Introduction
The extent of lymph node involvement is one of the
major determinants for the staging and prognosis of melanoma
(1). Until recently, lymph node
colonization by tumor cells was proposed to be a passive process
involving tumor cell spread through pre-existing afferent lymphatic
vessels. However, it is now well established that tumor cells, as
inflammatory cells of the tumor environment, contribute to
lymphatic dissemination through the de novo formation of
lymphatic capillaries, a phenomenon termed lymphangiogenesis
(2–4). Among the members of the vascular
endothelial growth factor (VEGF) family, VEGF-C has been identified
as the major lymphangiogenic growth factor required for lymphatic
development (5,6). VEGF-C-overexpressing tumors not only
exhibit an increase in intratumoral lymphangiogenesis, but also an
increased number of functional peritumoral lymphatic vessels
(7). Furthermore, patients with
tumors expressing high levels of VEGF-C have been reported to be
more likely to have advanced disease and lymph node metastasis
compared with those with tumors expressing low levels of VEGF-C
(8). Among the inflammatory host
cells, tumor-associated macrophages (TAMs) have been found to
release high levels of VEGF-C (9)
and VEGF-C has been reported to promote macrophage recruitment, in
addition to lymphangiogenesis, in VEGF-C-overexpressing human
melanoma cells transplanted into nude mice (10). Kerjaschki (11) demonstrated that macrophages promote
lymphangiogenesis either through transdifferentiating and
incorporating into the endothelial layer or through stimulating the
proliferation of pre-existing local lymphatic endothelial cells
(11).
The present study aimed to elucidate the mechanisms
involved in VEGF-C expression in TAMs, in order to identify novel
approaches to inhibit lymph node dissemination. In vivo
tumor cell-macrophage interactions were mimicked through
co-culturing B16 murine melanoma cells with syngeneic peritoneal
macrophages.
Materials and methods
Materials
Unless specified, all reagents were obtained from
Sigma-Aldrich (St. Louis, MO, USA). All products for reverse
transcription polymerase chain reaction (RT-PCR) analysis were
purchased from Promega Corporation (Madison, WI, USA). Recombinant
human and murine cytokines were purchased from PeproTech (Rocky
Hill, NJ, USA).
Cell lines and culture conditions
In the present study, a clone isolated from a
B16-F10 melanoma cell line (F10-M3 cells), kindly supplied by Dr.
S. Gattoni-Celli (Medical University of South Carolina, Charleston,
SC, USA), was used. Cells were cultivated in Dulbecco’s modified
Eagle’s medium (DMEM 4500; Gibco-BRL, Grand Island, NY, USA)
supplemented with 10% fetal calf serum (Boehringer Ingelheim,
Ingelheim, Germany), at 37°C in a humidified atmosphere containing
10% CO2. Cells were harvested from subconfluent cultures
through incubation with a trypsin-EDTA solution and propagated
every three days. B16 melanoma cells were exposed to TNF-α (400,
800 or 1200 U/ml) and/or IL-1β (300, 600 or 900 U/ml) for 24 h and
H2O2 (200 uM) for 4 h. Cell viability was
determined using the trypan blue exclusion test. Cultures were
periodically monitored for mycoplasma contamination using Chen’s
fluorochrome test (12).
Macrophage cultures and macrophage-tumor
cell co-cultures
Cultures of inflammatory macrophages were
established from peritoneal exudates collected through lavage from
C57Bl/6 mice (Charles River Laboratories, Wilmington, MA, USA)
between six and eight weeks old, that had been injected
intraperitoneally with 1 ml 3% thioglycollate broth 3–4 days
previously (13). Co-cultures were
prepared through seeding tumor cells on macrophage monolayers at a
1:1 ratio at a density of 250×103 cells/cm2.
This cell density was selected based on previous experiments and in
order to establish close contact between the macrophages and the
tumor cells (14).
Macrophage monolayers incubated for 2 h in DMEM 4500
containing 250 μg/ml bovine serum albumin were exposed to the
following cytokines: Interferon γ (50 U/ml), TNF-α (50 ng/ml),
transforming growth factor (TGF)-β (25 ng/ml), IL-4 (100 U/ml),
IL-10 (10 ng/ml), lipopolysaccharide (10 ng/ml) or IL-1β (100
U/ml). For antibody treatment, tumor cell-macrophage co-cultures
were exposed to either 1 μg/ml rabbit anti-mouse polyclonal
anti-IL-1β receptor 1 (sc-689; Santa Cruz Biotechnology, Inc.,
Santa Cruz, CA, USA) and hamster anti-mouse monoclonal anti-TNF
R1-p55 (sc-12746; Santa Cruz Biotechnology, Inc.) antibodies or
irrelevant IgG (Santa Cruz Biotechnology, Inc.) for 24 h.
RNA isolation and RT-PCR
Total RNA was extracted from cells using TRI
Reagent®. The quantity and purity of the RNA was
determined spectrophotometrically. Complementary DNA (cDNA)
synthesis was performed through incubating 1 μg total RNA with 4
U/μl M-MLV reverse transcriptase. Aliquots of 5 μl cDNA were used
for the PCR amplification. The RT-PCR reactions were performed in a
50 μl reaction volume containing specific primers (Table I) and 0.1 U/μl GoTaq®
Polymerase using a Perkin-Elmer Thermal cycler. Aliquots of 10 μl
PCR reaction were applied to a 2% agarose gel, electrophoresed and
visualized. cDNA products were analyzed on the basis of standard
PCR markers.
 | Table IPrimer sequences used for reverse
transcription polymerase chain reaction analysis. |
Table I
Primer sequences used for reverse
transcription polymerase chain reaction analysis.
| Gene | Abbreviation | Forward primer
5′-3′ | Reverse primer
5′-3′ | Tm (°C) |
|---|
| Transforming growth
factor beta | TGF-β |
GGCTTCTAGTGCTGACG |
GGGTGCTGTTGTACAAAG | 54 |
| Tumor necrosis factor
alpha | TNF-α |
GCGGTGCCTATGTCTCAGCC |
TGAGGAGCACGTAGTCGGGG | 60 |
| Vascular endothelial
growth factor C | VEGF-C |
CCATGCACTTGCTGTGCTTC |
ACCGGCAGGAAGTGTGATTG | 59 |
| Interleukin 1
beta | IL-1β |
CCTGCAGCTGGAGAGTGTGGA |
CCCATCAGAGGCAAGGAGGAA | 60 |
| Beta 2
microglobulin | β2m |
TGCTATCCAGAAAACCCCTC |
GTCATGCTTAACTCTGCAGG | 58 |
| Interleukin 1
receptor 1 | IL-1R1 |
ACCCCCATATCAGCGGACCG |
TTGCTTCCCCCGGAACGTAT | 58 |
| Tumor necrosis factor
alpha receptor 1 | TNFaR1 |
GGATACAGTCTGCAGGGAGTG |
TCCACCGGGGATATCGGCACATTAA | 60 |
| Transforming growth
factor beta receptor 1A | TGFβR1A |
GAGCTCTGCAGTTGAGACGTTTAG |
AACAAAACACTGCTTTGATCAAGTA | 58 |
In vivo experiments
Subconfluent cultures of B16 melanoma cells were
harvested using EDTA/trypsin solution, centrifuged at 200 × g and
resuspended in DMEM medium at 2×106 cells/ml. A total of
0.5 ml cell suspension was injected into the peritoneal cavity of
syngeneic C57Bl/6 mice. Mice were sacrificed by cervical
dislocation after seven days and the peritoneal lymph nodes were
removed. Lymph node colonization by tumor cells was determined
using a dissecting microscope, with lymph nodes which were
colonized by murine melanoma cells observed to be enlarged and
brown for melanin. B16 melanoma cell-colonized lymph nodes were
weighed and formalin fixed (5% in phosphate-buffered saline) to
assess the tumor burden, as well as for histological examination
following hematoxylin and eosin staining. In vivo
experiments were performed in agreement with the national
guidelines and approved by the ethical committee of Animal Welfare
Office of Italian Work Ministry (Rome, Italy) and conform to the
legal mandates and Italian guidelines for the care and maintenance
of laboratory animals
Statistical analysis
Statistical analysis was conducted using the SPSS
software, version 16.0 (SPSS, Inc, Chicago, IL, USA). Densitometric
data are expressed as the mean ± standard error of the mean
depicted by vertical error bars from at least three independent
experiments. Statistical analyses of the data were performed using
Student’s t-test and P≤0.05 was considered to indicate a
statistically significant difference.
Results
Inflammatory cytokine and VEGF-C
expression in macrophage cultures and macrophage-tumor cell
co-cultures
In order to mimic the multiple interactions that
tumor cells establish with macrophages during tumor growth, F10-M3
murine melanoma cells were co-cultured with syngeneic C57Bl/6
thioglycollate-elicited peritoneal macrophages at a high cell
density. After 24 h, the tumor cells were removed from the
co-cultures and collected. Macrophages adherent to the tissue
culture dishes were analyzed for VEGF-C expression. Trypan blue
exclusion test revealed that the tumor cells and macrophages had
95–97% cell viability. The number of tumor cells present in the
macrophage preparations was <0.1%, demonstrating that tumor
cells are easily removed from culture dishes using trypsin-EDTA
solution, whereas adherent macrophages require specific treatment,
for example using the anesthetic lidocaine, or scraping (15). Fig.
1A shows that VEGF-C expression in macrophages is stimulated
through contact with tumor cells, whereas there is no significant
change in VEGF-C expression in the tumor cells following
co-cultivation with macrophages.
 | Figure 1Inflammatory cytokines and VEGF-C
expression in murine peritoneal macrophages co-cultivated with
syngeneic B16 melanoma cells. (A) VEGF-C mRNA expressed by C57Bl/6
thioglycollate-elicited macrophages and B16 cells collected from
standard cultures or co-cultures. (B) mRNA expression of TNF-α,
IL-1β, IL-6, TGF-β and their receptors in murine macrophages grown
with tumor cells, as well as the relative densitometric analyses.
(C) VEGF-C mRNA expressed by murine thioglycollate-elicited
macrophages stimulated in vitro by several pro- and
anti-inflammatory cytokines and growth factors. (D) VEGF-C mRNA
expression in macrophages grown in media conditioned by
macrophage-tumor cell co-cultures in the absence or presence of
anti-IL-1βR1 and -TNF-αR1 antibodies. Densitometric analyses were
performed using Image J software and standardized to
β2-microglobulin mRNA expression. *P<0.05 vs.
control. VEGF, vascular endothelial growth factor; IL, interleukin;
TNF, tumor necrosis factor; TGF, transforming growth factor; β2-m,
β2-microglobulin; R, receptor; SEM, standard error of the mean. |
Based on the finding that macrophages express tumor
promoting properties associated with changes in cytokine production
upon contact with tumor cells and that VEGF-C is stimulated by
cytokines, the levels of a series of inflammatory cytokines and
their receptors were assessed in macrophages co-cultivated with
tumor cells. Macrophages exposed to tumor cells were observed to
express an enhanced level of IL-1β, TNF-α and theirs receptors, and
a reduced level of TGF-β receptor 1 (Fig. 1B). As shown in Fig. 1C, exogenous TNF-α and IL-1β were
found to promote VEGF-C expression in murine macrophages.
Furthermore, antibodies against IL-1β and TNF-α receptors added to
media conditioned by macrophage/tumor cell co-cultures were
observed to inhibit the enhanced expression of VEGF-C in
macrophages (Fig. 1D).
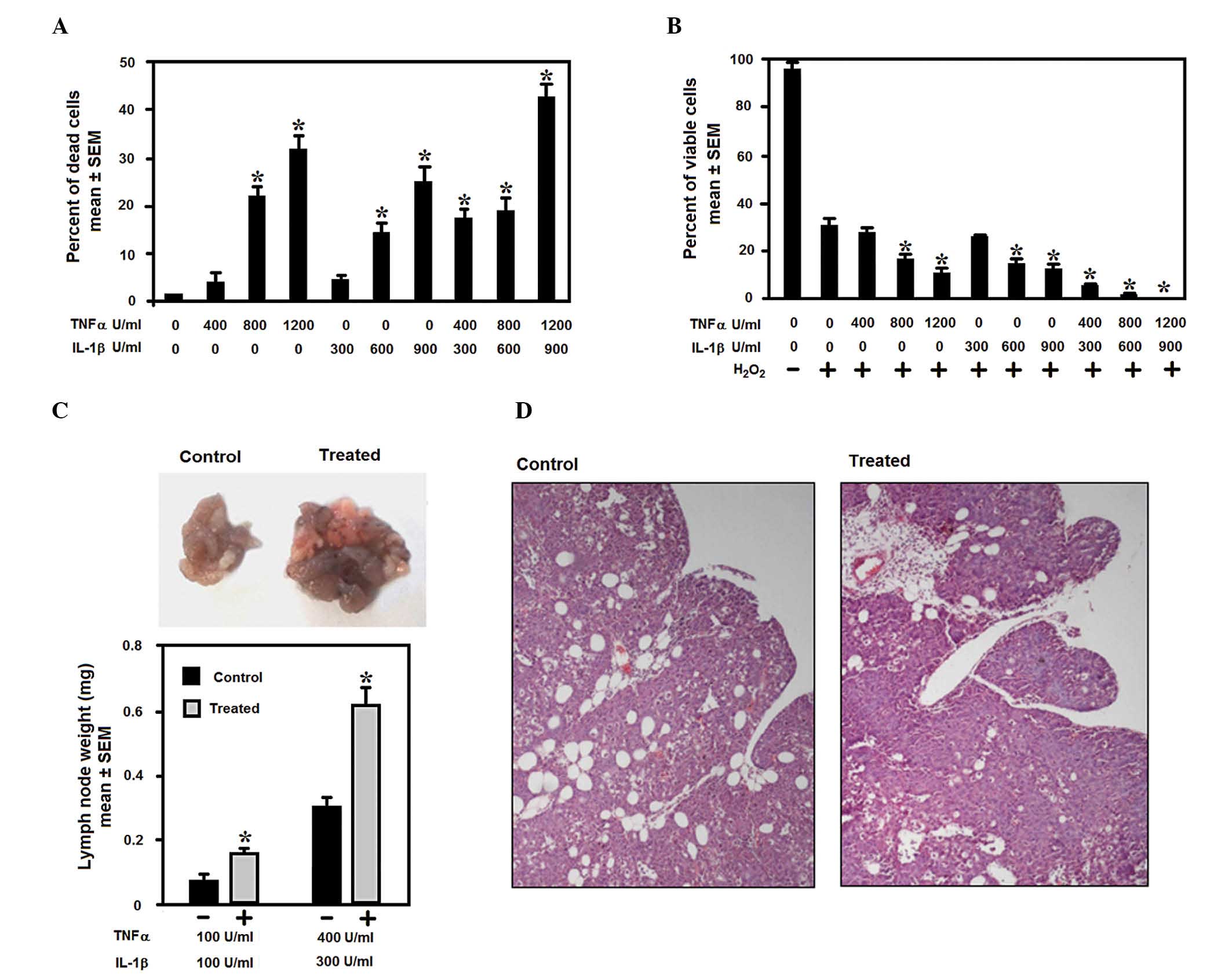
Effect of TNF-α and IL-1β on murine
melanoma cell viability and lymph node metastasis
Experimental data support the hypothesis that
inflammatory cytokines may either inhibit or facilitate tumor cells
depending on their concentration. Fig.
2A and B show a dose-response curve demonstrating that low
levels of IL-1β and TNF-α have minimal effect on cell viability,
whereas when the levels of IL-1β and TNF-α increase, a significant
reduction in cell proliferation and an increase in cell death is
promoted in melanoma cells, either in the absence or in the
presence of the pro-apoptotic signal, H2O2.
In the present study, following stimulation with low doses of IL-1β
and TNF-α, the melanoma cells were observed to exhibit an increased
capacity to colonize the peritoneal lymph nodes, as demonstrated by
the weight of the lymph nodes collected from the peritoneal
cavities of the injected mice, as well as histological examination
of the lymph nodes (Fig. 2C and D).
These findings suggest that macrophage IL-1β and TNF-α are
responsible for VEGF-C expression in TAMs, which may lead to
lymphangiogenesis and melanoma lymph node metastasis.
Discussion
Cancer cells acquire the capacity to recruit various
stromal cells, including endothelial precursor cells, fibroblasts
and monocytes during their progression to malignancy. Monocytes are
recruited from the peripheral blood in response to tumor-derived
chemokines, including monocyte chemotactic protein-1, which
stimulate adhesion to endothelial cells, transendothelial migration
and differentiation into macrophages (16). Macrophages in tumors are termed TAMs
and are versatile cells which may reversibly express diverse
functional states under the influence of host inflammatory cells
and tumor cells. TAMs may function as effector cells against tumor
growth, or may promote tumor aggression through releasing growth
factors, cytokines and proteinases (17,18).
TAMs are also the major contributor to tumor angiogenesis through
the secretion of VEGF-A and have a crucial role in
lymphangiogenesis through the secretion of VEGF-C.
The present study shows that, inflammatory
macrophages co-cultivated with B16 murine melanoma cells express
enhanced VEGF-C mRNA which was found to be associated with an
autocrine loop of activity between macrophage IL-1β and TNF-α and
their receptors. Furthermore, antibodies against the IL-1β and
TNF-α receptors, which were added to the media conditioned by
macrophages and tumor cells, were found to inhibit the enhancement
of VEGF-C expression in macrophages co-cultivated with tumor cells.
Thioglycollate-elicited peritoneal macrophages are inflammatory
macrophages which release no, or very low quantities of TNF-α and
IL-1β under unstimulated conditions. Thus, it is possible that
contact with tumor cells promotes a more advanced state of
activation in these macrophages. However, in the present study,
this level of activation was not observed to be cytotoxic for tumor
cells, as the viability of the tumor cells collected from the
macrophage-tumor cell co-cultures was not significantly different
compared with that of the cells grown in standard conditions.
Schoppmann et al (9)
revealed that a particular subfraction of cluster of
differentiation 14-positive and VEGFR-3-expressing monocytes
isolated from the peripheral blood of patients with cervical
cancer, acquire the capacity to express VEGF-C following
stimulation with TNF-α. By contrast, it has also been reported that
macrophages co-cultivated with colon or breast carcinoma cells do
not exhibit an increase in VEGF-C mRNA expression (19). It is possible that VEGF-C expression
in TAMs may be associated with the different tumor histotype. It
has been demonstrated that TNF-α and IL-1β may stimulate VEGF-C
expression in human fibroblasts and vascular endothelial cells
primarily through nuclear factor κ-light-chain-enhancer of
activated B cells (20). In
addition, an unexpected inducer of VEGF-C expression in human
melanoma cells was the low extracellular pH (21), which has been demonstrated to
enhance IL-1β production by monocytes (22).
In the present study, TNF-α and IL-1β were found to
cooperate to promote the colonization of peritoneal lymph nodes by
murine melanoma cells, when they were used at a non-toxic dose. A
high dose of TNF-α and IL-1β was found to induce tumor cell
cytotoxicity and reduce tumor cell resistance to the apoptotic
agent H2O2, in a synergistic manner. TNF-α
and IL-1β are the most potent pro-inflammatory cytokines produced
by TAMs which, depending on their concentration, may either induce
the expression of several genes resulting in the promotion of tumor
cell aggression, or exert a cytotoxic effect. In tumor cells, IL-1β
induces the secretion of growth- and invasion-promoting factors,
matrix-metalloproteases and angiogenic molecules, including VEGF-A
and basic fibroblast growth factor (23). Vidal-Vanaclocha et al
(24) showed that IL-1β promotes
experimental liver metastasis in B16 melanoma cells, while a
reduction in liver metastases was observed following treatment with
IL-1Ra. Moreover, experimental models of invasion and metastasis
have shown that TNF-α promotes melanoma dissemination (25). However, hemorrhagic necrosis of
tumors following TNF-α treatment was common and high doses of TNF-α
had to be injected locally and repeatedly (25). While IL-1β is not generally
considered to be a cytokine which mediates cytotoxic activity, a
previous study found that IL-1β had a direct cytotoxic effect on
tumor cells, with human monocyte-derived IL-1β found to be
growth-inhibitory and cytocidal in A375 melanoma cells (26).
In conclusion, the present study has shown that
macrophage IL-1β and TNF-α may promote VEGF-C expression in TAMs
and may have a role in melanoma lymph node colonization. Targeting
the signaling between TAMs and tumor cells in an inflammatory
environment may be critical for inhibiting the progression of
melanoma cells.
Acknowledgements
The present study was supported by grants from the
Tuscan Tumor Institute (Florence, Italy) and Ente Cassa di
Risparmio di Firenze (ECRF).
References
|
1
|
Dadras SS, Paul T, Bertoncini J, et al:
Tumor lymphangiogenesis: a novel prognostic indicator for cutaneous
melanoma metastasis and survival. Am J Pathol. 162:1951–1960.
2003.
|
|
2
|
Tammela T and Alitalo K:
Lymphangiogenesis: Molecular mechanisms and future promise. Cell.
140:460–76. 2010.
|
|
3
|
Alitalo K, Tammela T and Petrova TV:
Lymphangiogenesis in development and human disease. Nature.
438:946–953. 2005.
|
|
4
|
Achen MG, McColl BK and Stacker SA: Focus
on lymphangiogenesis in tumor metastasis. Cancer Cell. 7:121–127.
2005.
|
|
5
|
Karkkainen MJ, Haiko P, Sainio K, et al:
Vascular endothelial growth factor C is required for sprouting of
the first lymphatic vessels from embryonic veins. Nat Immunol.
5:74–80. 2004.
|
|
6
|
Su JL, Yen CJ, Chen PS, et al: The role of
the VEGF-C/VEGFR-3 axis in cancer progression. Br J Cancer.
96:541–545. 2007.
|
|
7
|
Ji RC: Lymphatic endothelial cells, tumor
lymphangiogenesis and metastasis: New insights into intratumoral
and peritumoral lymphatics. Cancer Metastasis Rev. 25:677–694.
2006.
|
|
8
|
Su JL, Yang PC, Shih JY, et al: The
VEGF-C/Flt-4 axis promotes invasion and metastasis of cancer cells.
Cancer Cell. 9:209–223. 2006.
|
|
9
|
Schoppmann SF, Birner P, Stöckl J, et al:
Tumor-associated macrophages express lymphatic endothelial growth
factors and are related to peritumoral lymphangiogenesis. Am J
Pathol. 161:947–956. 2002.
|
|
10
|
Skobe M, Hamberg LM, Hawighorst T, et al:
Concurrent induction of lymphangiogenesis, angiogenesis, and
macrophage recruitment by vascular endothelial growth factor-C in
melanoma. Am J Pathol. 159:893–903. 2001.
|
|
11
|
Kerjaschki D: The crucial role of
macrophages in lymphangiogenesis. J Clin Invest. 115:2316–2319.
2005.
|
|
12
|
Chen TR: In situ detection of mycoplasma
contamination in cell cultures by fluorescent Hoechst 33258 stain.
Exp Cell Res. 104:255–262. 1977.
|
|
13
|
Calorini L, Bianchini F, Mannini A, Mugnai
G and Ruggieri S: Enhancement of nitric oxide release in mouse
inflammatory macrophages co-cultivated with tumor cells of a
different origin. Clin Exp Metastasis. 22:413–419. 2005.
|
|
14
|
Bianchini F, Massi D, Marconi C, et al:
Expression of cyclo-oxygenase-2 in macrophages associated with
cutaneous melanoma at different stages of progression.
Prostaglandins Other Lipid Mediat. 83:320–328. 2007.
|
|
15
|
Marconi C, Bianchini F, Mannini A, et al:
Tumoral and macrophage uPAR and MMP-9 contribute to the
invasiveness of B16 murine melanoma cells. Clin Exp Metastasis.
25:225–231. 2008.
|
|
16
|
Varney ML, Johansson SL and Singh RK:
Tumour-associated macrophage infiltration, neovascularization and
aggressiveness in malignant melanoma: role of monocyte chemotactic
protein-1 and vascular endothelial growth factor-A. Melanoma Res.
15:417–425. 2005.
|
|
17
|
Condeelis J and Pollard JW: Macrophages:
obligate partners for tumor cell migration, invasion, and
metastasis. Cell. 124:263–266. 2006.
|
|
18
|
Qian BZ and Pollard JW: Macrophage
diversity enhances tumor progression and metastasis. Cell.
141:39–51. 2010.
|
|
19
|
Barbera-Guillem E, Nyhus JK, Wolford CC,
Friece CR and Sampsel JW: Vascular endothelial growth factor
secretion by tumor-infiltrating macrophages essentially supports
tumor angiogenesis, and IgG immune complexes potentiate the
process. Cancer Res. 62:7042–7049. 2002.
|
|
20
|
Ristimäki A, Narko K, Enholm B, Joukov V
and Alitalo K: Proinflammatory cytokines regulate expression of the
lymphatic endothelial mitogen vascular endothelial growth factor-C.
J Biol Chem. 273:8413–8418. 1998.
|
|
21
|
Peppicelli S, Bianchini F, Contena C,
Tombaccini D and Calorini L: Acidic pH via NF-κB favours VEGF-C
expression in human melanoma cells. Clin Exp Metastasis.
30:957–967. 2013.
|
|
22
|
Jancic CC, Cabrini M, Gabelloni ML,
Rodríguez Rodrigues C, Salamone G, Trevani AS and Geffner J: Low
extracellular pH stimulates the production of IL-1β by human
monocytes. Cytokine. 57:258–268. 2012.
|
|
23
|
Stetler-Stevenson WG and Yu AE: Proteases
in invasion: matrix metalloproteinases. Semin Cancer Biol.
11:143–152. 2001.
|
|
24
|
Vidal-Vanaclocha F, Alvarez A, Asumendi A,
Urcelay B, Tonino P and Dinarello CA: Interleukin 1
(IL-1)-dependent melanoma hepatic metastasis in vivo; increate
endothelial adherence by IL-1-induced mannose receptors and growth
factor production in vitro. J Natl Cancer Inst. 88:198–205.
1996.
|
|
25
|
Balkwill F: Tumour necrosis factor and
cancer. Nat Rev Cancer. 9:361–371. 2009.
|
|
26
|
Lachman LB, Dinarello CA, Llansa ND and
Fidler IJ: Natural and recombinant human interleukin 1-beta is
cytotoxic for human melanoma cells. J Immunol. 136:3098–3102.
1986.
|