Introduction
Ampullary carcinoma is a rare entity accounting for
only 0.2% of all gastrointestinal malignancies and <6% of all
periampullary cancers (1,2). Signet ring cell carcinoma (SRC) is
extremely uncommon in the ampulla of Vater; few cases have been
previously described and only mini-reviews are available (3–11). In
the current study, owing to its location at the ampulla,
obstructive jaundice was the most common symptom. The majority of
patients also exhibited dilation of the common bilary duct and the
main pancreatic duct, as well as an enhanced mass lesion in the
ampulla of Vater, as determined by helical computed tomography
(CT). At present, surgical resection is performed as the only
curative treatment, with pancreatoduodenectomy or ampullectomy the
most common options. As for prognosis, Hara et al (12) showed that SRC localized in the
ampulla of Vater has a poor prognosis and lymph node involvement is
also regarded as a key prognostic factor. Certain authors have also
demonstrated that initial surgical resection with adjuvant therapy
may not provide a survival benefit in patients without lymph node
invasion (5). Due to its marked
association with gastric mucosa, Blundell et al (13) hypothesized the origin of SRC only in
the presence of gastric mucosa/metaplasia. Owing to SRCs expression
of the caudal-related homeobox transcription factor 2 (CDX2) and
mucin (MUC) 2, certain authors have also suggested that they are
considered to be variants of intestinal (I)-type adenocarcinoma
(14). Gheza et al (15) reported an additional histogenesis of
SRC differentiation from the pancreatobiliary (PB) type of
adenocarcinoma. In the present study, to discuss the histological
origin and explore the correlation between the immunohistochemical
phenotypes and survival of ampullary SRC, the establishment of a
simple ampullary SRC classification system was attempted based on
the immunohistochemical staining of cytokeratin (CK) and MUC in
eight cases.
Patients and methods
Patients
A retrospective review of the records of all the
patients diagnosed with ampullary cancer at the First Affiliated
Hospital, (Hangzhou, China) was performed between January 2008 and
December 2012. The study was approved by the ethics committee of
The First Affiliated Hospital, College of Medicine, Zhejiang
University (Hangzhou, China) and written informed consent was
obtained for each patient. A total of 162 patients were identified
with ampullary neoplasms and their final histopathological
diagnosis was determined from pancreaticoduodenectomy specimens. Of
these, 152 (93.8%) were adenocarcinoma, eight (4.9%) were SRC and
two (1.2%) were neuroendocrine tumors. Of the patients with SRC,
four were male and four were female with an average age of 60 years
(range, 42–75 years). The patients’ clinical details, surgical
outcome with five-year follow-up and treatment were reviewed. All
patients underwent pancreaticoduodenectomy and one patient
underwent extended lymphadenectomy.
Antibodies
The signet ring cells were stained
immunohistochemically using the following antibodies: Monoclonal
mouse anti-human CK7 (clone OV-TL 12/30; 1:200; Dako Agilent
Technologies Company, Copenhagen, Denmark), CK20 (clone Ks20.8;
1:80; Dako Agilent Technologies Company), CDX2 (clone DAK-CDX2;
1:200; Dako Agilent Technologies Company), MUC1 (clone MRQ-17;
1:80; Cell Marque Corporation, Rocklin, CA, USA), MUC2 (clone
CCP58; 1:100; Beijing Zhongshan Golden Bridge Biotechnology Co.,
Ltd., Beijing, China), MUC5AC (clone MRQ-19; 1:100; Beijing
Zhongshan Golden Bridge Biotechnology Co., Ltd.), MUC6 (clone
MRQ-20; 1:100; Beijing Zhongshan Golden Bridge Biotechnology Co.,
Ltd.), E-cadherin (clone NCH-38; 1:100; Dako Agilent Technologies
Company), β-catenin (clone β-catenin-1; 1:200; Dako Agilent
Technologies Company) and CD10 (clone 56C6; 1:50; Dako Agilent
Technologies Company).
Immunohistochemisty
After being fixed in 10% natural-buffered formalin
for ~24 h, all tissue specimens were embedded in paraffin,
sectioned (4-μm thick), and then stained with hematoxylin and
eosin. The histopathological variables included lymphovascular and
tumor size, and all tumors were staged according to the WHO
classification for ampullary carcinoma (16); cases in which signet ring cells
constituted >50% of the adenocarcinoma were regarded as SRC. The
histology of all tumors was examined by experienced pathologists
with no prior knowledge of the clinical or pathological outcomes.
Positive and negative controls were produced for all of the
antibodies tested. The staining score was evaluated as the
percentage ratio of stained signet ring cells to the total number
of cells evaluated. The signet ring cells showing <10% tumor
cell positivity were regarded as negative, 10–50% tumor cell
positivity were designated as focal positive and >50% tumor cell
positivity were considered diffuse positive.
Results
Clinical and pathological features
The pertinent clinical features are summarized in
the Tables I and II. Of all 162 patients with ampullary
lesions, eight cases (4.9%) were diagnosed as SRC, with a mean age
at presentation of 60 years. The presenting symptoms information
was available for all eight cases. Five patients presented with
jaundice, weight loss and abdominal pain associated with the tumor,
while two patients had fever and vomiting. The remaining case was
asymptomatic and the disease was recognized by routine abdominal
ultrasound and CT with diffuse ampulla of Vater wall thickening.
The predominant radiological observations were long segmental wall
thickening, dilatation of the common biliary tract and the main
pancreatic duct, and an enhanced mass lesion. All patients
underwent surgical pancreaticoduodenectomy and one patient
underwent extended lymphadenectomy. The overall tumor size ranged
between 1.2 and 9.5 cm, and the mean size was 3.8 cm. Postoperative
adjuvant chemotherapy was performed in four cases and one patient
received radiotherapy. Follow-up data were available for all eight
patients; five patients are alive (cases 1, 2, 6, 7 and 8),
including two with clinical evidence of lymph node metastatic
disease (cases 1 and 6); one patient succumbed to brain and bone
metastases at two years following diagnosis (case 4); one patient
succumbed to liver metastases at one and a half years following
diagnosis (case 3); and one patient succumbed to lymph node
metastases at nine months following diagnosis (case 5).
 | Table IClinical features of eight patients
with signet ring cell carcinoma lesions. |
Table I
Clinical features of eight patients
with signet ring cell carcinoma lesions.
| Case | Age,
years/gender | Clinical
presentation | CT/MRI | Treatment following
PD | Follow-up status
(months) |
|---|
| 1 | 40/F | Jaundice,
intermittent right abdominal pain | Dilated CBD with soft
tissue mass at ampulla | ACT | A (8) |
| 2 | 64/F | Fever, weight
loss | Dilated CBD mass in
ampullary region | None | A (76) |
| 3 | 75/F | Jaundice, weight
loss, upper abdominal pain | Peri-ampullary
mass | ACT/ART | D (16) |
| 4 | 62/M | Jaundice, fever,
abdominal pain, vomiting | Dilated CBD mass in
ampullary region | None | D (27) |
| 5 | 62/M | Fever, vomiting,
diarrhea | Dilated CBD with wall
thickening | None | D (9) |
| 6 | 53/M | Asymptomatic | Diffuse ampullary
wall thickening | ACT | A (45) |
| 7 | 66/F | Jaundice,
diarrhea | Dilated CBD mass in
ampullary region | None | A (54) |
| 8 | 68/M | Jaundice, weight
loss | Peri-ampullary
mass | ACT | A (72) |
 | Table IIPathological characteristics of signet
ring cell carcinoma lesions. |
Table II
Pathological characteristics of signet
ring cell carcinoma lesions.
| Case | Tumor size, cm | Lymphatic
invasion | Vascular
invasion | Histology | Stagea |
|---|
| 1 | 3.0×2.0 | P | P | Por | IIA |
| 2 | 6.5×4.2 | P | P | Pfm | III |
| 3 | 3.0×3.5 | P | N | Por | III |
| 4 | 2.4×2.0 | P | P | Pur | IIB |
| 5 | 3.0×2.0 | P | N | Por | IIB |
| 6 | 1.2×0.7 | N | N | Por | IIA |
| 7 | 1.5×1.4 | N | N | Pur | IIA |
| 8 | 9.5×5.5 | P | N | Pur | III |
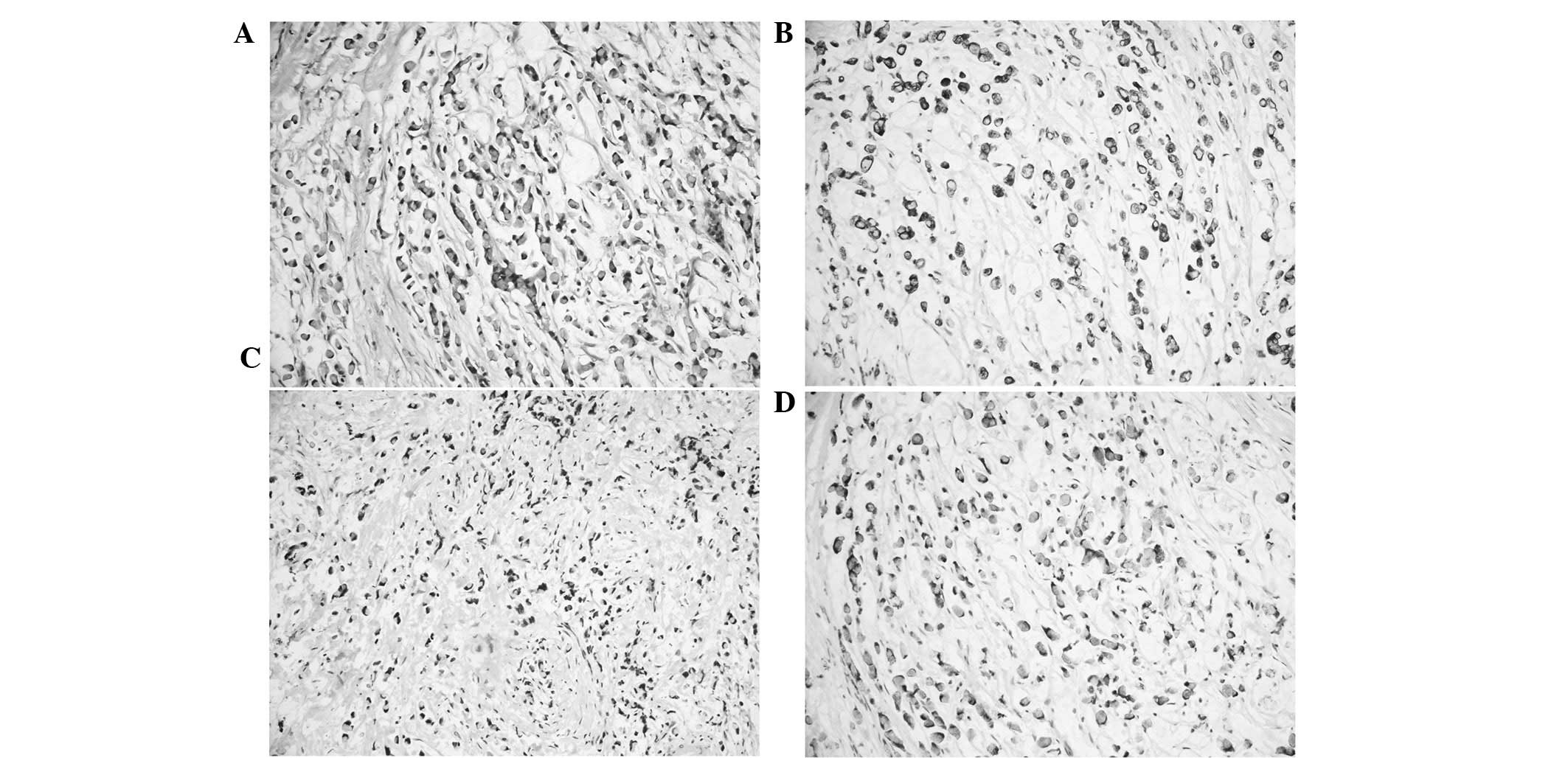
Immunohistochemical examination
The immunohistochemical results of the eight cases
are shown in Table III. In total,
four out of the eight ampullary SRCs showed poorly differentiated
adenocarcinoma with prominent signet ring cell features; of these,
three showed CK7+, CK19+ and MUC1+
staining (cases 5, 3 and 6; Fig. 1)
and one case showed positive immunoreactivity for CD10 (case 3).
Immunoreactivity for MUC5AC and MUC6 was evident in three cases
(cases 1, 3 and 5). Only one case showed prominent signet ring
cells floating in a pool of mucus which were positive for CK20,
CDX2 and MUC2 (case 2; Fig. 2). Two
pure SRCs were positive for CK7, CK19 and MUC1 (cases 4 and 7), and
one pure SRC was positive for CK19, MUC2 and MU5AC (case 8);
however, only one case was positive for MUC5AC and MUC6 (case 4).
Four cases showed loss of expression of the E-cadherin/β-catenin
complex (cases 1, 2, 6 and 8). The comparison between these
immunoprofiles and those of the eight cases of ampullary SRC are
shown in Table III. In the
present study, according to the immunohistochemical results,
ampullary SRC may be classified into the following four subtypes:
I-, PB-, gastric- and mixed-type (composed of I mucosa lining and
PB epithelium). One I-type tumor patient and one of the five
PB-type tumor patients, as well as two patients with mixed-type
tumors were classified as stage III according to the WHO criteria
(16). Two of the PB-type tumor
patients were classified as stage IIB, while one gastric-type and
two PB-type tumor patients were classified as stage IIA. No lymph
node metastasis was documented in two of the five cases of PB-type
ampullary SRC.
 | Table IIIImmunohistochemical findings. |
Table III
Immunohistochemical findings.
| Case | CK7 | CK19 | CK20 | CDX2 | MUC1 | MUC2 | MU5AC | MUC6 | β-catenin | CD10 | E-cad |
|---|
| 1 | − | + | − | − | − | − | +/− | + | − | − | − |
| 2 | − | − | + | + | − | + | − | − | − | − | − |
| 3 | +/− | + | − | − | + | − | +/− | +/− | − | + | − |
| 4 | + | + | − | − | + | − | + | + | + | − | + |
| 5 | + | + | − | − | + | − | + | + | + | − | + |
| 6 | + | + | − | − | +/− | − | + | − | − | − | − |
| 7 | + | + | − | − | +/− | − | + | − | + | − | + |
| 8 | − | + | − | − | − | + | + | − | − | − | − |
Discussion
SRC is a rare tumor that arises in a number of
organs, including the stomach, gallbladder, breast and urinary
bladder; particularly in the stomach (17). Sekoguchi and Mizumoto (3) first reported this histological pattern
in 1979, and individual case reports or small series of a few cases
have since been reported in the English language literature
(4–11). In accordance with the WHO
classification of the gastrointestinal tract (16), the present study defined SRC as
cases in which the adenocarcinoma constituted >50% signet ring
cells. Li et al (6) reported
14 patients presenting with ampullary SRC, including eight males
and six females. The median age of the published cases was 57 years
(range, 32–83 years) and the median tumor diameter was 1.8 cm
(range, 0.8–2.5 cm). The current study reports SRC of the ampulla
of Vater, which represents a rare entity accounting for only 4.9%
of all ampullary cancers. Furthermore, no sex predilection was
observed in the distribution of SRC; males and females were equally
affected in the eight cases. The mean patient age at diagnosis was
60 years (range, 42–75 years). Owing to its location at the
ampulla, obstructive jaundice was the most common symptom. The
majority of patients also exhibited dilation of the common biliary
duct and the main pancreatic duct, as well as an enhanced mass
lesion in the ampulla of Vater, as determined by helical CT. The
tumor size ranged between 1.0 and 9.5 cm (mean size, 3.8 cm).
It is well known that SRC is an extremely rare
histological subtype of adenocarcinoma, which is normally found in
the gastrointestinal tract, particularly in the stomach. Due to its
association with the gastric epithelium, Blundell et al
(13) hypothesized the origin of
ampullary SRC only in the presence of gastric mucosa/metaplasia.
Furthermore, Bakkelund et al (18) demonstrated that SRCs originate from
neuroendocrine cells in gastric cancers. One author has also
described a double-secreting amphicrine tumor with a large
population of neuroendocrine cells in the ampullary SRC (19). The majority of studies have
attempted to discuss the cellular origin and differentiation of
ampullary SRC based on the immunohistochemical staining of CK and
MUC. de Paiva Haddadd et al (20) demonstrated that MUC1 and CK7 were
associated with the PB phenotype; whereas the expression of CK20,
MUC2 and CDX2 were significantly in ampullary tumors of the I-type
than that in PB-type. In addition, the expression of CD10 was
significantly higher in I tumors (20). MUC5AC and MUC6 coexpression has been
regarded to represent gastric differentiation (21). According to immunohistochemical
classification systems, Kawabata et al (22) attempted to classify ampullary
carcinomas into the following three types: I, PB and unusual types.
With regard to the different histological phenotypes, the present
study attempted to establish a simple ampullary SRC classification
system based on the immunohistochemical staining of CK and MUC. In
total, four out of eight cases showed poorly differentiated
ampullary adenocarcinomas with prominent signet ring cells floating
in the pools of mucous, two of these cases were positive for CK7,
CK19 and MUC1, while only one was positive for CK7, CK19, MUC1 and
CD10. This was suggestive of SRC arising from the distal section of
the ductal pancreatic or biliary epithelium in the former cases
(cases 5 and 6) and SRC arising from the I mucosa lining and PB
epithelium in the latter (case 3). Additional results indicated
that the CK20+, CDX2+ and MUC2+
pattern fully corresponds to the immunohistochemical I type in the
one patient with prominent signet ring cells floating in the pool
of mucus (case 2). Two of the pure SRCs showed positive expression
of CK7, CK19 and MUC1, which indicated that the tumor cells had
arisen from PB differentiation (cases 4 and 7); while one case was
positive for CK19, MUC2 and MU5AC, which suggested that the pure
SRC had arisen from the mixed type, consisting of I muscosa linig
and PB epithelium (case 8). In this study, four of the eight
ampullary SRC patients were positive for gastric MUC5AC and MUC6,
and heterotopic or metaplastic gastric mucosa was observed
frequently in the peritumoral lesion in ampullary SRC. These
results indicated that specific ampullary SRC may arise from
gastric differentiation. According to these results, these tumors
were classified into the following four types: I, PB, gastric and
mixed types (composed of I and PB epithelium). The
gastric/pyloric-type epithelium is frequently observed in
intraductal lesions of the pancreas, particularly in intraductal
papillary mucinous neoplasm composed of the I and PB epithelium.
Chetty and Serra (23) suggested
that gastric/pyloric metaplasia of the pancreatic ductal epithelium
is a common and perhaps pivotal event in the pathogenesis of
intraductal lesions. It also indicated a close pathogenic
correlation between the I/PB and gastric phenotypes in ampullary
SRC. In this study, all of cases showed no neuroendocrine
differentiation.
Hsu et al (24) described that the loss of expression
of the E-cadherin/β-catenin complex does not correlate with less
differentiated histology and poor prognosis in ampullary cancer.
Patients whose tissues showed membranous staining of E-cadherin
exhibit a long-term result similar to that of aberrant cytoplasmic
expression. Park et al (25)
found that the nuclear accumulation of β-catenin expression
correlates with protruding growth and the well-differentiated type.
The authors also reported that the membranous loss of β-catenin is
associated with poor survival rate. In the gastrointestinal tract,
the majority of SRCs exhibit a loss of E-cadherin and β-catenin.
However, in the present study, three out of four of the PB-type
ampullary SRCs exhibited membranous staining of E-cadherin and
β-catenin (cases 4, 5 and 7). This may suggest that E-cadherin and
β-catenin coexpression results from the activation of an additional
oncogenic pathway that induces carcinogenesis in the PB-type
ampullary SRC. The carcinogenesis of ampullary SRC may differ from
that of other gastrointestinal malignancies.
In the current study, all cases underwent
pancreaticoduodenectomy and only one patient underwent extended
lymphadenectomy. The patients with pure SRC showed an improved
five-year survival time (mean, 51 months) than that of patients
with the mixed-type, poorly differentiated adenocarcinoma (mean, 26
months). The clinical follow-up of I-type ampullary SRC patients
revealed a more favorable prognosis than that for patients with
PB-type ampullary SRC differentiation. The patients with mixed-type
ampullary SRC may have a poorer prognosis than the other
phenotypes. Furthermore, the coexpression of E-cadherin and
β-catenin revealed a poor prognosis in ampullary SRC.
In conclusion, the current study presents eight
cases of SRC in the ampulla of Vater with regard to the detailed
clinicopathological features and immunohistochemical phenotypes. In
addition, the histological origin and prognosis is discussed and
the correlation between the immunohistochemical phenotypes and
survival is evaluated. Although the majority of the cases were
considered to be the PB type, as determined from the results of the
immunohistochemical staining, there is a possible pathogenic
correlation between gastric-type ampullary SRC, and MU5AC and MUC6
expression. In contrast to gastric SRC, E-cadherin and β-catenin
were positive in ampullary SRC, indicating that the carcinogenesis
of ampullary SRC may differ from that of other gastrointestinal
malignancies. However, a limitation of this study was the lack of
genetic study according to the suggested testing algorithm for
identifying the disease category. Furthermore, additional
investigation is required to confirm its histological origin and to
discuss the correlation between the clinicopathological features
and differentiation.
Acknowledgements
The authors would like to thank Dr Bing Wen for
excellent technical assistance.
References
|
1
|
Howe JR, Klimstra DS, Moccia RD, Conlon KC
and Brennan MF: Factors predictive of survival in ampullary
carcinoma. Ann Surg. 228:87–94. 1998.
|
|
2
|
Albores-Saavedra J, Henson DE and Klimstra
DS: Tumors of the gallbladder, extrahepatic bile duct, and ampulla
of Vater. Atlas of Tumor Pathology. 3rd series. Armed Forces
Institute of Pathology; Washington, DC: pp. 259–316. 2000
|
|
3
|
Sekoguchi T and Mizumoto R:
Clinicopathological study of papilla of Vater. Geka Chiryo. 41:1–5.
1979.(In Japanese).
|
|
4
|
Eriguchi N, Aoyagi S and Jimi A: Signet
ring cell carcinoma of the ampulla of Vater: report of a case. Surg
Today. 33:467–469. 2003.
|
|
5
|
Ramia JM, Mansilla A, Villar J, et al:
Signet-ring-cell carcinoma of the Vater’s ampulla. JOP. 5:495–497.
2004.
|
|
6
|
Li L, Chen QH, Sullivan JD, et al:
Signet-ring cell carcinoma of the ampulla of Vater. Ann Clin Lab
Sci. 34:471–475. 2004.
|
|
7
|
Bloomston M, Walker M and Frankel WL:
Radical resection in signet ring carcinoma of the ampulla of Vater:
report of an 11-year survivor. Am Surg. 72:193–195. 2006.
|
|
8
|
Akatsu T, Aiura K, Takahashi S, et al:
Signet-ring cell carcinoma of the ampulla of Vater: report of a
case. Surg Today. 37:1110–1114. 2007.
|
|
9
|
Gao JM, Tang SS, Fu W and Fan R:
Signet-ring cell carcinoma of ampulla of Vater: contrast-enhanced
ultrasound findings. World J Gastroenterol. 15:888–891. 2009.
|
|
10
|
Maekawa H, Sakurada M, Orita H and Sato K:
Signet-ring cell carcinoma co-existing with adenocarcinoma of the
ampulla of vater. JOP. 12:162–166. 2011.
|
|
11
|
Daoudi K, El Haoudi K, Bouyahia N, et al:
Signet ring cell carcinoma of the Vater’s ampulla: A very rare
malignancy. Case Rep Oncol Med. Sep 26–2012.(Epub ahead of
print).
|
|
12
|
Hara T, Kawashima H, Ishigooka M, et al:
Signet-ring-cell carcinoma of the ampulla of Vater: a case report.
Hepatogastroenterology. 49:561–563. 2002.
|
|
13
|
Blundell CR, Kanun CS and Earnest DL:
Biliary obstruction by heterotopic gastric mucosa at the ampulla of
Vater. Am J Gastroenterol. 77:111–114. 1982.
|
|
14
|
Zhou H, Schaefer N, Wolff M and Fischer
HP: Carcinoma of the ampullary of Vater: comparative
histologic/immunohistochemical classification and follow-up. Am J
Surg Pathol. 28:875–882. 2004.
|
|
15
|
Gheza F, Cervi E, Pulcini G, et al: Signet
ring cell carcinoma of the ampulla of Vater: demonstration of a
pancreatobiliary origin. Pancreas. 40:791–793. 2011.
|
|
16
|
Hamilton SR, Nakamura S, Bosman FT, Quirke
P, Boffetta P, Riboli E, IIyas M, Sobin LH and Morreau H: Carcinoma
of the colon and rectum. WHO Classifcation of Tumours of the
Digestive Organs. Bosman FT, Carneiro F, Hruban RH and Theise ND:
IARC Press; Lyon: pp. 134–146. 2010
|
|
17
|
Yokota T, Kunii Y, Teshima S, et al:
Signet ring cell carcinoma of the stomach: a clinicopathological
comparison with the other histological types. Tohoku J Exp Med.
186:121–130. 1998.
|
|
18
|
Bakkelund K, Fossmark R, Nordrum I and
Waldum H: Signet ring cells in gastric carcinomas are derived from
neuroendocrine cells. J Histochem Cytochem. 54:615–621. 2006.
|
|
19
|
Gardner HA, Matthews J and Ciano PS: A
signet-ring cell carcinoma of the ampulla of Vater. Arch Pathol Lab
Med. 114:1071–1072. 1990.
|
|
20
|
de Paiva Haddad LB, Patzina RA, Penteado
S, et al: Lymph node involvement and not the histophatologic
subtype is correlated with outcome after resection of
adenocarcinoma of the ampulla of vater. J Gastrointest Surg.
14:719–728. 2010.
|
|
21
|
Gürbüz Y and Klöppel G: Differentiation
pathways in duodenal and ampullary carcinoma: a comparative study
on mucin and trefoil peptide expression, including gastric and
colon carcinomas. Virchow Arch. 444:536–541. 2004.
|
|
22
|
Kawabata Y, Tanaka T, Nishisaka T, et al:
Cytokeratin 20 (CK20) and apomucin 1 (MUC1) expression in ampullary
carcinoma: Correlation with tumor progression and prognosis. Diagn
Pathol. 5:75–85. 2010.
|
|
23
|
Chetty R and Serra S: Intraductal tubular
adenoma (pyloric gland-type) of the pancreas: a reappraisal and
possible relationship with gastric-type intraductal papillary
mucinous neoplasm. Histopathology. 55:270–276. 2009.
|
|
24
|
Hsu HP, Shan YS, Jin YT, et al: Loss of
E-cadherin and beta-catenin is correlated with poor prognosis of
ampullary neoplasms. J Surg Oncol. 101:356–362. 2010.
|
|
25
|
Park S, Kim SW, Lee BL, et al: Expression
of E-cadherin and beta-catenin in the adenoma-carcinoma sequence of
ampulla of Vater cancer. Hepatogastroenterology. 53:28–32.
2006.
|