Introduction
Hydrogen/potassium adenosine triphosphatase
(H+/K+-ATPase), normally contained within the
lumen of gastric parietal cells, plays a vital role in the
maintenance of cellular pH homeostasis by exchanging luminal
K+ for cytoplasmic H+. This particular proton
pump also participates in the formation of the abnormal pH
gradients that are typical of gastric cancer cells during
tumorigenesis (1).
H+/K+-ATPase is composed of a 114-kDa
α-subunit and a 35-kDa β-subunit. The α-subunit functions as a
catalyst and a transporter of the proton pump, while the β-subunit
is responsible for endocytic retrieval of the complex from the
canaliculus (2).
Proton pump inhibitors (PPIs), substituted
2-pyridyl-methyl- sulfinyl-benzimidazole derivatives, exert their
anti-secretory effects through the inhibition of gastric
H+/K+-ATPase (3). PPIs are prodrugs that require
protonation in acidic conditions for full activation (4). In their acid-activated form, PPIs bind
covalently through disulfide bonds between cysteine residues
located in the luminal vestibule of the proton pumps, leading to
irreversible inhibition of the gastric proton pumps (5). Rabeprazole is a second-generation PPI
that inactivates the gastric pump through covalent binding, causing
a rapid and sustained inhibition of intracellular proton efflux, as
well as raising the extracellular pH (6,7).
As gastric cancer cells in vivo often exist
in an ischemic microenvironment with acidic conditions, it is of
great importance to maintain cellular pH homeostasis for the
function and survival of cancer cells (8,9). The
acidified microenvironment in tumors is a consequence of the
production of acidic by-products from rapid and large amounts of
glycolysis (10,11). To avoid the intracellular
accumulation of acidic moles, otherwise detrimental to cell
survival, cancer cells enhance their ability to eliminate
intracellular protons (12,13). Thus, intracellular proton extrusions
in gastric cancer cells can promote cancer cell survival under
acidic conditions. However, this protective mechanism can be
inhibited by PPIs. PPIs are able to convert into the active form
under hypoxic and acidic conditions in gastric cancer cells, a
result of the upregulated anaerobic glucose metabolism. PPIs target
gastric cancer cells and disturb cellular pH homeostasis. Previous
studies have indicated that gastric cancer cells are more
vulnerable to cell death than non-cancer cells following PPI
treatment (14).
Taken together, these data show that PPIs may target
gastric cancer cells and exert their antineoplastic effects locally
by taking advantage of the low extracellular pH of gastric cancers,
as a target and a way to specifically activate drugs within the
tumor tissues. The present study investigated whether rabeprazole
could exert an antineoplastic effect on gastric cancer cells and
analyzed the possible anticancer mechanism of rabeprazole.
Materials and methods
Cell culture and reagents
Human gastric cancer cell lines, AGS, KATO III,
MKN-28 and MKN-45, were purchased from the Shanghai Institute of
Digestive Disease (Shanghai, China). The gastric cancer cell lines
were cultured in RPMI 1640 medium (Gibco BRL, Grand Island, NY,
USA) with 10% fetal bovine serum and 100 U/ml penicillin. The
non-tumorigenic human gastric epithelial cell line, GES-1, was
established from fetal stomach cells infected with the SV40 virus
(15). The GES-1 cells were grown
in DMEM with 10% fetal bovine serum. These cells were maintained in
a humidified incubator at 37°C in a 5% CO2
atmosphere.
Rabeprazole (H20020330) was obtained from Jiangsu
Hansoh Pharmaceutical Co., Ltd. (Jiangsu, China). The ERK 1/2
inhibitor, PD98059, was purchased from Selleck Chemicals LLC
(Shanghai, China).
Analysis of cell viability
To determine the effect of acidic media on cell
viability, three gastric cancer cell lines, KATO III, MKN-28 and
MKN-45, and one control human gastric cell line, GES-1, were
cultured in media with various pH levels (7.5, 6.5 and 5.5) for 24
h. The AGS cells were further cultured at various pH levels (7.4,
6.4, and 5.4) for 16 h following treatment with rabeprazole and
PD98059 for 2 h, respectively. The cell viability was determined by
a dye exclusion assay. The viability percentage was calculated
using the following formula: The number of viable cells counted
(unstained cells) / the number of total cells × 100.
Reverse transcription polymerase chain
reaction (RT-PCR) of α- and β-subunits of
H+/K+-ATPase
Total RNA from gastric cancer cell lines was
extracted using the TRIzol reagent (Beyotime Institute of
Biotechnology, Shanghai, China) according to the manufacturer’s
instructions. The RT reaction for the first-strand cDNA synthesis
was carried out with reverse transcriptase (Beyotime Institute of
Biotechnology) using 2 μg total RNA. Specific primers were as
follows: Human H+/K+-ATPase α-subunit forward, 5′-TCT CTC CGA GCA
GCG CA-3′ and reverse, 5′-CGT CGC CAC TCT TGC TGT CG-3′; human
H+/K+-ATPase β-subunit forward, 5′-ATG GCG GCT CTG CAG GAG AA-3′
and reverse, 5′-CGT GGA GAC TCT GTG TGA CG-3′; human GAPDH forward,
5′-AGG TCG GAG TCA ACG GAT TTG -3′ and reverse, 5′-GTG ATG GCA TGG
ACT GTG GT-3′. RT-PCR was performed using the Premix Ex Taq kit
(Takara Biotechnology (Dalian) Co., Ltd., Dalian, China). The
amplifications were performed in 50-μl reaction volumes with an
initial denaturation at 94°C for 5 min prior to 38 thermal cycles
of 94°C for 1 min, 55°C for 30 sec and 72°C for 1 min, with a final
extension at 72°C for 10 min. The amplified PCR products were
subjected to electrophoresis in 1% agarose gels at 80 V for 30 min
and visualized by ethidium bromide staining.
Annexin V-fluorescein isothiocyanate
(FITC)/propidium iodide (PI) staining
Induction of apoptosis was detected by an annexin
V-FITC/PI apoptosis assay kit (Major BioTech Co., Ltd., Shanghai,
China), according to the manufacturer’s instructions. Treated and
control cancer AGS cells were harvested following trypsinization
and washed twice with cold phosphate-buffered saline (PBS). The
cells were centrifuged at 500 × g for 5 min, and the supernatant
was removed. The suspensions were incubated with 20 ml annexin
V-FITC and 20 ml PI for 15 min, at room temperature and in the
dark. The cells were then evaluated by flow cytometry (Beckman
Coulter, Inc., Miami, FL, USA) and the data were analyzed using
WinMDI 2.9 (Purdue University Cytometry Laboratories, West
Lafayette, IN, USA). FITC-positive cells were classed as early
apoptotic cells and FITC- and PI-double positive cells were
interpreted as late apoptotic cells. All experiments were conducted
in triplicate.
Western blot analysis
The treated and control cells were harvested, washed
with cold PBS and lysed in cold lysis buffer. Subsequent to
incubation on ice for 30 min, lysates were centrifuged at 12,000 ×
g for 10 min at 4°C, and supernatants were then collected. Protein
concentrations were determined by the Bicinchoninic Acid Protein
Assay kit (Pierce, Rockford, IL, USA), following the manufacturer’s
instructions. Samples containing 50 μg protein were electrophoresed
on 12% sodium dodecyl sulphate-polyacrylamide gels, and transferred
to polyvinylidene difluoride (PVDF) membranes using a semidry
transfer system (Bio-Rad Laboratories, Hercules, CA, USA). The PVDF
membranes were blocked with a 5% bovine serum albumin and
Tris-buffered saline with Tween® 20 buffer for 2 h at
room temperature and incubated with specific polyclonal rabbit
anti-human antibodies corresponding to phosphorylated-ERK and total
ERK (Cell Signaling Technology, Beverly, MA, USA) overnight at
4°C.
Statistical analysis
Results are expressed as the mean ± standard
deviation. The apoptotic rates in the rabeprazole and control
groups were compared by Student’s t-test. One-way analysis of
variance was performed for multiple comparisons and the statistical
significance between the groups was determined by Duncan’s multiple
range test. P<0.05 was considered to indicate a statistically
significant difference. All analyses were performed using SPSS
v15.0 (SPSS, Inc., Chicago, IL, USA).
Results
Gastric cancer cells show more tolerance
to acidic culture media than non-cancer cells
To investigate whether gastric cancer cells
exhibited better resistance to the acidic microenvironment, three
gastric cancer cell lines, KATO III, MKN-28 and MKN-45, and one
non-cancer cell line, GES-1, were cultured in media at pH values of
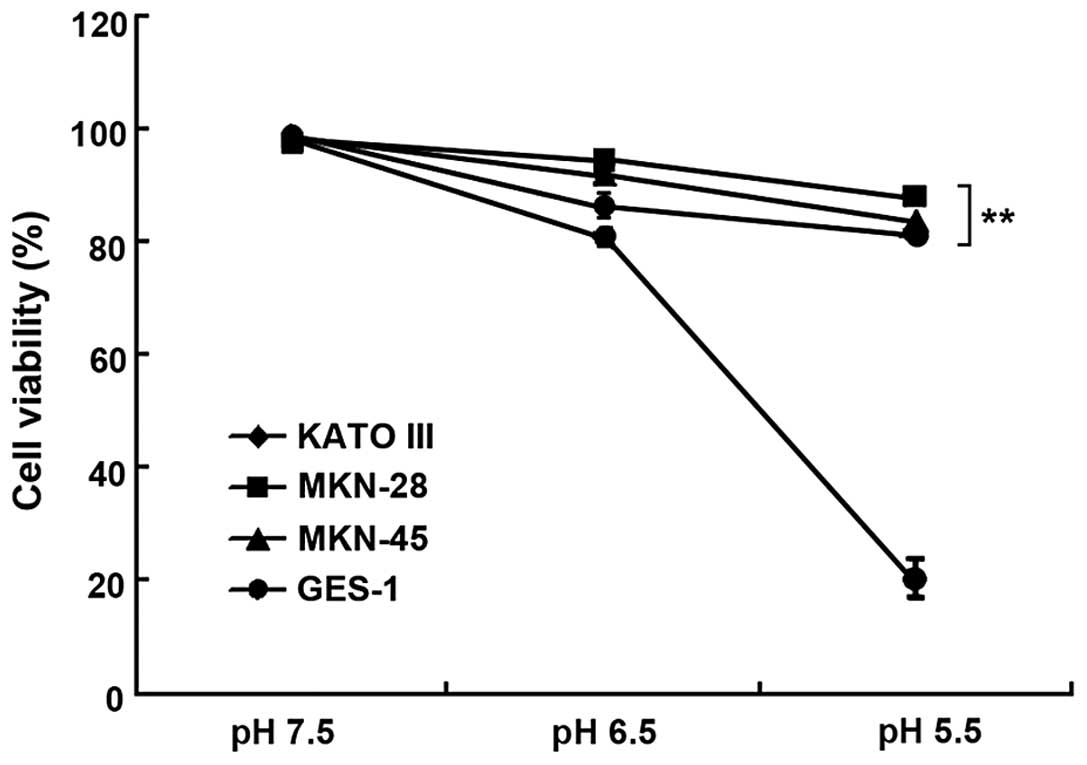
7.5, 6.5 and 5.5. As shown in Fig.
1, the gastric cancer cells were more tolerant to acidity,
whereas the viability of the non-cancer cells significantly
decreased in lower pH conditions (P<0.01). The viability of the
GES-1 cells rapidly declined to 20.30±4.05%, while the gastric
cancer cell lines retained high viability at pH 5.5, suggesting
that gastric cancer cells adapt better to acidic conditions than
non-cancer cells.
Rabeprazole exerts a strong
antiproliferative effect on gastric cancer cells under acidic
conditions
As aforementioned, the gastric cancer cells
sustained high viability when cultured in acidic media. The
increased proton efflux of cancer cells may play a protective role
in maintaining their viability in adverse microenvironments.
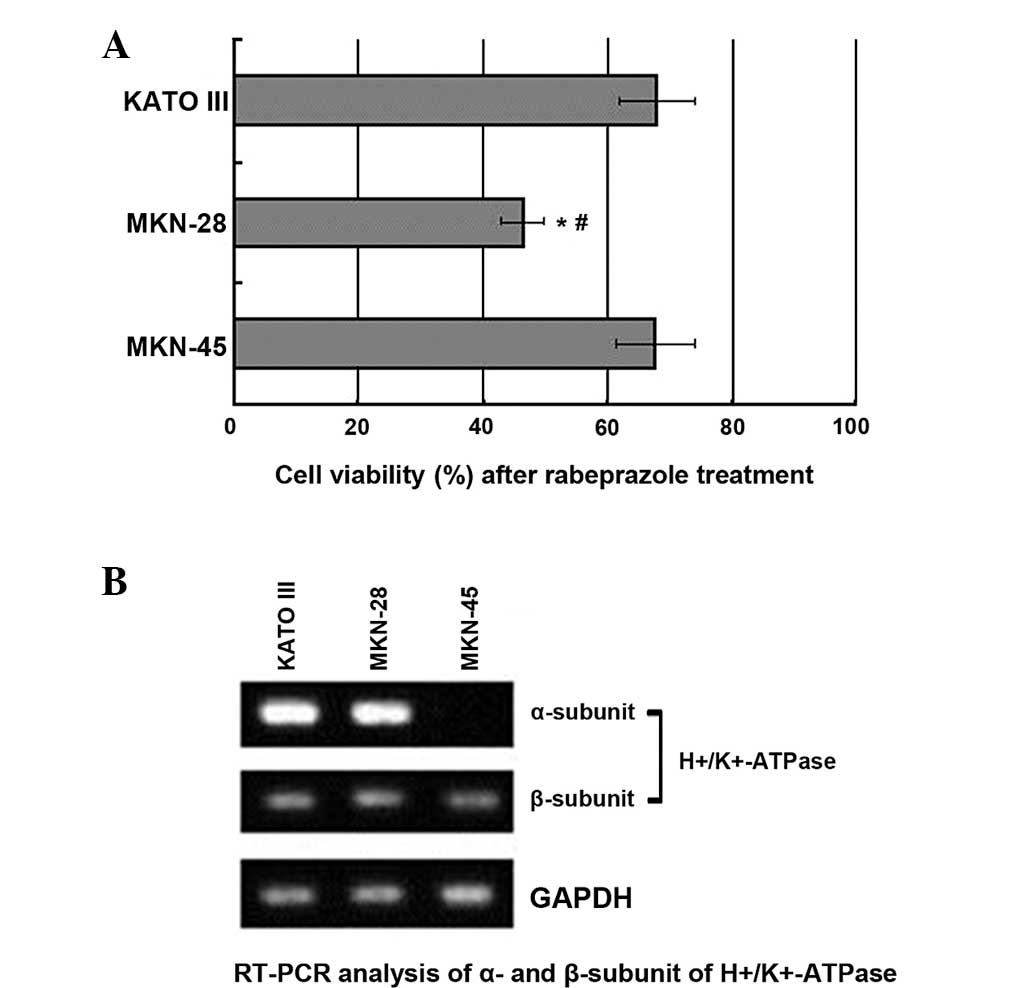
Rabeprazole was administrated to three gastric cancer cell lines,
KATO III, MKN-28 and MKN-45, at a dosage of 0.2 mM for 16 h. The
viability of these cells was determined by a trypan blue exclusion
assay. Although rabeprazole treatment resulted in the attenuation
of viability in all cancer cell lines tested (Fig. 2A), the ability of rabeprazole to
induce cell death differed considerably between the cell lines. The
viability of the MKN-28 cells was significantly lower than that of
the KATO III and MKN-45 cells (P<0.05). In addition to changes
in viability, the expression level of
H+/K+-ATPase, which is widely acknowledged as
the target of rabeprazole, was examined. As Fig. 2B shows, the α-subunit of
H+/K+-ATPase was highly expressed in the KATO
III and MKN-28 cells, while being weakly expressed in the MKN-45
cells. However, the β-subunit of H+/K+-ATPase
was equally expressed in all the cancer cell lines, based on the
similar expression level of total proteins controlled by GAPDH.
These results suggest that, in terms of rabeprazole treatment, the
ability to induce cell death was not clearly correlated with the
expression level of H+/K+-ATPase.
Rabeprazole attenuates cell viability via
induction of apoptotic cell death in gastric cancer cells
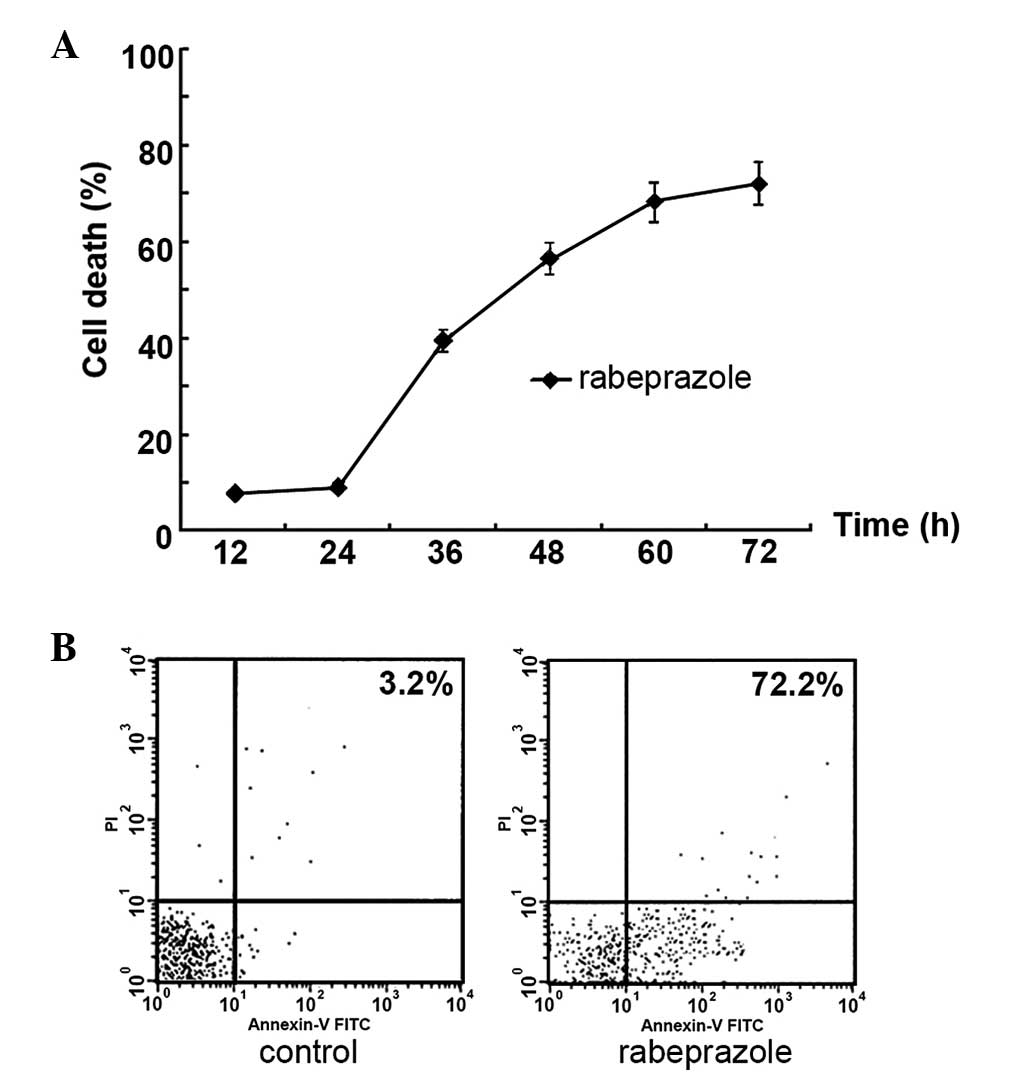
The results indicated that the treatment of
rabeprazole led to increasing cell death in a time-dependent manner
(Fig. 3A). To determine whether
rabeprazole affected gastric cancer cell viability via the
induction of apoptosis, the gastric cancer AGS cells were treated
with 0.2 mM rabeprazole for 24 h. Following annexin V-FITC/PI
staining, gastric cancer cell apoptosis was detected by flow
cytometry. Rabeprazole treatment induced apoptosis in the treated
cells (Fig. 3B). The AGS cells
displayed significant apoptosis following treatment with
rabeprazole for 72 h compared with the control group (P<0.01).
The administration of rabeprazole resulted in an increased rate of
apoptosis over time, reaching 72.21±3.24% subsequent to treatment
for 72 h. Simultaneous staining of cells with annexin V-FITC/PI
distinguished between the intact cells, early apoptosis, late
apoptosis and cell death. These results indicated that exposure to
rabeprazole mainly led to early apoptosis in the AGS cells
(Fig. 3B).
Rabeprazole inhibits the phosphorylation
of ERK1/2 in human gastric cancer cells
The human gastric cancer cells showed strong
resistance to hypoxic and acidic environments, while the non-cancer
cells could hardly tolerate such adverse conditions. It has been
reported that ERK 1/2, a subgroup of the mitogen-activated protein
kinases (MAPKs), may contribute to cancer cell survival within
acidic environments (14). The
present study therefore examined the effect of rabeprazole on
signal transduction through the ERK 1/2 pathway, which regulates
many cellular activities, particularly cell proliferation and
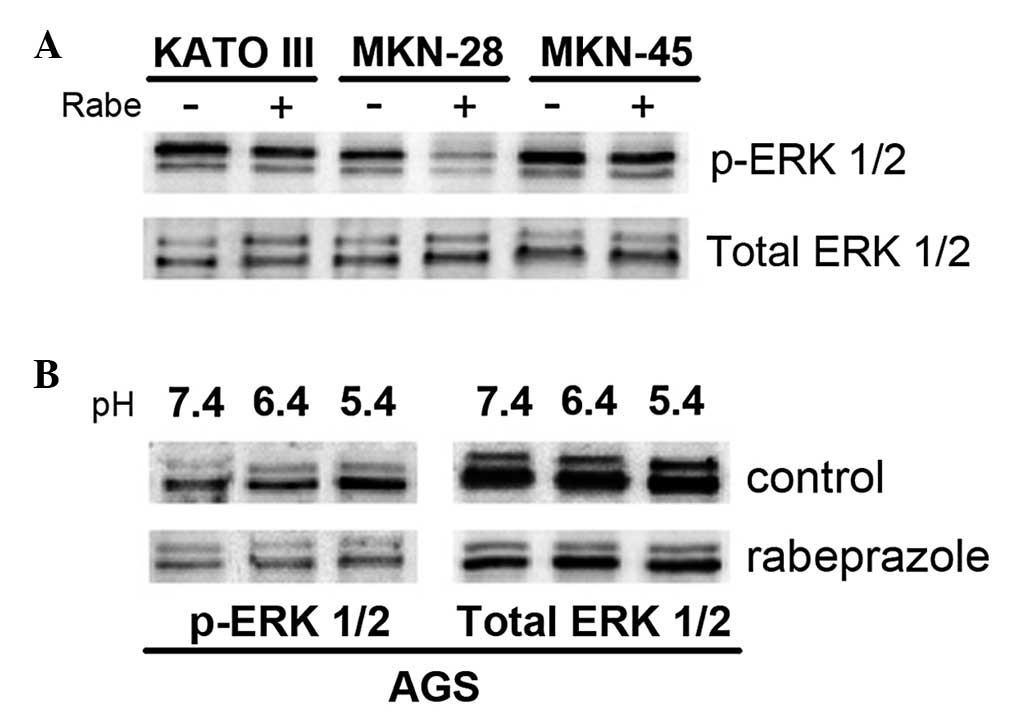
apoptosis. The KATO III, MKN-28 and MKN-45 gastric cell lines were
treated with 0.2 mM of the PPI rabeprazole at pH 5.4. Although the
phosphorylation of ERK 1/2 in the MKN-28 cells was completely
suppressed by the administration of rabeprazole, the same effect
was not observed in the KATO III and MKN-45 cells (Fig. 4A). The MKN-28 cells also displayed
significant attenuation in cell viability compared with the KATO
III and MKN-45 cells following rabeprazole treatment (Fig. 2A). These results indicated that the
sensitivity to rabeprazole-induced apoptosis in gastric cancer
cells may be correlated with the inhibitory efficacy of rabeprazole
on ERK 1/2 phosphorylation. Furthermore, the study evaluated the
inhibitory effect of rabeprazole on ERK 1/2 phosphorylation at
various pH levels, including pH 7.4, 6.4 and 5.4. Treatment with
rabeprazole suppressed ERK 1/2 phosphorylation in the AGS cells.
However, ERK 1/2 activation increased as pH declined in the control
cells (Fig. 4B). To determine
whether the induction of apoptosis in the gastric cancer cells was
due to the inhibitory actions of rabeprazole on ERK 1/2
phosphorylation, PD98059, a potent ERK 1/2 inhibitor, was
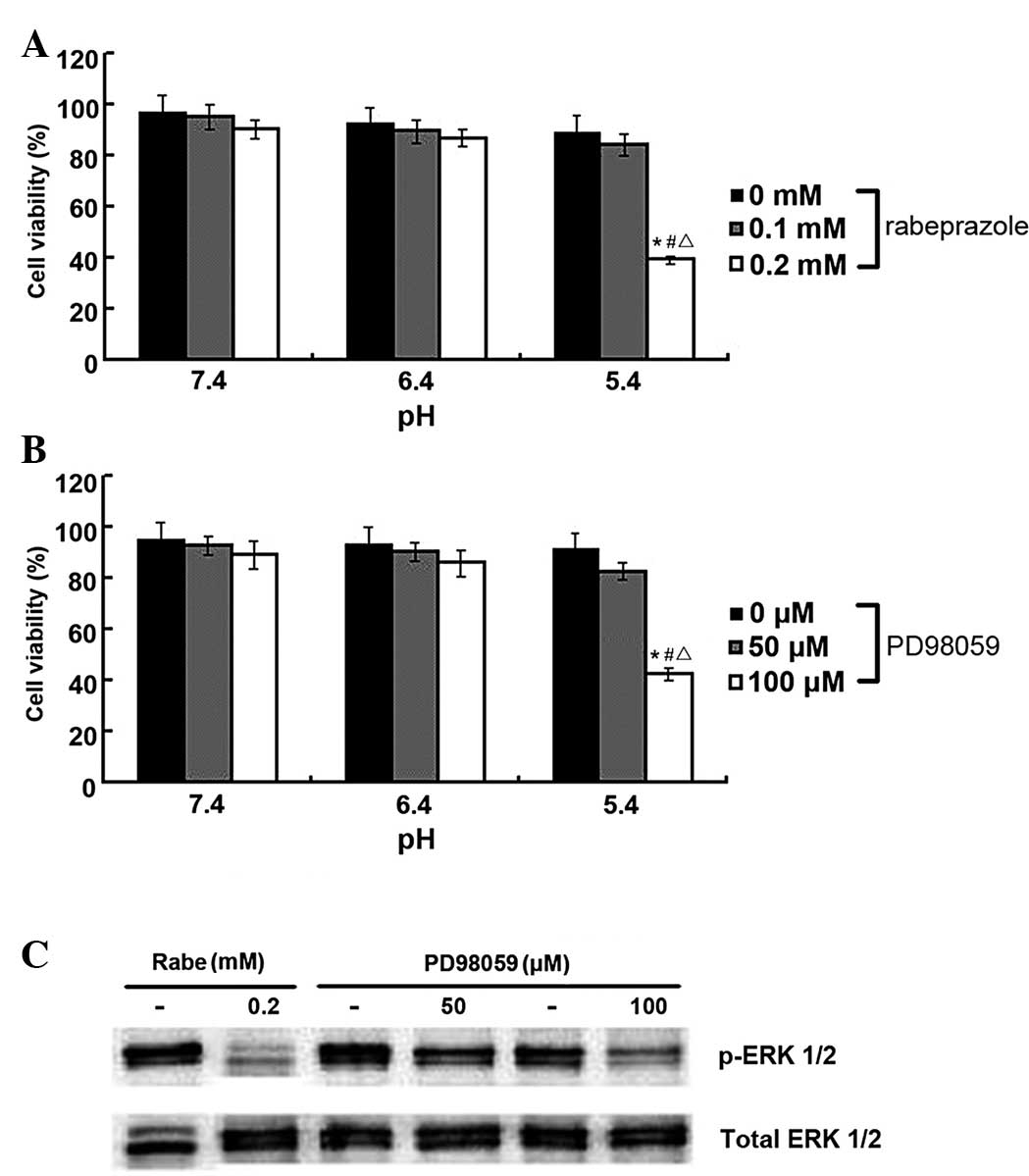
administered to the AGS cells. As shown in Fig. 5B, the viability of the AGS cells
significantly decreased following treatment with PD98059 at pH 5.4
(P<0.05). Similar antiproliferative effects were observed in the
AGS cells within the rabeprazole treatment group (Fig. 5A). In addition, these results
indicated that PD98059 and rabeprazole could each exert strong
inhibitory effects on ERK 1/2 phosphorylation in the gastric cancer
cells (Fig. 5C), thus suggesting
that PD98059-induced cell death may follow a similar pattern of
apoptotic cell death induced by rabeprazole. Consequently, it is
conceivable that rabeprazole-induced inhibition of the
phosphorylation of ERK 1/2 participates in the attenuation of cell
viability and the induction of apoptosis in gastric cancer
cells.
Discussion
Current antineoplastic strategies are aimed at
promoting the efficiency and specificity of therapies for gastric
cancer. The present study indicated that rabeprazole, a
second-generation PPI, could efficaciously induce apoptosis in
human gastric cancer cells by blocking the intracellular proton
extrusion, thus supporting the idea of PPIs as a potential
treatment for gastric cancer (16,17).
Highly proliferative cancer cells produce a large amount of
H+ generated by aerobic glycolysis, lactic acid
production and proton extrusion, leading to a lower extracellular
pH level compared with normal tissues (18,19).
As promising anticancer pro-drugs, PPIs require protonation in
acidic conditions to become fully activated prior to acting on
H+/K+-ATPase (20). Specific drug delivery to the acidic
compartments and specific transformation into the active form
within the acidic microenvironment are therefore permitted by this
chemical property, thus inducing selective apoptosis in gastric
cancer cells (21). Additionally,
rabeprazole can be activated faster and has a higher accumulation
even in the weakly acidic environment compared with other
frequently used PPIs, owing to its relatively higher pKa (6,7,22,23).
Therefore, it can be rapidly converted into its active form in
acidic conditions within tumors and possibly serve as a novel
approach, which is of high efficiency and specificity, for
antineoplastic therapy.
As a potent PPI, rabeprazole is able to inactivate
H+/K+-ATPase, causing a rapid and sustained
inhibition of the gastric acidification (24). The present results suggested that
rabeprazole is able to exert an antineoplastic effect on gastric
cancer cells via the induction of apoptosis, and that the molecular
mechanism of this effect involves its inhibitory action on the
phosphorylation of ERK 1/2 in human gastric cancer cells. It is
therefore possible that rabeprazole can be used as an adjuvant
anticancer agent.
Despite the specific antiproliferative action of
rabeprazole on cancer cells, a similar effect was not observed in
the non-cancer cells in the present study. This discrepancy can be
attributed to the induction of anti-apoptotic molecules HSP70 and
HSP27 (13). Consequently,
rabeprazole can induce apoptotic cell death in human gastric cancer
cells without exerting any potential side-effects on non-cancer
cells. Overall, these results suggest that rabeprazole may
specifically target gastric cancer cells within acidic
microenvironments, generated by upregulated glycolysis, without
significant systemic toxicity.
In the present study, the gastric PPI rabeprazole
attenuated the viability of three gastric cancer cell lines, KATO
III, MKN-28 and MKN-45. However, the efficacy of this inhibitory
action differed between the three cancer cell lines. The viability
of the MKN-28 cells was markedly reduced when compared with that of
the KATO III and MKN-45 cells. The present results also
demonstrated that administration of rabeprazole induced significant
apoptosis of the AGS cells in a time-dependent manner and caused
early apoptotic cell death. Since the inhibition of intracellular
proton efflux by PPI plays a participating role in the induction of
apoptosis in gastric cancer cells, it is highly possible that the
expression level of proton transporters determines the sensitivity
to rabeprazole-induced apoptosis in anticancer therapy (14). However, the present study suggested
that the ability of rabeprazole to induce cell death does not
strongly correlate with the expression level of
H+/K+-ATPase. Following treatment with
rabeprazole, the viability of the MKN-45 cells was not as decreased
as the viability of the MKN-28 cells, despite the strong expression
of H+/K+-ATPase. This result indicates that,
besides inhibitory effects on H+/K+-ATPase,
rabeprazole may affect the activities of the other proton
transporters that contribute to cell function and survival. In
cancer cells, various intra- and extracellular pH regulatory
mechanisms operate simultaneously to maintain cellular pH
homeostasis. Among these mechanisms, vacuolar H+-ATPase
(V-ATPase) is another crucial proton transporter due to its
distinctive role in determining the acidification of the tumor
microenvironment and therefore, the elimination of toxic molecules,
such as H+ or reactive oxygen species (25). V-ATPase participates in the proton
efflux by extruding protons into the extracellular environment or
lumen of particular membrane-bound organelles to avoid the
accumulation of H+ within the cytosol, resulting in
extracellular acidification (26–28).
Therefore, V-ATPase may also participate in the enhancement of
tumor cell viability under hypoxic and acidic conditions via the
extrusion of protons, providing another potential approach for an
antineoplastic strategy.
Previous studies have shown that the MAPK pathway
may contribute to cancer cell apoptosis and proliferation (29). As a subgroup of the MAPKs, ERK 1/2
plays a crucial role in the regulation of cell proliferation
(30). In the present study,
pretreatment with rabeprazole inhibited the ERK 1/2 phosphorylation
and the viability of these cells, indicating that the inhibition of
ERK 1/2 phosphorylation may result in the induction of apoptosis in
gastric cancer cells. Moreover, the sensitivity to
rabeprazole-induced apoptotic cell death in the gastric cancer
cells appeared to be correlated with the inhibitory efficacy of
rabeprazole on ERK 1/2 phosphorylation. ERK 1/2 phosphorylation was
completely inhibited by rabeprazole in the MKN-28 cells, consistent
with its high sensitivity to rabeprazole-induced apoptosis.
However, significant ERK 1/2 phosphorylation inhibition was not
observed in either the KATO III or MKN-45 cells, whose cell
viability decreased slightly compared with the MKN-28 cells treated
within the same protocol. Furthermore, the rabeprazole-induced
inhibition of ERK 1/2 phosphorylation plays a participating role in
the attenuation of cancer cell survival under acidic conditions.
The potent ERK 1/2 inhibitor, PD98059, also exerted an inhibitory
effect on ERK 1/2 phosphorylation and simultaneously induced
apoptosis in the gastric cancer cells. Additionally, treatment with
rabeprazole led to similar effects in the same cancer cell lines.
Thus, it is conceivable that rabeprazole can induce apoptosis in
human gastric cancer cells through inhibition of the
phosphorylation of ERK 1/2.
In summary, the present results demonstrated that
rabeprazole can attenuate cell viability via the induction of
apoptosis in human gastric cancer cells. The mechanism underlying
this antiproliferative effect of rabeprazole involves the
inhibition of ERK 1/2 phosphorylation in gastric cancer cells.
Thus, this study provides mechanistic insight and supports the use
of rabeprazole as a promising agent for an anticancer strategy.
However, the potential antiproliferative effects of the agent in
vivo require investigation in the future.
Acknowledgements
The authors would like to thank the State Key
Laboratory for the Diagnosis and Treatment of Infectious Diseases
of The First Affiliated Hospital of Zhejiang University and Jiangsu
Hansoh Pharmaceutical Co., Ltd., for providing excellent technical
assistance.
References
|
1
|
Fais S: Proton pump inhibitor-induced
tumour cell death by inhibition of a detoxification mechanism. J
Intern Med. 267:515–525. 2010.
|
|
2
|
Rabon EC and Reuben MA: The mechanism and
structure of the gastric H,K-ATPase. Annu Rev Physiol. 52:321–344.
1990.
|
|
3
|
Sachs G, Shin JM and Howden CW: Review
article: the clinical pharmacology of proton pump inhibitors.
Aliment Pharmacol Ther. 23(Suppl 2): 2–8. 2006.
|
|
4
|
Shin JM, Cho YM and Sachs G: Chemistry of
covalent inhibition of the gastric (H+,
K+)-ATPase by proton pump inhibitors. J Am Chem Soc.
126:7800–7811. 2004.
|
|
5
|
Roche VF: The chemically elegant proton
pump inhibitors. Am J Pharm Educ. 70:1012006.
|
|
6
|
Pantoflickova D, Dorta G, Ravic M, Jornod
P and Blum AL: Acid inhibition on the first day of dosing:
comparison of four proton pump inhibitors. Aliment Pharmacol Ther.
17:1507–1514. 2003.
|
|
7
|
Besancon M, Simon A, Sachs G and Shin JM:
Sites of reaction of the gastric H,K-ATPase with extracytoplasmic
thiol reagents. J Biol Chem. 272:22438–22446. 1997.
|
|
8
|
Stubbs M, McSheehy PM and Griffiths JR:
Causes and consequences of acidic pH in tumors: a magnetic
resonance study. Adv Enzyme Regul. 39:13–30. 1999.
|
|
9
|
Stubbs M, Rodrigues L, Howe FA, et al:
Metabolic consequences of a reversed pH gradient in rat tumors.
Cancer Res. 54:4011–4016. 1994.
|
|
10
|
Holm E, Hagmüller E, Staedt U, et al:
Substrate balances across colonic carcinomas in humans. Cancer Res.
55:1373–1378. 1995.
|
|
11
|
Vaupel P, Kallinowski F and Okunieff P:
Blood flow, oxygen and nutrient supply, and metabolic
microenvironment of human tumors: a review. Cancer Res.
49:6449–6465. 1989.
|
|
12
|
Tannock IF and Rotin D: Acid pH in tumors
and its potential for therapeutic exploitation. Cancer Res.
49:4373–4384. 1989.
|
|
13
|
Helmlinger G, Yuan F, Dellian M and Jain
RK: Interstitial pH and pO2 gradients in solid tumors in
vivo: high-resolution measurements reveal a lack of correlation.
Nat Med. 3:177–182. 1997.
|
|
14
|
Yeo M, Kim DK, Kim YB, et al: Selective
induction of apoptosis with proton pump inhibitor in gastric cancer
cells. Clin Cancer Res. 10:8687–8696. 2004.
|
|
15
|
Ke Y, Ning T and Wang B: Establishment and
characterization of a SV40 transformed human fetal gastric
epithelial cell line-GES-1. Zhonghua Zhong Liu Za Zhi. 16:7–10.
1994.(In Chinese).
|
|
16
|
Pérez-Sayáns M, García-García A,
Reboiras-López MD and Gándara-Vila P: Role of V-ATPases in solid
tumors: importance of the subunit C (Review). Int J Oncol.
34:1513–1520. 2009.
|
|
17
|
Pérez-Sayáns M, Somoza-Martín JM,
Barros-Angueira F, Rey JM and García-García A: V-ATPase inhibitors
and implication in cancer treatment. Cancer Treat Rev. 35:707–713.
2009.
|
|
18
|
Trédan O, Galmarini CM, Patel K and
Tannock IF: Drug resistance and the solid tumor microenvironment. J
Natl Cancer Inst. 99:1441–1454. 2007.
|
|
19
|
De Milito A and Fais S: Tumor acidity,
chemoresistance and proton pump inhibitors. Future Oncol.
1:779–786. 2005.
|
|
20
|
Mullin JM, Gabello M, Murray LJ, et al:
Proton pump inhibitors: actions and reactions. Drug Discov Today.
14:647–660. 2009.
|
|
21
|
Fais S, De Milito A, You H and Qin W:
Targeting vacuolar H+-ATPases as a new strategy against
cancer. Cancer Res. 67:10627–10630. 2007.
|
|
22
|
Kromer W, Krüger U, Huber R, Hartmann M
and Steinijans VW: Differences in pH-dependent activation rates of
substituted benzimidazoles and biological in vitro correlates.
Pharmacology. 56:57–70. 1998.
|
|
23
|
Kirchheiner J, Glatt S, Fuhr U, et al:
Relative potency of proton-pump inhibitors - comparison of effects
on intragastric pH. Eur J Clin Pharmacol. 65:19–31. 2009.
|
|
24
|
Marelli S and Pace F: Rabeprazole for the
treatment of acid-related disorders. Expert Rev Gastroenterol
Hepatol. 6:423–435. 2012.
|
|
25
|
De Milito A, Marino ML and Fais S: A
rationale for the use of proton pump inhibitors as antineoplastic
agents. Curr Pharm Des. 18:1395–1406. 2012.
|
|
26
|
Nishi T and Forgac M: The vacuolar
(H+)-ATPases - nature’s most versatile proton pumps. Nat Rev Mol
Cell Biol. 3:94–103. 2002.
|
|
27
|
Hinton A, Bond S and Forgac M: V-ATPase
functions in normal and disease processes. Pflugers Arch.
457:589–598. 2009.
|
|
28
|
Sennoune SR, Luo D and Martínez-Zaguilán
R: Plasmalemmal vacuolar-type H+-ATPase in cancer biology. Cell
Biochem Biophys. 40:185–206. 2004.
|
|
29
|
Kim EK and Choi EJ: Pathological roles of
MAPK signaling pathways in human diseases. Biochim Biophys Acta.
1802:396–405. 2010.
|
|
30
|
Ballif BA and Blenis J: Molecular
mechanisms mediating mammalian mitogen-activated protein kinase
(MAPK) kinase (MEK)-MAPK cell survival signals. Cell Growth Differ.
12:397–408. 2001.
|