Introduction
Prostate cancer (PCa) is the second most commonly
diagnosed cancer. Accounting for 14% (903,500) of the total number
of new cancer cases and 6% (258,400) of the total number of
cancer-related mortalities in males in 2008, PCa is the sixth
leading cause of cancer-related mortality in males (1). The growth and maintenance of cancerous
cells in the early stages of PCa is androgen-dependent, and therapy
often involves androgen deprivation (2). In the later stages, therapeutic
alternatives are not available, as the tumor becomes
androgen-independent. There is an association between our poor
understanding of the molecular mechanisms that underlie disease
progression with regard to invasion and metastasis, and the lack of
successful therapies for advanced PCa (3). PCa has a significant impact on
survival and patient quality of life due to its high potential for
metastasis to other areas of the body (4). Clinical observations have shown that
approximately one-third of PCas invade the surrounding tissues,
metastasize to distant organs and cause subsequent mortality,
despite current therapies. The survival of a patient with PCa is
directly associated with the spread of the tumor (5).
As ultrasound is non-invasive and perceived as safe,
with relatively low costs, the technique is widely used for the
imaging of soft tissues. Ultrasound can also be used
therapeutically; specifically, the technique has been investigated
in certain preclinical therapeutic studies and shown to mediate
apoptosis in numerous in vitro and in vivo
experimental systems (6–8). Furthermore, the greater susceptibility
of cancer cells to ultrasound therapy has been demonstrated in
comparison to normal cells (9,10),
resulting in its eventual application in the treatment of cancer.
In addition, enhancement of the apoptotic effect that ultrasound
induces in tumors may occur through the use of porphyrins,
anticancer drugs and microbubbles (10,11).
Ultrasound combined with microbubbles exhibits the ability to
induce tumor apoptosis, however, it remains unclear whether the
technique can inhibit cell invasion and metastasis.
In cancer cells, the matrix metalloproteinases
(MMPs) are overexpressed and are involved in the invasion and
metastasis of various cancer cells (12). MMP-2 and -9 are components of the
basement membrane and thus, their involvement is indicated in the
invasion and metastasis of malignant cancers. Therefore, the
inhibition of MMP activity is significant for the prevention of
cancer, particularly the cancer promotion process (13).
In the present study, an ultrasound frequency of 21
kHz was used, with a spatial-average temporal-average intensity
(ISATA) of 46 mW/cm2. Human PCa PC-3 cells were treated
with low-frequency and low-energy ultrasound combined with
microbubbles. Following treatment, the antimetastatic effect of the
ultrasound combined with microbubbles was determined in the PC-3
cells using migration and invasion assays. Additionally, changes in
the MMP-2 and MMP-9 mRNA and protein levels were evaluated.
Materials and methods
Cell culture
The androgen-independent human PCa PC-3 cell line
was obtained from the Cell Bank of the Chinese Academy of Sciences
(Shanghai, China). The cells were grown in Dulbecco’s modified
Eagle’s medium (DMEM; Gibco-BRL, Grand Island, NY, USA)
supplemented with 10% heat-inactivated fetal bovine serum
(Invitrogen Life Technologies, Carlsbad, CA, USA) at 37°C in a
humidified atmosphere containing 5% CO2. The PC-3 cells
were resuspended at a density of 1×106 cells/ml and
placed into 1.5-ml polystyrene test tubes. Each tube contained a
1-ml suspension of PC-3 cells. The tubes were 13 mm in diameter,
with planar bottoms, allowing them to be positioned closer to the
ultrasound probe.
Ultrasound apparatus and
microbubbles
Ultrasound treatment was performed using a FS-450
ultrasonic processor (Shanghai Institute of Ultrasound in Medicine,
Shanghai, China) in combination with the SonoVue microbubble
echo-contrast agent (Bracco Imaging SpA, Milan, Italy). The FS-450
ultrasonic processor was equipped with a built-in digital timer and
an intensity regulator. The probe frequency was fixed at 21 kHz,
with an intensity of 46 mW/cm2. In all experiments,
ultrasound was generated by a 21-kHz ultrasound probe using the
continuous wave mode. The duration of treatment was 30 sec. The
ultrasound probe was cylindrical with a diameter of 13 mm,
identical to that of the test tubes. The experimental setup for
ultrasound exposure has been shown in our previous study (14).
The SonoVue agent used was a lipid-shelled
ultrasound contrast agent composed of microbubbles filled with
sulfur hexafluoride gas. The microbubbles were 2.5–6.0 μm in
diameter. Prior to use, the SonoVue agent was reconstituted in 5 ml
of phosphate-buffered saline at a concentration of
2–5×108 microbubbles/ml.
In all experiments, the cells were divided into
three groups: The control group (no treatment); the ultrasound
group (US); and the ultrasound combined with microbubbles group (US
+ MB; 200 μl SonoVue). Each group contained three samples.
Measurement of cell proliferation
Following treatment, each group of cells was seeded
at a density of 3×103 cells/well in 96-well plates.
Following incubation for 12, 24, 48, or 72 h, 100 μl cell counting
kit-8 (Dojindo Laboratories, Kumamoto, Japan) was added. The plates
were then incubated for an additional 3 h. The optical density of
each well was measured using a microculture plate reader (Bio-Tek
Instruments, Inc., Winooski, VT, USA) at a wavelength of 450 nm
(15).
In vitro migration and invasion
assays
For the Transwell migration assays, following
treatment, 5×104 PC-3 cells were plated in the top
chamber with the non-coated membrane (24-well insert; pore size, 8
μm; Corning Inc., Lowell, MA, USA). For the invasion assays,
5×104 cells were plated in the top chamber with a
membrane (24 well insert; pore size, 8 μm; Corning Inc.) coated in
Matrigel (1 mg/ml; BD Biosciences, San Jose, CA, USA). In the two
assays, the cells were plated in serum-free DMEM medium, and medium
containing 10% serum was used as a chemoattractant in the lower
chamber. The cells were incubated for 12 h at 37°C in an atmosphere
of 5% CO2. Following incubation, a cotton swab was used
to remove the non-migrated cells in the upper chamber and the
filters were individually stained with 2% crystal violet (Beyotime
Institute of Biotechnology, Nantong, China). The migrated cells
adhering to the underside of the filter were examined, counted and
images were captured under a light microscope (magnification, ×200;
Olympus IX70; Olympus Corporation, Osaka, Japan) (16).
Quantitative polymerase chain reaction
(qPCR)
To quantitatively determine the mRNA expression
level qPCR was performed. The total RNA of each clone was extracted
using TRIzol (Invitrogen Life Technologies) according to the
manufacturer’s instructions. Reverse-transcription was carried out
using M-MLV, and cDNA amplification was performed using the SYBR
Green Master Mix kit (Invitrogen Life Technologies) according to
the manufacturer’s instructions. MMP-2 and MMP-9 genes were
amplified using specific oligonucleotide primers, and the human
glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene was used as
an endogenous control. The PCR primer sequences used were as
follows: MMP-2 forward, 5′-TTGGTGGGAACTCAGAAG-3′ and reverse,
5′-TTGCGGTCATCATCGTAG-3′; MMP-9 forward,
5′-GTGGCACCACCACAACATCAC-3′ and reverse,
5′-CGCGACACCAAACTGGATGAC-3′; and GAPDH forward,
5′-CAACGAATTTGGCTACAGCA-3′ and reverse, 5′-AGGGGTCTACATGGCAACTG-3′.
Data were analyzed using the comparative Ct method
(2−ΔΔCt) (17). Three
separate experiments were performed for each clone.
Western blot analysis
Subsequent to 24 h, the treated and untreated cells
were harvested and lysed, and the supernatants were separated from
the cell debris by centrifugation at 13,000 × g for 15 min at 4°C.
Aliquots containing 30 μg of total protein were separated by
SDS-PAGE and transferred onto nitrocellulose membranes. The
membranes were then probed with primary rabbit monoclonal
antibodies against MMP-2 and -9 (Santa Cruz Biotechnology, Inc.,
Santa Cruz, CA, USA) at 4°C overnight. The membranes were
subsequently probed with a goat anti-rabbit secondary antibody
conjugated with horseradish peroxidase (Santa Cruz Biotechnology,
Inc.) and visualized by electrochemiluminescence. Protein band
densities were quantified using Bio-Rad Quantity One software
(Bio-Rad, Hercules, CA, USA).
Statistical analysis
Data are expressed as the mean ± standard deviation.
Different groups were compared using a paired t-test and P<0.05
was considered to indicate a statistically significant
difference.
Approval
This study was approved by the Ethics Committee of
Shanghai Jiao Tong University Affiliated Sixth People’s Hospital
(Shanghai, China).
Results
Measurement of cell proliferation
Cell reproduction levels in the US and US + MB
groups were significantly suppressed when compared with the control
group (P<0.01) following treatment for 24 h and this suppression
was significantly higher in the US + MB group compared with the US
group (P<0.01). However, no significant difference in cell
reproduction levels was identified between the three groups
following 12 h of treatment (P>0.05) (Fig. 1).
In vitro migration and invasion
assays
To evaluate the migration potential of the PC-3
cells using ultrasound combined with microbubbles in vitro,
migration assays were performed. The result revealed that PC-3 cell
motility was suppressed in the US and US + MB groups compared with
the control group (Fig. 2A;
Table I; P<0.01). However, this
suppression was significantly higher in the US + MB group than in
the US group (P<0.01). To evaluate the effect of ultrasound
combined with microbubbles on the invasive ability of the PC-3
cells, an in vitro invasion assay was performed. The number
of cells passing through the filter was markedly less in the US and
US +MB groups compared with the control group (Fig. 2B; Table
I; P<0.01). Furthermore, this suppression was significantly
higher in the US+MB group than in the US group (P<0.01). These
results indicated that ultrasound combined with microbubbles
suppresses PC-3 cell migration and invasion in vitro.
 | Table INumber of migrating and invasive cells
following treatment. |
Table I
Number of migrating and invasive cells
following treatment.
| Group | Migration | Invasion |
|---|
| Control | 509.67±18.62 | 271.33±65.14 |
| US | 386.67±44.23a | 180.67±13.29a |
| US + MB | 190.83±14.63a,b | 86.67±10.60a,b |
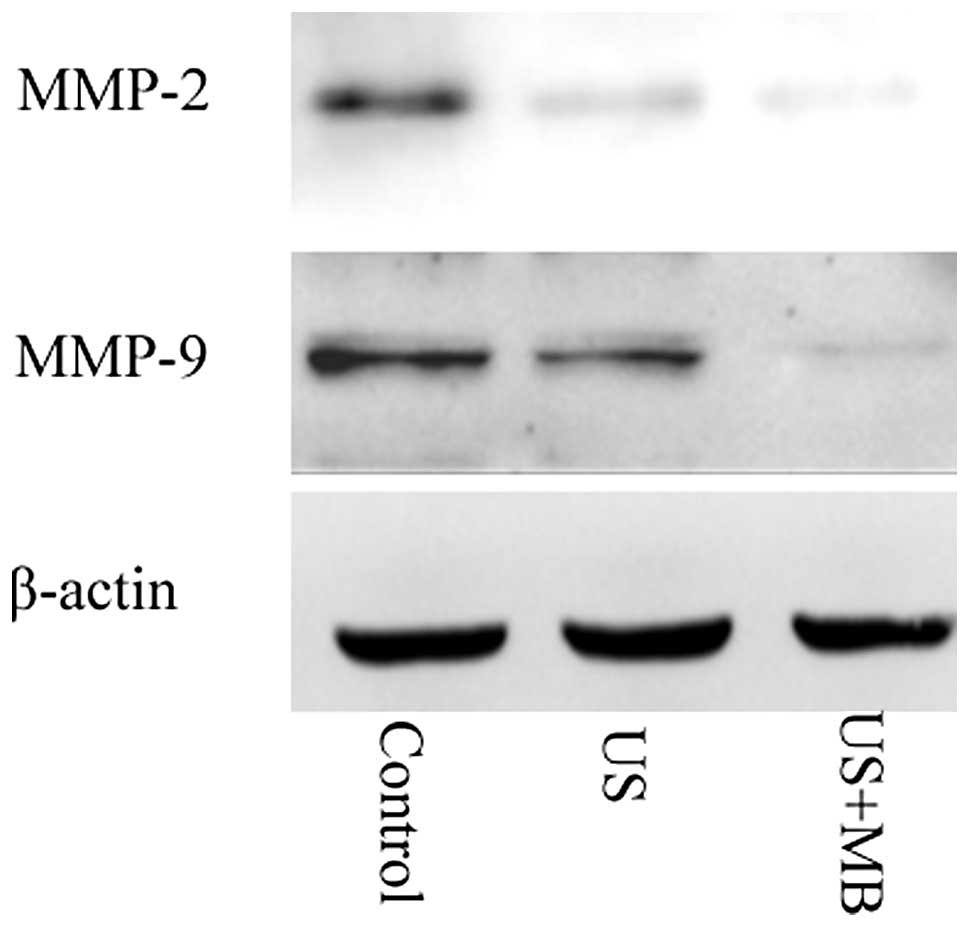
MMP-2 and MMP-9 expression following
treatment
The expression of MMP-2 and MMP-9 mRNA and protein
was investigated following treatment. It was found that the MMP-2
and MMP-9 levels in the US and US + MB groups were significantly
suppressed compared with the control group (P<0.01), and that
this suppression was significantly greater in the US + MB group
than in the US group (P<0.01). (Tables II and III; Fig.
3).
 | Table IIExpression of MMP-2 and MMP-9 mRNA
following treatment. |
Table II
Expression of MMP-2 and MMP-9 mRNA
following treatment.
| Group | MMP-2 mRNA | MMP-9 mRNA |
|---|
| Control | 11.64±1.02 | 12.69±1.80 |
| US | 5.65±1.17a | 3.05±0.49a |
| US + MB | 1.47±0.51a,b | 0.15±0.07a,b |
 | Table IIIExpression of MMP-2 and MMP-9 protein
following treatment. |
Table III
Expression of MMP-2 and MMP-9 protein
following treatment.
| Group | MMP-2 | MMP-9 |
|---|
| Control | 0.80±0.06 | 0.73±0.08 |
| US | 0.55±0.09a | 0.47±0.08a |
| US + MB | 0.25±0.05a,b | 0.15±0.05a,b |
Discussion
The biophysical modes of ultrasound exhibit three
types of effect: Thermal, cavitational and mechanical (18). Low-frequency ultrasound induces
predominantly mechanical and cavitational effects, as the thermal
effect results in only a neglible temperature increase (19). In the present study, regular cell
media that had not been degassed or air-saturated was used to avoid
any resulting cell changes. Thus, the cell media may have contained
air bubbles that were able to produce cavitation (20). Following ultrasound exposure,
insonation may be used to intentionally collapse microbubbles
suspended in liquid, resulting in the production of a mechanical
force on the cellular membrane, and the destruction of adjoining
cellular membrane integrity. The cavitation effect is more intense
following the addition of microbubbles to the cell suspension
compared with administration of ultrasound alone. Collapsing
microbubbles and the resultant cavitation bubbles create impulsive
pressures, including liquid jets and shock waves, which result in
cell apoptosis, enzyme inactivation and the denaturation of
proteins. Neighboring cells are also affected by these pressures,
as the propagation distance of the shock-wave from the center of a
cavitation bubble with the potential to damage a cell membrane is
greater than the maximum radius of the cavitation bubble (21).
Clinically, ultrasound is of low intensity at a
value of 0–0.5 W/cm2, of medium intensity at a value of
0.5–3 and of high intensity at a value of >3 W/cm2
(22). As high energies are
involved in ultrasound treatment, cell lysis forms the predominant
effect, which possibly masks additional effects on surviving cells
(23,24).
In the present study, cell proliferation was
measured at 12, 24, 48 and 72 h post-ultrasound treatment. The
results indicated that ultrasound combined with microbubbles
suppressed the proliferation of the PC-3 cells following 24 h of
treatment. In our previous study, an ultrasound frequency of 21
kHz, with an ISATA of 46 mW/cm2 and a continuous wave
mode was used to treat PC-3 cells for 30 sec. It was found that
ultrasound combined with microbubbles was able to induce apoptosis
in PC-3 cells (25). These results
indicated that ultrasound combined with microbubbles inhibits cell
proliferation via a mechanism involving apoptotic induction. In our
previous study, cell viability was evaluated immediately following
treatment. The results indicated that ultrasound alone and
ultrasound combined with microbubbles exhibited minimal effects on
the viability of the PC-3 cells and cause minimal induction of cell
lysis (25).
One of the key stages in cancer invasion and
metastasis is the degradation of the extracellular matrix. Notably,
MMP-2 and MMP-9 have been demonstrated to be significant in this
process (26). Numerous studies
have shown that the inhibition of MMP expression and/or the
inhibition of the activities of MMP enzymes may be used as early
targets for preventing cancer metastasis (27–29).
In addition, in vitro studies have revealed that the
expression of MMP-2 and MMP-9 is associated with the high invasive
and metastatic potential of PCa cell lines (30). The results from the migration and
invasion assays of the current study also demonstrated that
ultrasound combined with microbubbles inhibited the invasion and
migration of PC-3 cells in vitro. In addition, the results
revealed that the antimetastastic effects of ultrasound combined
with microbubbles were associated with the inhibition of
enzymatically degradative metastatic processes in the PC-3 cells.
Ultrasound combined with microbubbles inhibited the activities of
MMP-2 and -9, which are involved in the degradation of the
extracellular matrix and thus, are important in cancer cell
migration and invasion.
Collectively, ultrasound treatment combined with
microbubbles exhibits antimetastatic activity and thus has the
potential to be developed into an antimetastatic agent for PCa. The
possible signal pathways targeted by ultrasound combined with
microbubble treatment may inhibit migration and invasion in PC-3
cells via the downregulation MMP-2 and -9. Therefore, ultrasound
treatment combined with microbubbles presents considerable promise
as an antimetastatic treatment for androgen-independent PCa.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant no. 81271597) and the Major
Infrastructure Projects of Shanghai Science and Technology (grant
no. 10JC1412600).
References
|
1
|
Jemal A, Bray F, Center MM, et al: Global
cancer statistics. CA Cancer J Clin. 61:69–90. 2011.
|
|
2
|
McCulloch DR, Harvey M and Herington AC:
The expression of the ADAMs proteases in prostate cancer cell lines
and their regulation by dihydrotestosterone. Mol Cell Endocrinol.
167:11–21. 2000.
|
|
3
|
Saleem M, Adhami VM, Zhong W, et al: A
novel biomarker for staging human prostate adenocarcinoma:
overexpression of matriptase with concomitant loss of its
inhibitor, hepatocyte growth factor activator inhibitor-1. Cancer
Epidemiol Biomarkers Prev. 15:217–227. 2006.
|
|
4
|
Yu W, Wang Y, Gong M, Pei F and Zheng J:
Phosphoprotein associated with glycosphingolipid microdomains 1
inhibits the proliferation and invasion of human prostate cancer
cells in vitro through suppression of Ras activation. Oncol Rep.
28:606–614. 2012.
|
|
5
|
Xiao LJ, Lin P, Lin F, et al: ADAM17
targets MMP-2 and MMP-9 via EGFR-MEK-ERK pathway activation to
promote prostate cancer cell invasion. Int J Oncol. 40:1714–1724.
2012.
|
|
6
|
Tabuchi Y, Takasaki I, Zhao QL, et al:
Genetic networks responsive to low-intensity pulsed ultrasound in
human lymphoma U937 cells. Cancer Lett. 270:286–294. 2008.
|
|
7
|
Tang W, Liu Q, Zhang J, et al: In vitro
activation of mitochondria-caspase signaling pathway in sonodynamic
therapy-induced apoptosis in sarcoma 180 cells. Ultrasonics.
50:567–576. 2010.
|
|
8
|
Feng Y, Tian Z and Wan M: Bioeffects of
low-intensity ultrasound in vitro: apoptosis, protein profile
alteration, and potential molecular mechanism. J Ultrasound Med.
29:963–974. 2010.
|
|
9
|
Ashush H, Rozenszajn LA, Blass M, et al:
Apoptosis induction of human myeloid leukemic cells by ultrasound
exposure. Cancer Res. 60:1014–1020. 2000.
|
|
10
|
Rosenthal I, Sostaric JZ and Riesz P:
Sonodynamic therapy - a review of the synergistic effects of drugs
and ultrasound. Ultrason Sonochem. 11:349–363. 2004.
|
|
11
|
Kolarova H, Tomankova K, Bajgar R, Kolar P
and Kubinek R: Photodynamic and sonodynamic treatment by
phthalocyanine on cancer cell lines. Ultrasound Med Biol.
35:1397–1404. 2009.
|
|
12
|
Liu KC, Huang AC, Wu PP, et al: Gallic
acid suppresses the migration and invasion of PC-3 human prostate
cancer cells via inhibition of matrix metalloproteinase-2 and -9
signaling pathways. Oncol Rep. 26:177–184. 2011.
|
|
13
|
Hwang ES and Park KK: Magnolol suppresses
metastasis via inhibition of invasion, migration, and matrix
metalloproteinase-2/-9 activities in PC-3 human prostate carcinoma
cells. Biosci Biotechnol Biochem. 74:961–967. 2010.
|
|
14
|
Bai WK, Wu ZH, Shen E, Zhang JZ and Hu B:
The improvement of liposome-mediated transfection of pEGFP DNA into
human prostate cancer cells by combining low-frequency and
low-energy ultrasound with microbubbles. Oncol Rep. 27:475–480.
2012.
|
|
15
|
Sicklick JK, Li YX, Jayaraman A, et al:
Dysregulation of the Hedgehog pathway in human
hepatocarcinogenesis. Carcinogenesis. 27:748–757. 2006.
|
|
16
|
Xinzhou H, Ning Y, Ou W, et al: RKIp
inhibits the migration and invasion of human prostate cancer PC-3M
cells through regulation of extracellular matrix. Mol Biol (Mosk).
45:1004–1011. 2011.
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
|
|
18
|
Furusawa Y, Zhao QL, Hassan MA, et al:
Ultrasound-induced apoptosis in the presence of Sonazoid and
associated alterations in gene expression levels: a possible
therapeutic application. Cancer Lett. 288:107–115. 2010.
|
|
19
|
Samuel S, Miller DL and Fowlkes JB: The
relationship of acoustic emission and pulse-repetition frequency in
the detection of gas body stability and cell death. Ultrasound Med
Biol. 32:439–447. 2006.
|
|
20
|
Chumakova OV, Liopo AV, Evers BM and
Esenaliev RO: Effect of 5-fluorouracil, Optison and ultrasound on
MCF-7 cell viability. Ultrasound Med Biol. 32:751–758. 2006.
|
|
21
|
Kodama T, Tomita Y, Koshiyama K and
Blomley MJ: Transfection effect of microbubbles on cells in
superposed ultrasound waves and behavior of cavitation bubble.
Ultrasound Med Biol. 32:905–914. 2006.
|
|
22
|
Jackson JK, Pirmoradi FN, Wan CP, et al:
Increased accumulation of paclitaxel and doxorubicin in
proliferating capillary cells and prostate cancer cells following
ultrasound exposure. Ultrasonics. 51:932–939. 2011.
|
|
23
|
Kawai N and Iino M: Molecular damage to
membrane proteins induced by ultrasound. Ultrasound Med Biol.
29:609–614. 2003.
|
|
24
|
Marentis TC, Kusler B, Yaralioglu GG, et
al: Microfluidic sonicator for real-time disruption of eukaryotic
cells and bacterial spores for DNA analysis. Ultrasound Med Biol.
31:1265–1277. 2005.
|
|
25
|
Bai W, Yang S, Shen E, et al: Treatment of
PC-3 cells with ultrasound combined with microbubbles induces
distinct alterations in the expression of Bcl-2 and Bax. Chin Sci
Bull. 58:3535–3540. 2013.
|
|
26
|
Hara T, Miyazaki H, Lee A, Tran CP and
Reiter RE: Androgen receptor and invasion in prostate cancer.
Cancer Res. 68:1128–1135. 2008.
|
|
27
|
Guruvayoorappan C and Kuttan G:
Amentoflavone inhibits experimental tumor metastasis through a
regulatory mechanism involving MMP-2, MMP-9, prolyl hydroxylase,
lysyl oxidase, VEGF, ERK-1, ERK-2, STAT-1, NM23 and cytokines in
lung tissues of C57BL/6 mice. Immunopharmacol Immunotoxicol.
30:711–727. 2008.
|
|
28
|
Roy R, Louis G, Loughlin KR, et al:
Tumor-specific urinary matrix metalloproteinase fingerprinting:
identification of high molecular weight urinary matrix
metalloproteinase species. Clin Cancer Res. 14:6610–6617. 2008.
|
|
29
|
Wang Q, Diao X, Sun J and Chen Z:
Regulation of VEGF, MMP-9 and metastasis by CXCR4 in a prostate
cancer cell line. Cell Biol Int. 35:897–904. 2011.
|
|
30
|
Aalinkeel R, Nair BB, Reynolds JL, et al:
Overexpression of MMP-9 contributes to invasiveness of prostate
cancer cell line LNCaP. Immunol Invest. 40:447–464. 2011.
|