Introduction
Basaloid squamous cell carcinoma (BSCC) is an
uncommon variant of squamous cell carcinoma (SCC), which was first
described by Wain et al (1)
in 1986. BSCC generally present in the upper aerodigestive tract,
particularly in the larynx, hypopharynx and the base of the tongue
(2). Ide et al (3) reported 46 cases of BSCC in the oral
mucosa, where only one case occurred in the gingival tissues. In
addition, Hirai et al (4)
described two cases of BSCC in the mandibular gingiva. In the
current study, an additional case of BSCC in the maxillary gingiva
is presented and the clinical features of BSCC are reviewed
according to the current literature. Patient provided written
informed consent.
Case report
In October 2009, a 40-year-old male visited the
outpatient clinic of Peking University, Shenzhen Hospital
(Shenzhen, China) presenting with a painless irregular mass of the
right maxillary gingiva as well as nasal obstruction following the
presentation of the initial symptoms two months previously. The
patient’s medical history was unremarkable. The patient had a
history of smoking for a period of 15 years (frequency, 10/day),
however, denied excessive alcohol consumption. The intraoral
examination revealed a gray, irregular mass (size, 3×2.5×2 cm) in
the right maxillary posterior buccal gingiva, which elicited
marginal pain on palpation. An extraoral clinical examination
identified various palpable, mobile lymph nodes in the right
submandibular region, measuring ~1×1×1 cm that were firm and
non-tender on palpation. A computed tomography (CT) scan
demonstrated a tumor, which involved the right maxillary sinus and
infiltrated the central region of the hard palate (Fig. 1). The chest CT was negative for
distant metastatic lesions. The lesion was clinically and
radiologically classified as cT4 cNx cM0, according to the American
Joint Committee on Cancer (AJCC) staging manual (5). The treatment comprised of an extended
surgical excision of the tumor, which involved a partial
maxillectomy with mandibulectomy and ipsilateral functional neck
dissection at levels I-III, where four enlarged lymph nodes were
removed at level Ib.
To investigate the excised tissue, the tissues were
fixed in 10% buffered formalin and processed via the usual methods
for paraffin embedding. The paraffin sections were stained with
hematoxylin and eosin. Immunohistochemical staining was performed
using the BOND-MAX automated immunostainer (Vision BioSystems,
Melbourne, Australia) to further define the diagnosis of the tumor.
The antibodies that were used for immunohistochemistry are
presented in Table I. The antigen
retrieval method was heat-induced epitope retrieval in EDTA at an
alkaline pH (pH 8.0). Adequate positive and negative tissue
controls were used.
 | Table IPrimary antibodies adopted for
immunohistochemical staining. |
Table I
Primary antibodies adopted for
immunohistochemical staining.
| Antibody | Clone | Source | Dilution | Result |
|---|
| p63 | 4A4 | Santa Cruza | 1:75 | + |
| CK7 | OV-TL12/30 | Dakob | 1:50 | − |
| CK14 | LL002 | Novocastrac | 1:50 | − |
| CK-H | 34βE12 | Dakob | 1:50 | + |
| Vimentin | V9 | Dakob | 1:50 | − |
| S-100 | Antiserum | Dakob | 1:4,000 | − |
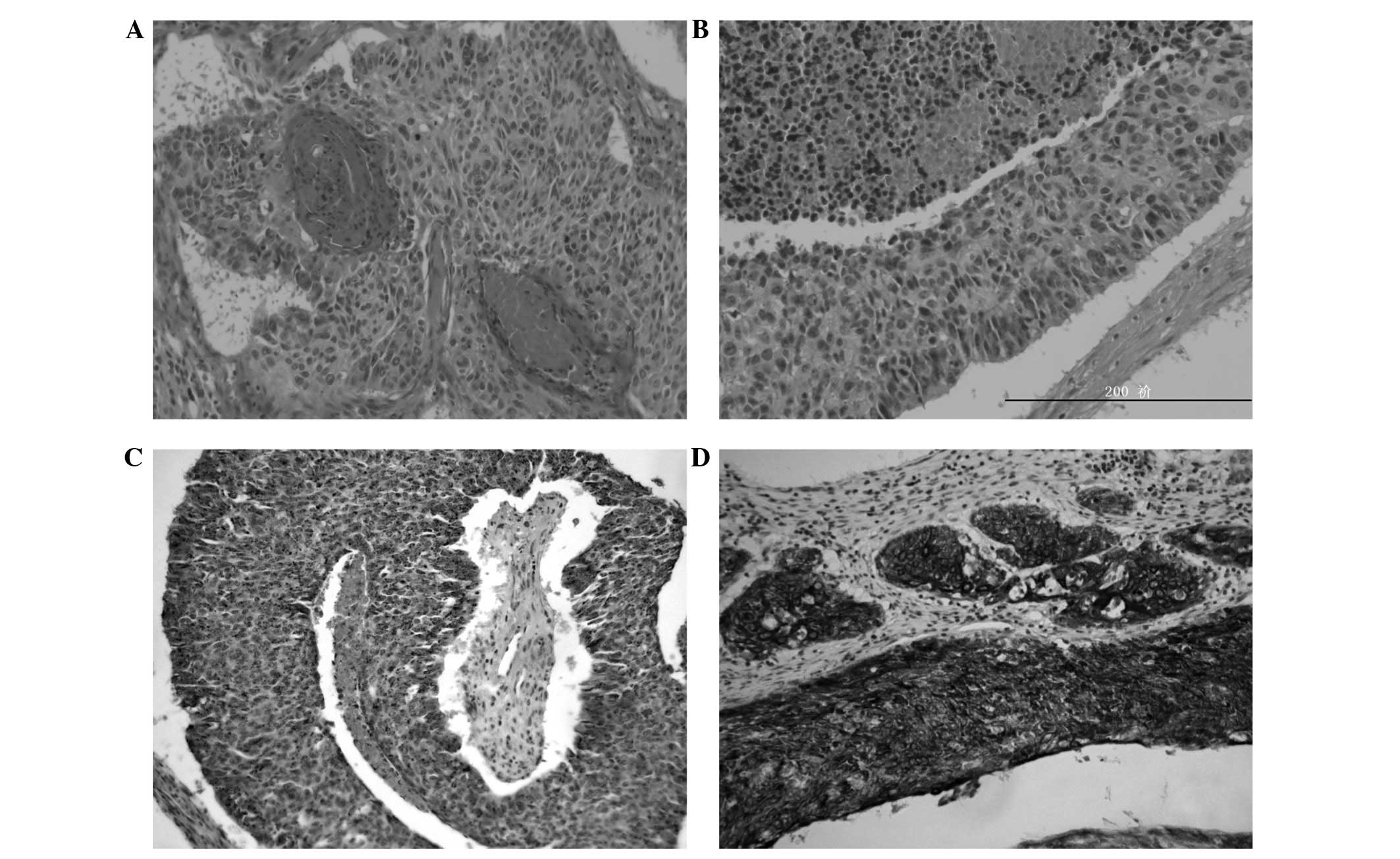
Microscopically, the tissues from the primary site
demonstrated that there were two cellular populations;
epithelial-like and basaloid tumor cells. The basaloid cells formed
the primary invasive component of the cancer nests and were
arranged in cords, trabeculae and lobules that occasionally
demonstrated a pseudoglandular formation. These cells exhibited
peripheral palisading and hyperchromatic nuclei with a high
nucleus-cytoplasm ratio as well as a scant cytoplasm (Fig. 2A and B). Mitotic figures were
observed within the nests and necrotic foci were scattered
throughout the visual field. Components of SCC exhibiting
keratinization were scarce, which differs to the features of
conventional SCC. Immunohistochemically, the basaloid carcinoma
cells were weakly positive for p63 (Fig. 2C) and focally positive for high
molecular weight cytokeratin (CK-H; Fig. 2D), and negative for cytokeratin
(CK)7, CK14, S-100 protein and vimentin. According to the clinical
presentation, histopathological features and immunohistochemical
findings, a final diagnosis of BSCC in the maxillary gingiva was
determined. The surgical excision margins were healthy and the neck
lymph node histopathology did not reveal any positive lymph nodes.
The pathological staging of pT4 pN0 pM0 was established according
to the AJCC staging manual.
However, the patient experienced recurrence half a
year later and presented with a painful mass of the right cheek
without enlarged lymph nodes on palpation. Following a failed tumor
response to chemotherapy (dose, 16 mg pingyangmycin per day for
seven days) and dimensional conformal radiotherapy (dose, 50 Gy), a
second surgical intervention comprising of an extended resection of
the neoplasm was conducted. Three years following treatment the
patient remains free from tumor recurrence.
Discussion
BSCC is a rare and malignant tumor that presents in
the head and neck region, including the oral mucosa, and has been
defined as an aggressive and distinct variant of SCC, which is
composed of basaloid and squamous components, according to the
World health Organization (6). BSCC
is particularly uncommon in the oral cavity and more so in the
gingiva. According to Hirai et al (4), only eight cases of BSCC in the gingiva
have been reported in the English literature (4,7–9), with
only one case of BSCC occurring in the maxillary gingiva.
The clinical features of the BSCC cases that
presented in the gingiva are reviewed and summarized in Table II. Two patients were female and
seven were male with an age range of 40–79 years (mean age, 60.1
years). The most frequent site of origin was the mandible (n=7)
followed by the maxilla (n=2). According to the standard
tumor-node-metastasis (TNM) staging, provided by the AJCC, three
patients presented in stage I, two in stage II, three in stage III,
and one in stage IV. All of the patients were treated using
surgery, four underwent neck dissections and three received
adjuvant radiotherapy. Five patients had survived at the median
follow-up time of 56 months.
 | Table IIReported cases of BSCC of the
gingiva. |
Table II
Reported cases of BSCC of the
gingiva.
| First author
(ref) | Year | Age/gender | Stage | Location of
lesion | Treatment | Final outcome | Follow-up period,
months |
|---|
| Wedenberg et
al (7) | 1997 | 55/M | I | Maxilla | S | A | 5 |
| Abiko et al
(8) | 1998 | 79/F | I | Mandible | S | A | 24 |
| Yu et al
(9) | 2008 | 61/M | II | Mandible | S + FND | A | 120 |
| Yu et al
(9) | 2008 | 56/M | IV | Mandible | S | D | 180 |
| Yu et al
(9) | 2008 | 65/M | III | Mandible | S | D | 2.5 |
| Subramania et
al (10) | 2009 | 72/F | III | Mandible | S + FND + RT | A | 12 |
| Hirai et al
(4) | 2009 | 55/M | II | Mandible | S + FND | A | 79 |
| Hirai et al
(4) | 2009 | 65/M | I | Mandible | S + RT | A | 60 |
| Present case | 2010 | 40/M | III | Maxilla | S + FND + RT | A | 25 |
The prognosis of patients with BSCC compared with
patients with conventional SCC remains uncertain. Winzenburg et
al (10) first identified that
distant metastases occurred in 52% of patients with BSCC and in 13%
of patients with poorly differentiated SCC. Soriano et al
(11) showed that patients with SCC
were associated with notably higher survival rates when compared
with patients with BSCC; furthermore, the rate of distant
metastasis was six times higher in the cases of BSCC. However, de
Sampaio Góes et al (12)
declared that the prognosis did not differ between patients with
BSCC of the oral cavity and those with conventional SCC.
The diagnosis of BSCC is currently based on
histological criteria, including focal squamous differentiation, a
basaloid pattern that is associated with frank invasive SCC or
carcinoma in situ. However, the histopathological diagnosis
of BSCC is difficult to differentiate from that of adenoid cystic
carcinoma (ACC), poorly differentiated carcinoma and basal cell
adenocarcinoma. BSCC may easily be misdiagnosed as ACC,
particularly when a small biopsy sample is used.
Previous studies have advocated that
immunohistochemical markers, including CK7, CK14, p63, CK-H, S-100
and vimentin aid with distinguishing BSCC from ACC (13,14).
Colleta et al (13) reported
that the majority of cancer cells of ACC express CK7, which
indicates a ductal-pattern possibly of salivary gland origin, while
in BSCC, the basaloid cells exhibit positive expression for CK17
and negative expression for vimentin, S-100, CK7, CK8 or CK20. The
p63 staining pattern of BSCC and ACC is markedly different; the
positive p63 staining is diffused in ~100% of the BSCC cancer
cells, while ACC demonstrates a compartmentalized pattern within
the tumor nests (15). In addition,
the expression of CK-H is positive in BSCC cases (14). In the present case the
immunohistochemical staining was positive for p63 and CK-H, and
negative for S-100, vimentin, CK14 and CK7. Thariat et al
(16) also advocated another
criterion (in addition to the original criteria and
immunohistochemical findings reported by Wain et al
[1]) and proposed that, owing to
its dual behavior that is marginally dependent on its association
with the human papilloma virus (HPV), the BSCC patients should be
systematically examined for their HPV status as this may determine
the treatment response (17).
BSCC require aggressive multimodality treatment,
including radical surgical excision, neck dissection, radiotherapy
and regular chemotherapy due to the high overall mortality rate.
Although chemotherapy is recommended by certain authors due to the
high incidence of distant metastasis and the relatively poor
prognosis (17), a standard
chemotherapy regimen for BSCC has not yet been established.
Furthermore, investigation of a greater number of patients is
required to determine the efficacy of chemotherapy for BSCC of the
head and neck. Bonner et al (18) advocated that immunotherapy elicited
an improved treatment effect when compared with radiotherapy alone
and resulted in a reduced mortality rate.
In conclusion, the aggressive behavior of BSCC has
been presented using a rare case of the maxillary gingiva. The
potential difficulties of a histological diagnosis were discussed
and the possible obstacles to an accurate diagnosis were
emphasized. Further studies with uniform reporting are required in
order to optimize the establishment of a diagnosis and define the
optimum treatment strategies.
Acknowledgements
The present study was supported by the Department of
Cranio-Maxillofacial and Oral Surgery, University Hospital Zürich
(Zürich, Switzerland). In addition, financial support was provided
by the Guangdong Province Nature Science Foundation (grant no.
S2012010010382) and the Shenzhen Science and Research Innovation
Foundation (grant no. JCY20130402114702120).
Abbreviations:
|
BSCC
|
basaloid squamous cell carcinoma
|
|
SCC
|
squamous cell carcinoma
|
|
ACC
|
adenoid cystic carcinoma
|
|
CK
|
cytokeratin
|
References
|
1
|
Wain SL, Kier R, Vollmer RT and Bossen EH:
Basaloid-squamous carcinoma of the tongue, hypopharynx, and larynx:
report of 10 cases. Hum Pathol. 17:1158–1166. 1986.
|
|
2
|
Sundharam BS and Krishnan PA: Basaloid
squamous cell carcinoma report of a case and review of literature.
Indian J Dent Res. 14:184–186. 2003.
|
|
3
|
Ide F, Shimoyama T, Horie N and Kusama K:
Basaloid squamous cell carcinoma of the oral mucosa: a new case and
review of 45 cases in the literature. Oral Oncol. 38:120–124.
2002.
|
|
4
|
Hirai E, Yamamoto K, Yamamoto N, et al:
Basaloid squamous cell carcinoma of the mandible: report of two
cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod.
108:e54–e58. 2009.
|
|
5
|
Brandwein-Gensler M and Smith RV:
Prognostic indicators in head and neck oncology including the new
7th edition of the AJCC staging system. Head Neck Pathol. 4:53–61.
2010.
|
|
6
|
Shanmugaratnam K and Sobin LH: The World
Health Organization histological classification of tumours of the
upper respiratory tract and ear. Cancer. 71:2689–2697. 1993.
|
|
7
|
Wedenberg C, Jesslén P, Lundqvist G,
Lundgren J and Hellquist HB: Basaloid squamous cell carcinoma of
the maxilla. Oral Oncol. 33:141–144. 1997.
|
|
8
|
Abiko Y, Muramatsu T, Tanaka Y, et al:
Basaloid-squamous cell carcinoma of the oral mucosa: report of two
cases and study of the proliferative activity. Pathol Int.
48:460–466. 1998.
|
|
9
|
Subramanian B, Agrawal K and Panda K:
Basaloid squamous carcinoma of mandible. J Craniofac Surg.
20:151–153. 2009.
|
|
10
|
Winzenburg SM, Niehans GA, George E, Daly
K and Adams GL: Basaloid squamous carcinoma: a clinical comparison
of two histologic types with poorly differentiated squamous cell
carcinoma. Otolaryngol Head Neck Surg. 119:471–475. 1998.
|
|
11
|
Soriano E, Faure C, Lantuejoul S, et al:
Course and prognosis of basaloid squamous cell carcinoma of the
head and neck: a case-control study of 62 patients. Eur J Cancer.
44:244–250. 2008.
|
|
12
|
de Sampaio Góes FC, Oliveira DT, Dorta RG,
et al: Prognoses of oral basaloid squamous cell carcinoma and
squamous cell carcinoma: a comparison. Arch Otolaryngol Head Neck
Surg. 130:83–86. 2004.
|
|
13
|
Coletta RD, Cotrim P, Almeida OP, et al:
Basaloid squamous carcinoma of oral cavity: a histologic and
immunohistochemical study. Oral Oncol. 38:723–729. 2002.
|
|
14
|
Madur BP and Jambhekar NA: Basaloid
squamous carcinoma simulating adenoid cystic carcinoma: Diagnostic
dilemma. Oral Oncol Extra. 42:227–230. 2006.
|
|
15
|
Emanuel P, Wang B, Wu M and Burstein DE:
p63 immunohistochemistry in the distinction of adenoid cystic
carcinoma from basaloid squamous cell carcinoma. Mod Pathol.
18:645–650. 2005.
|
|
16
|
Thariat J, Badoual C, Faure C, et al:
Basaloid squamous cell carcinoma of the head and neck: role of HPV
and implication in treatment and prognosis. J Clin Pathol.
63:857–866. 2010.
|
|
17
|
Raslan WF, Barnes L, Krause JR, et al:
Basaloid squamous cell carcinoma of the head and neck: a
clinicopathologic and flow cytometric study of 10 new cases with
review of the English literature. Am J Otolaryngol. 15:204–211.
1994.
|
|
18
|
Bonner JA, Harari PM, Giralt J, et al:
Radiotherapy plus cetuximab for squamous-cell carcinoma of the head
and neck. N Engl J Med. 354:567–578. 2006.
|