of Tumours of the
Breast and Female Genital Organs. IARC Press; Lyon: 2003
|
|
7
|
Tomas D, Janković D, Marusić Z, Franceschi
A, Mijić A and Kruslin B: Low-grade periductal stromal sarcoma of
the breast with myxoid features: Immunohistochemistry. Pathol Int.
59:588–591. 2009.
|
|
8
|
Chen CM, Chen CJ, Chang CL, Shyu JS, Hsieh
HF and Harn HJ: CD34, CD117, and actin expression in phyllodes
tumor of the breast. J Surg Res. 94:84–91. 2000.
|
|
9
|
Masbah O, Lalya I, Mellas N, et al:
Periductal stromal sarcoma in a child: a case report. J Med Case
Rep. 5:2492011.
|