Introduction
Pancreatic cancer is known to be difficult to
diagnose in its early stages and to treat medically. Pancreatic
malignant tumors are common, latent, highly lethal and extremely
difficult to be surgically treated. A previous report has suggested
that ~90% of patients succumb to the disease within one year after
diagnosis while the five-year survival rate is <5% (1). The incidence of pancreatic cancer has
been on the increase in recent years, thus demonstrating the
importance of studying the pathogenesis.
Alterations in gene and protein expression, and the
activation of signaling pathways, are associated with the
occurrence and progression of pancreatic cancers (2). JWA, a newly identified
tubulin-associated protein, encodes a cytoskeleton-associated
protein (AF 070523, 1998) that shares similar biological functions
with tubulin-associated proteins. JWA may regulate the tubulin and
actin system, affect cell migration, and may be associated with the
biological functions of a number of tumor promoters and inhibitors,
and involve the corresponding signaling pathways (3). JWA appears to have a significant role
in both the directed and non-directed tumor cell migration
(2).
The MAPK signaling cascade is a highly conserved
pathway that transfers extracellular signals to cellular
proliferation signals. The MAPK pathway triggers a genetic
signaling cascade to the nucleus, resulting in regulation of cell
proliferation, differentiation, apoptosis, gene expression and
cellular response to the external environment (4). The MAPK signal transduction pathways
of cell proliferation, apoptosis, invasion and migration converge,
an event that is significant to the occurrence and progression of
hematopoietic malignancies, epithelial tumors and choriocarcinoma
(5,6). There have been no previous reports as
to whether JWA gene affects the proliferation, invasion and
migration of pancreatic tumors through the MAPK pathway. The
present study therefore provides. to the best of our knowledge,
evidence for the first time for the treatment and prognosis of
pancreatic cancer.
In order to determine the function of JWA in PANC-1
human pancreatic cancer cells, the expression level of the
JWA gene was downregulated using JWA-specific small
interfering RNA (siRNA). Subsequently the proliferation, apoptosis,
invasion and migration of PANC-1 cells were analyzed. Additionally,
the present study analyzed the association between these cell
functions with the MAPK signaling pathway, in order to identify
molecular mechanisms in the pathogenesis of pancreatic cancer.
Materials and methods
Cell culture
Human PANC-1 pancreatic cancer cells were purchased
from ATCC and cultured in Dulbecco’s modified Eagle’s medium (DMEM)
(HyClone, Logan, UT, USA) supplemented with 10% fetal bovine serum
(FBS; Hangzhou Sijiqing Biological Engineering Materials Co. Ltd,
Hangzhou, China), 100 U/ml penicillin and 100 mg/l streptomycin
(Beyotime Institute of Biotechnology, Shanghai, China). The cells
were grown at 37°C with 5% CO2 in a humidified
incubator.
JWA siRNA transfection
Human JWA-specific siRNA was purchased from Santa
Cruz (sc-60874; Santa Cruz Biotechnology Inc., Santa Cruz, CA,
USA). Transfections of siRNA were carried out using
Lipofectamine® 2000 (Invitrogen Life Technologies, CA,
USA). The final concentration of siRNA used was 150 nM for a
transfection period of 6 h. Cells were collected for subsequent
analyses following 48 h incubation. Nonsense siRNA was used as a
negative control (NC) and untransfected PANC-1 cells were used as a
blank control.
Measurement of cell proliferation by MTT
assay
PANC-1 cell proliferation was measured by MTT assay
in 96-well micro-culture plates. The cells were collected 5 h after
transfection, and seeded at a density of 2×104
cells/well in 96-well plates in DMEM containing 10% FBS. Five
duplicate wells were set up for each group and the experiment was
repeated three times. The PANC-1 untransfected and nonsense
siRNA-transfected cells were used as controls. After 48 h
incubation, 20 μl of 5 mg/ml MTT solution in phosphate-buffered
saline, was added to each well for 4 h. The absorbance of each well
was analyzed using an Infinite® F50 Microplate Reader
(Tecan Group Ltd., Männedorf, Switzerland) at a wavelength of 570
nm. Proliferation curves were plotted according to the optical
density and the cell growth before and after transfection was
compared.
Measurement of cell invasion and
migration by the Transwell® assay
A cell invasion assay was performed using Transwell
chambers. A volume of 100 μl Matrigel® (BD Biosciences,
Franklin Lakes, NJ, USA) was added to a 24-well Transwell chamber.
An untreated Transwell chamber was used for the cell migration
assay and a Matrigel-coated chamber was used for the cell invasion
assay. A total of 100 μl cell suspension (diluted in DMEM) with a
density of 2×105 cells/ml, was added to the upper
chamber while 600 μl DMEM with 10% FBS was added to the lower
chamber. The chamber was incubated at 37°C for 24 h and then the
non-migratory cells were subsequently removed from the upper
surface of the filter using a cotton swab. The invasive cells that
penetrated through the pores and migrated to the underside of the
membrane were stained with 1% crystal violet solution for 15 min
and then fixed using 4% paraformaldehyde. Nine random fields were
counted for penetrating cells using a light microscope at ×200
magnification (Olympus BX41; Olympus Corporation, Tokyo,
Japan).
Western blotting
Total protein was extracted from PANC-1 cells using
RIPA buffer (Beyotime Institute of Biotechnology) 72 h after
transfection and 40 μg protein was separated by SDS-PAGE. Following
electro-transfer of the proteins to a Hybond enhanced
chemiluminescence (ECL) nitrocellulose membrane, the membrane was
blocked using skim milk powder at room temperature (15–25°C) for
1.5 h. The membrane was then incubated at 4°C overnight with rabbit
polyclonal BAX, Bcl-2 (Abcam, Cambridge, UK), phospho-p38,
phospho-ERK1/2, phospho-JNK, phospho-MEK, p38, ERK1/2, JNK, and MEK
(Cell Signaling Technology, Inc., Danvers, MA, USA) antibodies and
mouse anti-human GAPDH monoclonal antibody (Beyotime Institute of
Biotechnology), respectively. The membranes were then washed prior
to incubation with secondary IgG antibody (Merck KGaA, Whitehouse
Station, NJ, USA) labelled with alkaline phosphatase and visualized
by ECL. The membranes were scanned and the relative level of
protein expression was analyzed.
Statistical analysis
Data were processed using SPSS 14.0. Data are
presented as the means ± standard deviation, using Student’s
t-tests or one-way analysis of variance. P<0.05 was considered
to indicate a statistically significant difference.
Results
JWA-specific siRNA transfection
downregulates JWA gene expression in PANC-1 cells
For the purpose of studying the association between
the JWA and MAPK pathways in pancreatic cancer cells, an siRNA for
JWA was prepared and transfected into PANC-1 cells. Nonsense siRNA
transfected into PANC-1 cells was used as a NC and untransfected
PANC-1 cells were used as a blank control. The protein expression,
analyzed by western blotting, of JWA in PANC-1 cells transfected
with JWA siRNA was significantly lower as compared with the
negative and blank controls. This indicated that the JWA siRNA was
effective in silencing the JWA gene and protein expression
(Fig. 1). As a result, subsequent
experiments investigating the effects of JWA knockdown should be
performed using the JWA siRNA in PANC-1 cells.
Cell proliferation following JWA
siRNA-mediated knockdown
A previous study has shown that all-trans retinoic
acid (ATRA) is crucial in inhibiting the cell proliferation of HeLa
cells (7). An MTT assay was
therefore used to measure the proliferation of PANC-1 cells.
Proliferation was observed to be enhanced following JWA siRNA
transfection for 24 h, as compared with the NC and blank controls.
The change in proliferation, however, was not significant (Fig. 2).
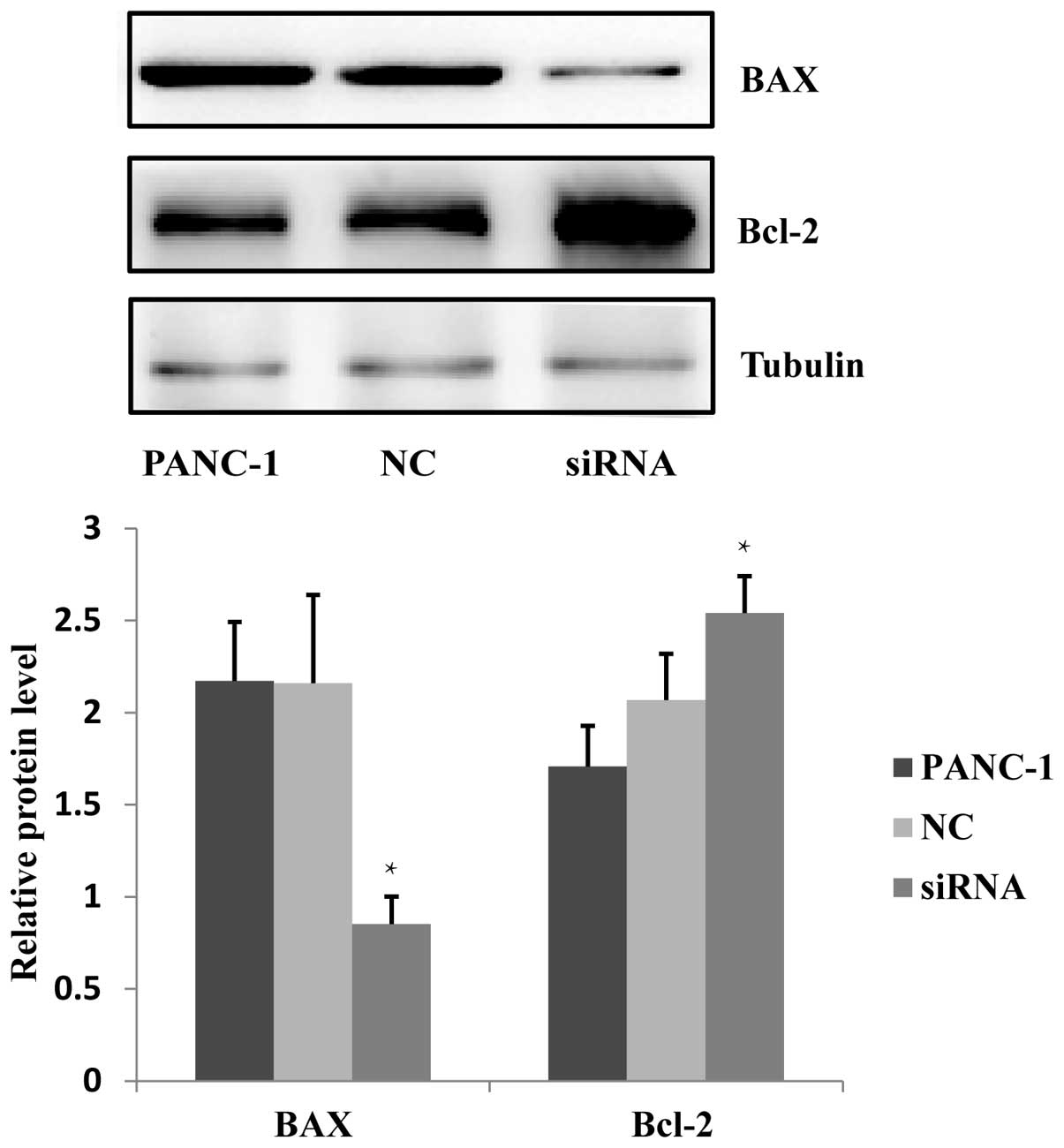
The effect of JWA siRNA on the apoptosis
of PANC-1 cells
Previous studies have indicated that JWA functions
in the process of As2O3 and C/EBPα-induced
apoptosis (8,9), and JWA overexpression has been shown
to enhance the apoptosis of esophageal cancer cells (10). It has been additionally reported
that the BAX protein expression in neoplasm of the digestive
system, including liver and colorectal cancers, is downregulated
and conversely, Bcl-2 protein is upregulated (11). The effects on apoptosis following
JWA-specific siRNA transfection in PANC-1 cells was investigated.
Western blotting was used to examine the BAX and Bcl-2 protein
expression in cells treated with JWA siRNA, NC and blank control.
The results indicated that the expression level of BAX protein was
significantly downregulated and the expression level of Bcl-2
protein was increased (Fig. 3).
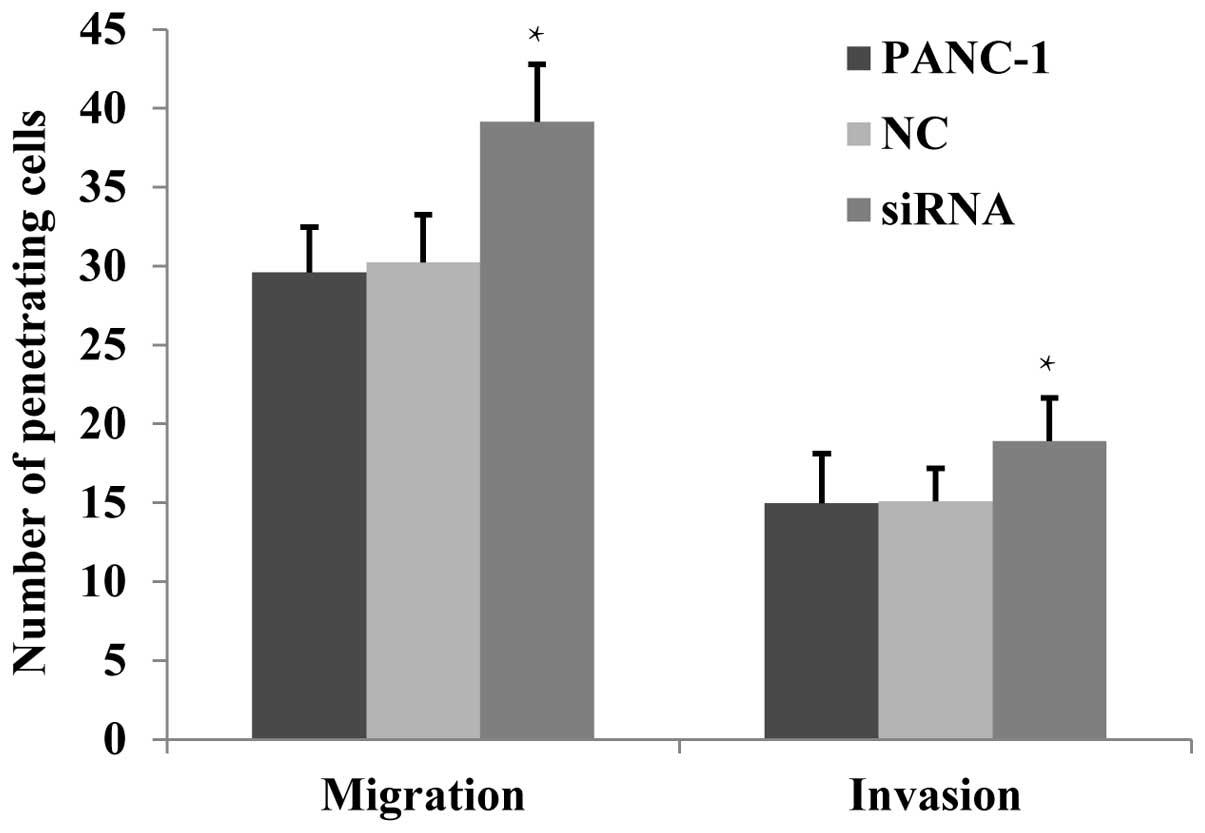
The effects of JWA siRNA on the migration
and invasion of PANC-1 cells by Transwell assay
It has been previously reported (10) that JWA downregulation enhances the
migration of numerous tumor cells, whereas JWA overexpression
inhibits cell migration. This suggests that JWA functions as a
tumor suppressor gene (3). The
migration and invasion ability of PANC-1 cells was analyzed using a
Transwell assay. It was identified that the number of penetrating
cells of the JWA siRNA-transfected group was found to be
significantly increased (P<0.05) in both the non-basement
membrane chamber and the Matrigel-coated chamber (Fig. 4). This suggested that following JWA
expression downregulation, the migration and invasion of PANC-1
cells was significantly enhanced.
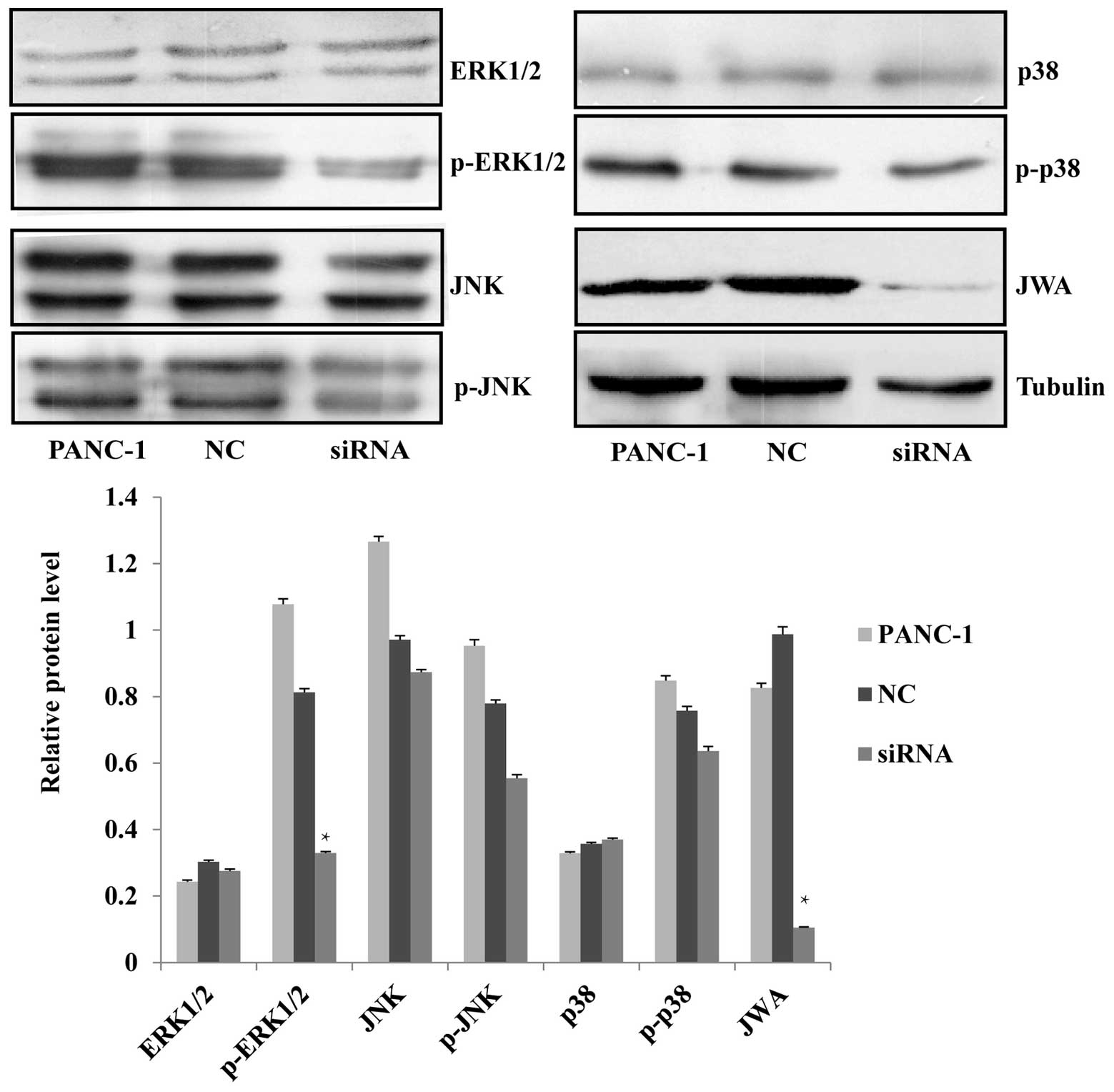
The MEK-ERK1/2 pathway is activated
following JWA knockdown
Mao et al (7)
reported that inhibition of the proliferation and induction of
apoptosis of HeLa cells by ATRA was due to the induction of ERK
phosphorylation, while the downregulation of JWA inhibits
ATRA-induced ERK phosphorylation (7). JWA is an essential factor of the
Raf/MEK/MAPK signaling pathway, involved in the regulation of cell
proliferation, apoptosis, migration, and invasion (3). The phosphorylated and
non-phosphorylated forms of predominant proteins of the three MAPK
pathways, were analyzed by western blotting. It was found that
knockdown of JWA by siRNA resulted in the significant
downregulation of p-ERK1/2 while the level of its
non-phosphorylated form was not affected. The expression of JNK and
p38, and their phosphorylated forms, was not significantly
different (Fig. 5). It was observed
that the protein expression level of the upstream factor of ERK1/2,
p-MEK, was decreased after siRNA-mediated knockdown of JWA
(Fig. 6). This suggested that the
MEK-ERK1/2 pathway was activated and that the regulation of cell
proliferation, apoptosis, migration and invasion may involve the
MEK-ERK1/2 signaling cascade of the MAPK pathway.
Discussion
The invasion and migration of tumor cells is a
process subject to dynamic change, and is closely associated with
the dynamic circulation of the cytoskeleton. A new cytoskeletal
protein, JWA, was previously identified from the tissues of human
primary tracheal and bronchial epithelial cells by Xu et al
(12), who showed it regulates
various biological functions including cell proliferation,
differentiation and migration. In the present study, siRNA was used
to knock down the expression of JWA in PANC-1 human pancreatic
cancer cells and the association between JWA and the MAPK signal
pathway was investigated.
Studies have identified that JWA is an important
signaling molecule in the regulation of migration and
differentiation of tumor cells, functioning as a tumor suppressor
(10). In addition, JWA is
associated with the occurrence and metastasis of malignant tumors
(13). Studies carried out using
liver cells with different metastatic potential have indicated that
the higher the metastasis potential, the lower the expression of
JWA mRNA and protein (14).
Furthermore, previous studies have shown the downregulation of the
expression of JWA protein in esophageal squamous cell carcinoma
(ESCC) tissues, suggesting that JWA overexpression may inhibit the
invasion and migration of tumor cells, including esophageal cancer
cells (10,15). In the present study, the
proliferation of PANC-1 cells was slightly enhanced, the protein
expression of BAX was significantly decreased, and the expression
of Bcl-2 was enhanced, following downregulation of JWA. Cell
migration and invasion was significantly enhanced, which may be
associated with cell proliferation and the involvement of JWA with
cytoskeletal actin. The downregulation of JWA expression affected
cell functions including migration, apoptosis and invasion.
MAPK signaling cascades are organized hierarchically
into three-tiered modules, which are MAPK, MAPK-kinase (MAPKK) and
MAPKK-kinase (MAPKKK). In eukaryotic cells, there are
downstream-associated pathways, which include ERK1/2, JNK and p38
regulating cell proliferation, differentiation, development,
apoptosis and inflammation (16).
The MAPK signaling pathways are closely associated with the
proliferation, apoptosis, invasion and migration of tumor cells,
and are of great importance with tumor development and
proliferation (17). It has been
shown that PMA and As2O3 induce migration and
invasion of tumor cells by activating the MAPK signaling pathways,
and is associated with the process of reconstruction of
cytoskeletal actin filaments (18).
A previous study has shown that the MAPK pathways have a
significant role in the development and differentiation of ovarian
cancer caused by KRAS and BRAF mutations (19). The study by Yao et al
(20) on breast cancer, suggested
that the phosphorylation level of ERK1/2 in breast cancer cells was
substantially higher as compared with normal breast cells,
suggesting that the overexpression of ERK1/2 protein is of great
importance in the occurrence and progression of breast cancer
(20). The data of the present
study have shown that the protein expression level of p-ERK1/2 and
its upstream MAPKK factor p-MEK, was significantly decreased
following JWA knockdown by siRNA. The expression level of ERK1/2,
MEK and the other two pathways showed only slight changes.
These data indicate that PANC-1 cells may function
through the MEK-ERK1/2 pathway of MAPK signaling cascades to
regulate proliferation, apoptosis, invasion and migration.
In conclusion, the JWA gene has a significant
function in the proliferation, apoptosis, invasion and migration of
PANC-1 human pancreatic cancer cells. The increase of JWA
gene expression may inhibit the invasion and migration of
pancreatic cancer cells and this function may be achieved through
the MEK-ERK1/2 pathway of the MAPK signaling cascades. These
findings provide new scientific evidence to facilitate the clinical
treatment of pancreatic cancer.
Acknowledgements
This study was supported in part by grants from the
Natural Science Foundation of Jiangsu Province (BK2012563), and the
Medical Research Project of the Health Department of Jiangsu
Province (Z201218).
References
|
1
|
Jin C, Yao L, Long J, et al: Effect of
multiple-phase regional intra-arterial infusion chemotherapy on
patients with resectable pancreatic head adenocarcinoma. Chin Med J
(Engl). 122:284–290. 2009.
|
|
2
|
Preis M and Korc M: Signaling pathways in
pancreatic cancer. Crit Rev Eukaryot Gene Expr. 21:115–129.
2011.
|
|
3
|
Chen H, Bai J, Ye J, et al: JWA as a
functional molecule to regulate cancer cells migration via MAPK
cascades and F-actin cytoskeleton. Cell Signal. 19:1315–1327.
2007.
|
|
4
|
Aguirre-Ghiso JA, Estrada Y, Liu D and
Ossowski L: ERK(MAPK) activity as a determinant of tumor growth and
dormancy; regulation by p38(SAPK). Cancer Res. 63:1684–1695.
2003.
|
|
5
|
Kyriakis JM and Avruch J: Sounding the
alarm: protein kinase cascades activated by stress and
inflammation. J Biol Chem. 271:24313–24316. 1996.
|
|
6
|
Zhang XQ, Zhao XS, Pang ZJ, et al: Role of
p38 pathway in PMA-induced in vitro invasion of JAR human
choriocarcinoma cell line. Di Yi Jun Yi Da Xue Xue Bao. 23:792–794.
2003.(In Chinese).
|
|
7
|
Mao WG, Liu ZL, Chen R, Li AP and Zhou JW:
JWA is required for the antiproliferative and pro-apoptotic effects
of all-trans retinoic acid in Hela cells. Clin Exp Pharmacol
Physiol. 33:816–824. 2006.
|
|
8
|
Wang GL, Shi X, Salisbury E and Timchenko
NA: Regulation of apoptotic and growth inhibitory activities of
C/EBPalpha in different cell lines. Exp Cell Res. 314:1626–1639.
2008.
|
|
9
|
Zhou J, Ye J, Zhao X, Li A and Zhou J: JWA
is required for arsenic trioxide induced apoptosis in HeLa and
MCF-7 cells via reactive oxygen species and mitochondria linked
signal pathway. Toxicol Appl Pharmacol. 230:33–40. 2008.
|
|
10
|
Shi GZ, Yuan Y, Jiang GJ, et al: PRAF3
induces apoptosis and inhibits migration and invasion in human
esophageal squamous cell carcinoma. BMC Cancer. 12:972012.
|
|
11
|
Charlotte F, L’Herminé A, Martin N, et al:
Immunohistochemical detection of bcl-2 protein in normal and
pathological human liver. Am J Pathol. 144:460–465. 1994.
|
|
12
|
Xu YQ, Li AP, Chen R and Zhou JW: The role
of JWA in N-methyl-N′-nitro-N-nitrosoguanidine induced human
bronchial epithelial cell apoptosis. Zhonghua Lao Dong Wei Sheng
Zhi Ye Bing Za Zhi. 24:205–208. 2006.(In Chinese).
|
|
13
|
Li CP, Zhu YJ, Chen R, et al: Functional
polymorphisms of JWA gene are associated with risk of bladder
cancer. J Toxicol Environ Health A. 70:876–884. 2007.
|
|
14
|
Wu X, Chen H, Gao Q, et al: Downregulation
of JWA promotes tumor invasion and predicts poor prognosis in human
hepatocellular carcinoma. Mol Carcinog. 53:325–336. 2014.
|
|
15
|
Zhou J, Ge Z, Tan Y, et al: Downregulation
of JWA expression in human esophageal squamous cell carcinoma and
its clinical significance. Oncol Res. 20:157–162. 2012.
|
|
16
|
Lawrence MC, Jivan A, Shao C, et al: The
roles of MAPKs in disease. Cell Res. 18:436–442. 2008.
|
|
17
|
Johnson GL and Lapadat R:
Mitogen-activated protein kinase pathways mediated by ERK, JNK, and
p38 protein kinases. Science. 298:1911–1912. 2002.
|
|
18
|
Woo SH, Park IC, Park MJ, et al: Arsenic
trioxide induces apoptosis through a reactive oxygen
species-dependent pathway and loss of mitochondrial membrane
potential in HeLa cells. Int J Oncol. 21:57–63. 2002.
|
|
19
|
Pohl G, Ho CL, Kurman RJ, et al:
Inactivation of the mitogen-activated protein kinase pathway as a
potential target-based therapy in ovarian serous tumors with KRAS
or BRAF mutations. Cancer Res. 65:1994–2000. 2005.
|
|
20
|
Yao Q, Luo JR, Chen JH, et al: Expression
and activation of MAPK pathway signaling molecules in human breast
cancer cell lines. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 20:328–330.
2004.(In Chinese).
|