Introduction
Extranodal natural killer/T-cell lymphoma (ENKL) is
a rare subtype of non-Hodgkin lymphoma (NHL) and is characterized
by distinct morphology, immunophenotype and biological behavior
(1). According to the current World
Health Organization (WHO) classification, extranodal natural
killer/T-cell lymphoma is divided into ENKL and the aggressive
natural killer cell leukemia (ANKL) (2). The tumor cells of ENKL derived from
natural killer (NK) cells and, more rarely, T cells closely
correlate with Epstein-Barr virus (EBV) infection (2,3). In
addition, ENKL is estimated to account for 3–8% of malignant
lymphomas in China and the incidence is much higher than that in
Western countries (4). According to
the original location, ENKL can be divided into two major subtypes;
nasal and extranasal diseases. The nasal and paranasal lesions,
including the upper aerodigestive tract, account for ≤80% of ENKLs
(5). Currently, immunosuppression
or immune disorders frequently occur in ENKL patients and ENKL is
recognized as a highly aggressive neoplasm with poor therapeutic
effects and prognosis. Thus, no uniform treatment regimen for ENKL
has been determined (5), and the
causes of the condition require investigation. We hypothesize that
the inhibitory signals expressed in tumor cells inhibit the
effective T-cell immune response, which aids the tumor cells to
evade and destruct the immune surveillance and, ultimately, promote
the pathogenesis and progression of ENKL.
The identification of the costimulatory molecules of
the B7-CD28 superfamily has led to tremendous advancements in
understanding the mechanism of T-cell immunity in numerous tumors.
The programmed death ligand (PD-L) 1 and PD-L2 are two ligands of
programmed death 1 (PD-1) and are members of the B7 immunoglobulin
superfamily. PD-1, an immune inhibitory receptor, is a member of
the CD28 family and, as a negative regulator, inhibits T-cell
activity by conjunction with signaling through the T-cell receptor
(6). In previous studies, the
PD-1-PD-L signaling system has been demonstrated to be pivotal in
immune tolerance, autoimmune diseases, chronic infections and
inflammatory diseases, and has demonstrated a negative role in
regulating the immune response (7,8).
Notably, the PD-1/PD-L pathway is involved in tumor immune evasion
of cancers, including melanoma, lung, esophageal, breast, renal and
ovarian cancers, as well as hematopoietic malignancies (9). Iwamura et al (10) demonstrated that the small
interfering RNA-mediated knockdown of PD-L1 or PD-L2 may enhance
tumor-specific human T-cell effector functions, such as interferon
(IFN)-γ production and antigen-specific cytotoxicity. However, a
series of clinical trials concerning the systemic administration of
therapeutic antibodies for blocking PD-1 or PD-L1 have shown a
promising clinical effect in several solid tumors (11,12).
PD-L1 and PD-L2 have an extensive expression pattern in NHL,
including T- and B-cell lymphoma (13); however, the expression has not yet
been characterized in ENKL. The current study addressed the role of
the PD-Ls, particularly PD-L1, in effective T-cell interactions in
ENKL. The results are likely to provide important evidence to
delineate the cellular immune deficiency mechanism in ENKL and a
potential strategy for immunotherapy against ENKL.
Materials and methods
Cell lines and peripheral blood
mononuclear cell (PBMC) separation
The human ENKL SNK-6 and YTS cell lines were used.
The SNK-6 cell line was a gift from Professor Norio Shimizu (Chiba
University, Chiba, Japan) and the cells were cultured in RPMI-1640
(Beijing Solarbio Science and Technology Co., Ltd., Beijing, China)
medium containing 2 mmol/l glutamine, 100 U/ml penicillin and 100
μg/ml streptomycin, supplemented with 1,000 U/ml interleukin (IL)-2
(Beijing SL Pharmaceutical Co., Ltd., Beijing, China) and 10% human
AB serum provided by the Blood Center of Henan Province (Zhengzhou,
China). The YTS cell line was a gift from Professor Scott Kaufmann
(Mayo Medical Center, Rochester, MN, USA) and the cells were
cultured in RPMI-1640, supplemented with 1% non-essential amino
acids and 10% fetal calf serum (FCS; Hangzhou Sijiqing Biological
Engineering Materials Co., Ltd, Hangzhou, China). The following
cell lines were stored in a liquid nitrogen container at the
Institute of Clinical Medicine of the First Affiliated Hospital of
Zhengzhou University (Zhengzhou, China) and cultured in RPMI-1640
supplemented with 10% FCS: Human acute T-lymphoblastic leukemia
Jurkat cell line (Shanghai Institute of Cellular Biology of Chinese
Academy of Science, Shanghai, China); human cutaneous T-cell
lymphoma Hut-78 cell line (gift from Professor Scott Kaufmann; Mayo
Medical Center); anaplastic large cell lymphoma (ALCL) Karpas-299
cell line (Shanghai Institute of Cellular Biology of Chinese
Academy of Science); diffuse large B-cell lymphoma LY-1 and LY-8
cell lines (Shanghai Institute of Cellular Biology of Chinese
Academy of Science); and Burkitt lymphoma Raji and Ramos cell lines
(Shanghai Institute of Cellular Biology of Chinese Academy of
Science). All cell lines were cultured at 37°C in a 5%
CO2 humidified atmosphere. The logarithmic growth phase
cells were collected for experiments.
The blood samples obtained from 20 ENKL patients
were collected at diagnosis prior to therapy and samples from six
of the 20 patients were collected at efficacy evaluation (one, two
and three cycles) during the chemotherapy. All patients provided
written informed consent. The blood samples of 10 healthy
volunteers (HVs) were provided by the Blood Bank of Henan Province
(Zhengzhou, China). The PBMCs were isolated by density-gradient
centrifugation using Ficoll-paque (Tianjin Biotechnology
Development, Tianjin, China) and used immediately for flow
cytometry (FCM) analysis. The study was approved by the First
Affiliated Hospital of Zhengzhou University (Zhengzhou, China).
Tissue samples
The specimens of 30 ENKL patients (21 males and nine
females; mean age, 47 years; age range, 15–68 years) and 25
rhinitis patients (15 males and 10 females; mean age, 52 years; age
range, 12–76 years) were obtained from the First Affiliated
Hospital of Zhengzhou University (Zhengzhou, China) between 2010
and 2012. The lesions were all located in the nasal cavity and the
pathological tissues were formalin-fixed and paraffin-embedded
(FFPE). The diagnosis and classification were performed according
to the 2008 WHO diagnosis and classification criteria (2). Clinical staging was conducted
according to Ann Arbor stage [stage I (n=6), stage II (n=15), stage
III (n=5) and stage IV (n=4)] (14). The specimens were selected according
to the following criteria: Patients who had not received
radiotherapy and chemotherapy; and availability of complete
clinical and pathological data. The slices were reviewed by two
senior pathologists.
Fluorescence-based quantitative
polymerase chain reaction (qPCR)
In total, 5×106 cells in logarithmic
growth phase were collected. The total RNA was extracted using a
RNA extraction kit spin column method (Qiagen, Beijing, China)
according to the manufacturer’s instructions. Finally, 60 μl RNA
was collected and RNA purity (D260/D280) was 1.8–2.0, tested using
an ultraviolet spectrophotometer [SMA4000; Merinton (Beijing)
Instrument, Ltd., Beijing, China]. Subsequently, reverse
transcription was conducted according to the RevertAid™ First
Strand cDNA synthesis kit (Fermentas, Shenzhen, China)
recommendations. In total, 20 μl cDNA was obtained and stored at
−20°C until use. The following primers (synthesized by Sangon,
Shanghai, China) were used for cDNA amplification system: Forward,
CAT CTT ATT ATG CCT TGG TGT AGC A and reverse, GGA TTA CGT CTC CTC
CAA ATG TG for PD-L1; forward, CAA CTT GGC TGC TTC ACA TTT T and
reverse, TGT GGT GAC AGG TCT TTT TGT TGT for PD-L2; and forward,
TGA CGT GGA CAT CCG CAA AG and reverse, CTG GAA GGT GGA CAG GG for
β-actin. The amplification products were 146, 110 and 205 bp,
respectively. qPCR was performed using the SYBR Green Mixture kit
(CoWin Biotech Co., Ltd., Beijing, China) according to the
manufacturer’s instructions. The data was normalized to the β-actin
expression of NK cells and analyzed using the ABI 7500 Fast system
(Applied Biosystems, Foster City, CA, USA). The qPCR was repeated
three times.
Immunohistochemistry (IHC)
The IHC streptavidin-peroxidase (SP) staining method
was performed on 4 μm-thick FFPE tissue sections containing NKTL
and rhinitis tissues. The sections were deparaffinized, dehydrated
in xylene and graded ethanol solutions. The antigen retrieval was
conducted in 0.01 mol/l citrate (pH 6.0) and the slides were
incubated overnight with rabbit anti-human PD-L1 polyclonal
antibody (1:120; Proteintech, Chicago, IL, USA), rabbit anti-human
PD-L2 polyclonal antibody (1:150) and mouse anti-human PD-1
monoclonal antibody (mAb; 1:100) (both ZSGB-BIO, Beijing, China)
and phosphate-buffered saline was used as a blank control.
Incubation of the biotinylated secondary antibody with horseradish
peroxidase (HRP) and 3,3′-diaminobenzidine chromogen (all from
ZSGB-BIO, Beijing, China) was performed sequentially. Next, the
slides were counterstained with hematoxylin and then covered with
neutral balsam. The PD-1, PD-L1 and PD-L2 protein expression in the
rhinitis tissue served as controls.
IHC scoring
PD-L1, PD-L2 and PD-1 expression were evaluated as
staining at the cell membrane and cytoplasm. Positive staining for
PD-L1 and PD-L2 was determined by staining intensity and the
percentage of positive cells was determined according to the
methods used in tumors (12,13,15).
The following staining intensity grading was used: 0, no staining;
1, faint yellow; 2, claybank; and 3, sepia. The percentage of the
positive tumor cells was scored as follows: 1, <10%; 2, 10–50%;
3, >50%. The positive cases were then classified according to
the product of the staining intensity and positive tumor cell
values as follows: Positive, >3 points; and negative, <3
points. The mean count of PD-1 positive cells of the 30 cases was
used as the threshold and the cases were divided into high
PD-1+ tumor-infiltrating T lymphocytes (TILs) and low
PD-1+ TIL groups according to the threshold.
Magnetic-activated cell sorting
(MACS)
The PBMCs were isolated from the HVs using the
previously described methods. The MACS was then conducted by a
standard method. CD8+ T cells were separated using CD8
and CD56 microbeads (BD Biosciences, Heidelberg, Germany) for NKs.
The purity of these cells was measured by FCM.
FCM
FCM was performed by a standard method and the
results were acquired using a FACSCanto II cytofluorimeter (BD
Biosciences) and analyzed using BD CellQuest Pro software (BD
Biosciences). The following antibodies were used to measure the
expression of PD-1 in the PBMCs: Mouse monoclonal
anti-human-CD3-peridinin (Percp) (Catalogue number: 347344, clone:
SK7), monoclonal mouse anti-human-CD4-fluorescein isothiocyanate
(FITC) (cat. no.: 340962, clone: SK3), monoclonal mouse
anti-human-CD8-allophycocyanin-cyanine 7 (Cy7) (cat. no.: 557760,
clone: RPA-T8) and monoclonal mouse anti-human-PD-1-phycoerythrin
(PE)-Cy7 (cat. no.: 561272, clone: EH12.1). The mAb was used to
measure the purity of CD8+ T and NK cells separated by
MACS, including monoclonal mouse anti-human-CD8-Percp (cat. no.:
341004, clone: SK7), monoclonal mouse anti-human-CD3-PE-Cy7 (cat.
no.: 557749, clone: SP34-2) and monoclonal mouse
anti-human-CD56-FITC (cat. no.: 340410, clone: NCAM16.2). The mouse
IgG-FITC isotype control antibodies were also used. The antibodies
used in FCM were all purchased from BD Pharmingen (San Diego, CA,
USA).
Enzyme-linked immunosorbent assays
(ELISA)
T-helper type 1 (Th1) cytokines (IL-2 and IFN-γ)
that had secreted into the serum of the 20 ENKL patients and 10 HVs
were detected by Quantikine HS ELISA kits (R&D Systems,
Minneapolis, MN, USA). Blood samples were collected and, following
centrifugation of the blood samples at 1,100 × g for 10 min, the
serum was obtained. The standard and blank wells were placed
separately in the coated ELISA plates and the test samples were
diluted with sample diluent (R&D Systems) and added to the test
sample wells. Following the addition of the HRP-conjugate reagent
(with the exception of the blank well), the samples were incubated
at 37°C in a humidified atmosphere of 5% CO2 for 72 h
and washed five times with scrubbing solution (R&D Systems)
subsequently the chromogen solutions A and B were added. Following
five times washing of the wells with scrubbing solution, the stop
solution was added to terminate the reaction. The blank well was
taken as zero and the absorbance optical density values were read
at 450 nm within 15 min. The concentrations of IL-2 and IFN-γ were
determined by standard curves. All experiments were repeated three
times.
Functional experiments
To delineate the role of the PD-Ls in tumor T-cell
interactions in ENKL, coculture experiments were performed by
simulating the tumor microenvironment in vivo. The
experiments were divided into the following groups: i)
SNK-6/CD8+ T-cell control group; ii) SNK-6 and activated
CD8+ T-cell group; and iii) SNK-6, activated
CD8+ T-cell and anti-PD-L1 antibody group. The purified
allogeneic CD8+ T cells were then plated in 24-well
plates at a density of 6×106 cells/well, and activated
with phytohemagglutinin (PHA; 2 μg/ml, Sigma-Aldrich, St. Louis,
MO, USA) for 48 h. Next, the allogeneic CD8+ T cells
were cocultured with 6×105 SNK-6 cells stained with 5
mmol/l carboxyfluorescein succinimidyl ester (CFSE; BD Pharmingen)
in the presence of the anti-PD-L1 antibody (6 μg/ml; MIH1 clone;
eBioscience, Inc., San Diego, CA, USA) or the mouse IgG1 isotype
control mAbs. The ratio of effective and target cells was 10:1. For
the measurement of the SNK-6 cell proliferation, the SNK-6 cells
were used as the control group. The cells in each group were
harvested at 0, 24, 48 and 72 h and analyzed by FCM gating
CFSE+ events. Following the coculture of the SNK-6 and
CD8+ T cells for 72 h, the supernatants were removed to
measure the Th1 cytokine (IL-2 and IFN-γ) secretion by ELISA, of
which the SNK-6 cells were used as the control group.
CD8+ T-cell apoptosis was analyzed at 72 h by FCM gating
Annexin V+ and 7-aminoactinomycin D (AAD)+
(Annexin-V-PE-A and 7-AAD-Percp-cy5-5-A; BD Pharmingen) cells, and
the activated CD8+ T cells were simultaneously used as
the control group.
Statistical analysis
SPSS 17.0 software (SPSS, Inc., Chicago, IL, USA)
was used for statistical analysis. Data are presented as the mean ±
standard deviation. Spearman’s rank correlation test was used to
examine the correlation between PD-L1 and PD-L2 and the
clinicopathological parameters. The χ2 test was used to
analyze the positive expression rate, and Student’s t-test and
one-way analysis of variance were used to analyze the differences
among the groups. For all comparisons, P<0.05 was considered to
indicate a statistically significant difference.
Results
mRNA expression of PD-L1 and PD-L2 in NHL
cell lines
The levels of PD-L mRNA in ENKL and other NHL cell
lines were detected by qPCR. The expression of PD-L1 and PD-L2 in
SNK-6 and YTS cells was significantly elevated compared with that
in the normal NK cells (P<0.05; Fig.
1A). In addition, PD-L1 and PD-L2 were expressed in the T- and
B-cell lymphoma cell lines. However, the expression of PD-L1 and
PD-L2 mRNA in the T-cell lymphoma cell lines was higher than that
in the B-cell lymphoma cell lines (Fig.
1A). The purity of NK cells separated by MACS was 97.8%
(Fig. 1B). In addition, the
expression of PD-1 was not detected in the ENKL cell lines, SNK-6
or YTS (data not shown).
Expression of PD-L1, PD-L2 and PD-1
proteins, and correlation between PD-L1 and PD-L2 and clinical
pathological parameters of ENKL
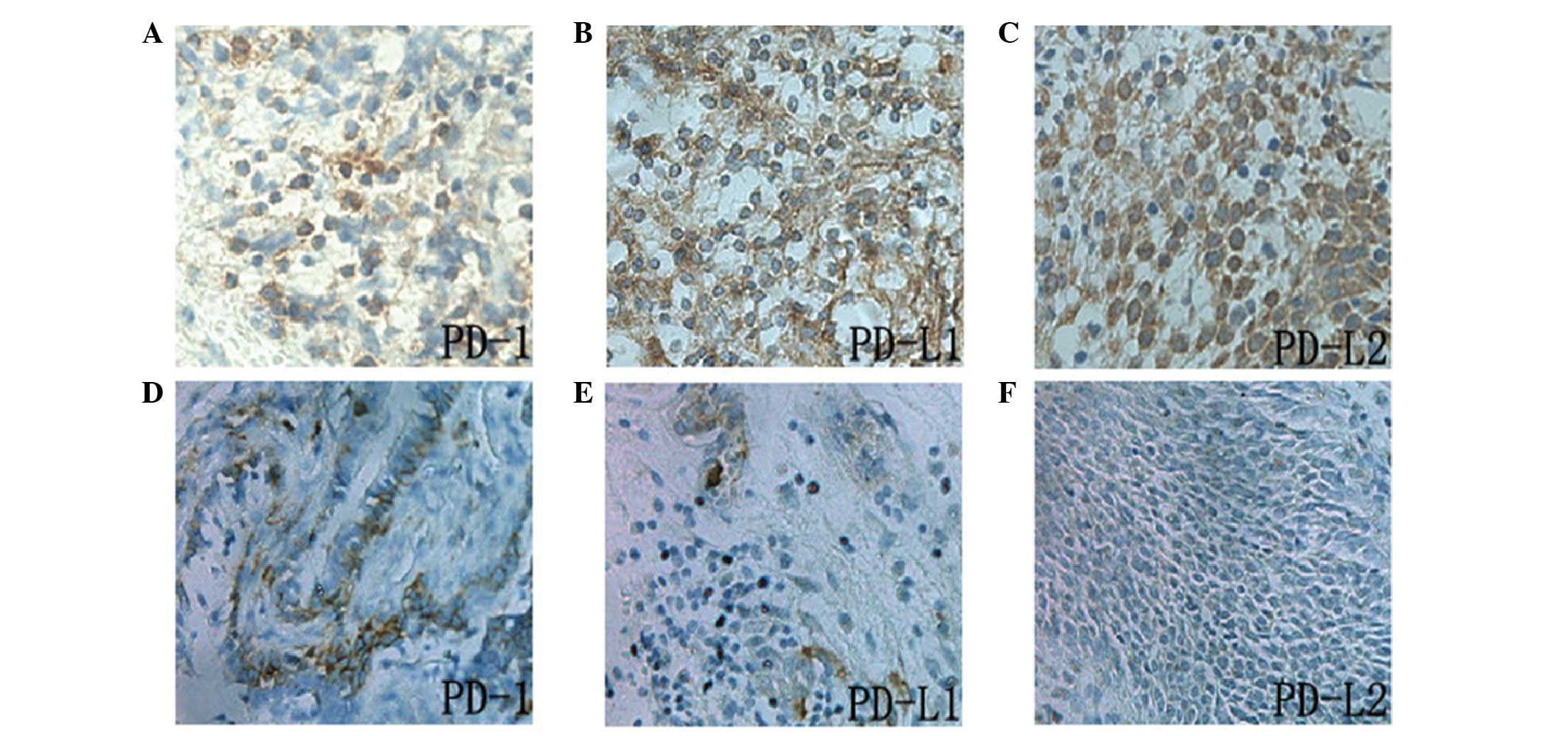
PD-L1, PD-L2 and PD-1 proteins were all located in
the cytoplasm and cell membrane, and the expression of PD-L1 and
PD-L2 was detected in tumor and stromal cells. However, PD-1 was
predominantly expressed in the tumor stromal lymphocytes (Fig. 2). The positive expression of PD-L1
and PD-L2 in ENKL tumor cells was 60.0 and 63.3%, respectively,
which was significantly increased compared with that in the
rhinitis tissues (16.0 and 12%; P<0.05). The count of
PD-1+ TILs in the 30 ENKL cases (range, 0–18; mean,
7.56) was significantly increased in contrast to that in the
rhinitis tissues (range, 0–9; mean, 1.52) (P<0.05). The mean
value of 7.56 was used as the threshold and, according to this, the
30 ENKL cases were divided into a PD-1+ TIL high-density
group (16 cases) and low-density group (14 cases). The expression
of PD-L1 and PD-L2 was found to inversely correlate with
PD-1+ TILs. Furthermore, the expression of PD-L1 and
PD-L2 was found to positively correlate with tumor stage. PD-L1 but
not PD-L2 expression was also found to positively correlate with
the international prognostic index (IPI) and lactate dehydrogenase
(LDH) and Ki-67 levels. However, no correlation was identified
between PD-L1 and PD-L2 expression and the other clinical
histopathological parameters (Table
I).
 | Table ICorrelation between PD-L1 or PD-L2
protein expression in ENKL tissues and the clinicopathological
parameters of the 30 ENKL patients. |
Table I
Correlation between PD-L1 or PD-L2
protein expression in ENKL tissues and the clinicopathological
parameters of the 30 ENKL patients.
| | PD-L1, n | PD-L2, n |
|---|
| |
|
|
|---|
| Variables | Total, n | − | + | P-value | − | + | P-value |
|---|
| Gender |
| Male | 21 | 10 | 11 | 0.193 | 8 | 13 | 0.804 |
| Female | 9 | 2 | 7 | | 3 | 6 | |
| Age, years |
| ≥45 | 17 | 7 | 10 | 0.880 | 6 | 11 | 0.858 |
| <45 | 13 | 5 | 8 | | 5 | 8 | |
| PS score |
| ≤80 | 15 | 7 | 8 | 0.456 | 5 | 10 | 0.705 |
| >80 | 15 | 5 | 10 | | 6 | 9 | |
| IPI score |
| ≤2 | 20 | 11 | 9 | 0.018 | 8 | 12 | 0.592 |
| >2 | 10 | 1 | 9 | | 3 | 7 | |
| Stage |
| I+II | 21 | 11 | 10 | 0.034 | 11 | 10 | 0.006 |
| III+IV | 9 | 1 | 8 | | 0 | 9 | |
| LDH |
| <281 | 23 | 12 | 11 | 0.014 | 10 | 13 | 0.161 |
| ≥281 | 7 | 0 | 7 | | 1 | 6 | |
| β2-MG |
| <3 | 20 | 9 | 11 | 0.429 | 7 | 13 | 0.789 |
| ≥3 | 10 | 3 | 7 | | 4 | 6 | |
| EBER |
| Positive | 25 | 9 | 16 | 0.317 | 8 | 17 | 0.236 |
| Negative | 5 | 3 | 2 | | 3 | 2 | |
| Ki-67, % |
| <60 | 15 | 9 | 6 | 0.025 | 5 | 10 | 0.705 |
| ≥60 | 15 | 3 | 12 | | 6 | 9 | |
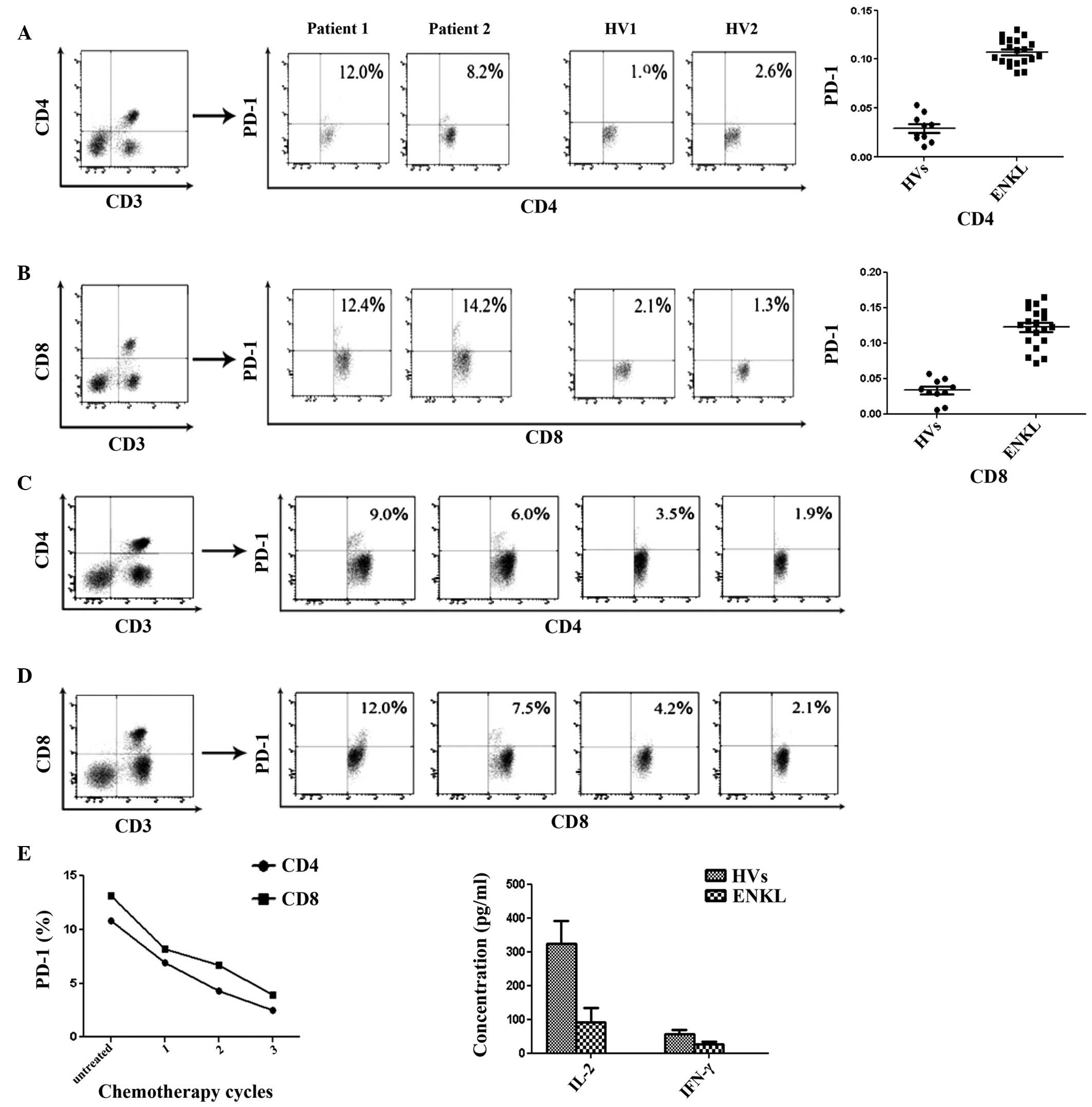
PD-1 expression in CD4+ and
CD8+ T-cell subsets prior to therapy, and PD-1
expression variation tendency with chemotherapy
By applying morphological parameters and multicolor
FCM, PD-1 expression in CD4+ (Fig. 3A) and CD8+ (Fig. 3B) T-cell subsets in 20 ENKL patients
was significantly increased compared with that in the 10 HVs
(P<0.05). Notably, PD-1 expression in the CD4+
(Fig. 3C) and CD8+
(Fig. 3D) T-cell subsets was
downregulated with chemotherapy (Fig.
3E).
Mean production levels of Th1 cytokines
(IL-2 and IFN-γ) in the serum of 20 ENKL patients
The concentration of Th1 cytokines (IL-2 and IFN-γ)
was obtained by standard curve. The mean production levels of IL-2
and IFN-γ in the serum of 20 ENKL patients were significantly lower
than that of the 10 HVs (Fig. 3F;
P<0.05).
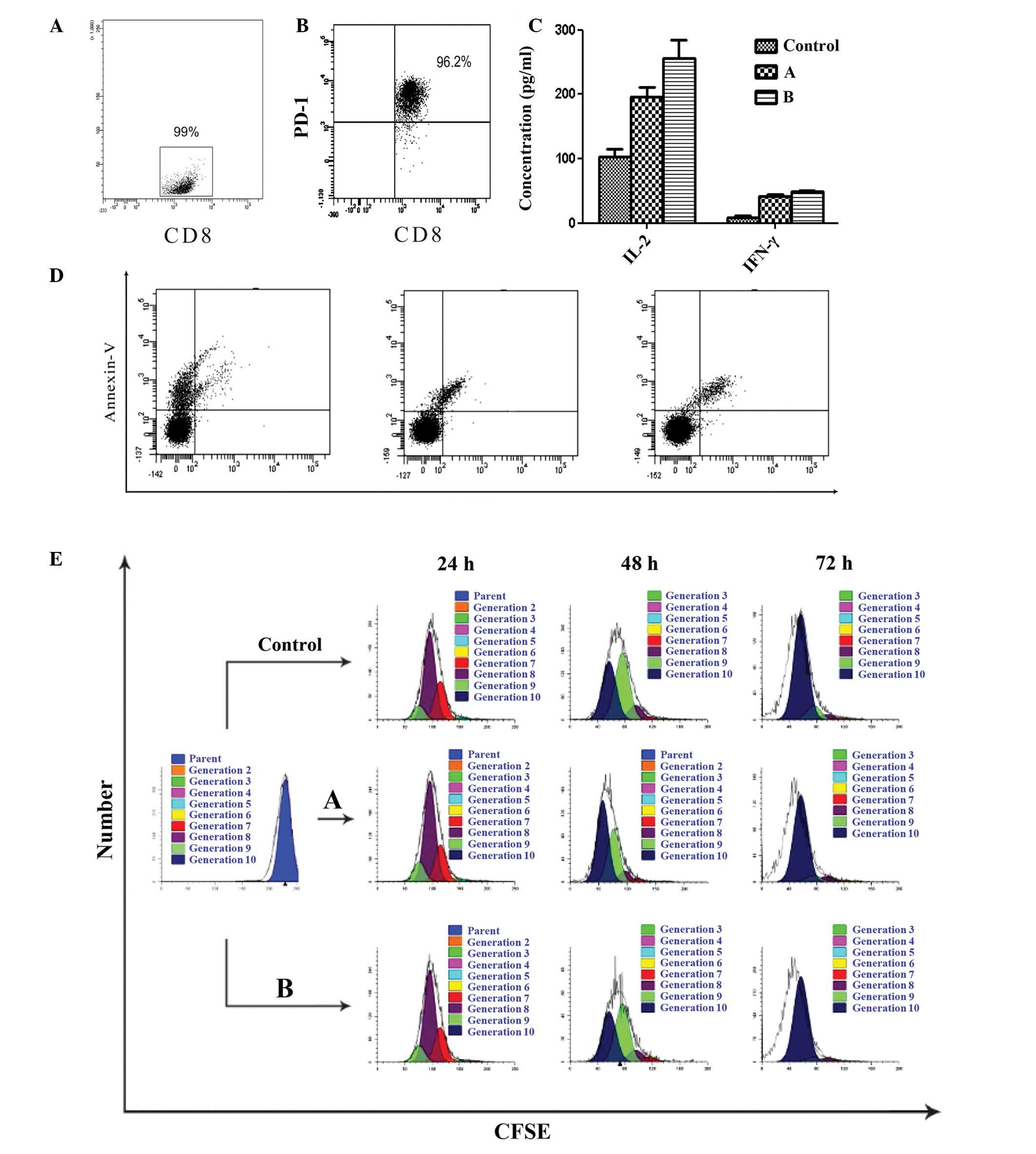
Functional significance of PD-L1
expression for purified allogeneic CD8+ T-cell
interactions
As PD-1 expression was elevated in the TILs of the
ENKL tissues, and PD-L1 and PD-L2 expression was elevated in the
tumor cells and cell lines, coculture experiments of the SNK-6
cells and purified allogeneic CD8+ T cells
(effector:target ratio, 10:1) were established by simulating the
tumor microenvironment in vivo. The purity of
CD8+ T cells separated by MACS was 99% (Fig. 4A). In the functional studies, PD-1
expression was characterized in the allogeneic CD8+ T
cells stimulated with PHA for 48 h, and the percentage of
CD8+ PD-1+ T cells was 96.2% (Fig. 4B). A significant inhibitory effect
of PD-L1 was identified for cytokine secretion (IL-2 and IFN-γ) in
the allogeneic CD8+ T-cell subset, and this inhibitory
effect was restored with the PD-L1 blocking antibody (P<0.05;
Fig. 4C). The CD8+
T-cell apoptosis in groups A and B was not altered significantly
compared with that of activated CD8+ T cells at 72 h
(P>0.05; Fig. 4D). The SNK-6
cell proliferation was detected using CD8+ T-cell
cytotoxic activity, and the proliferation index was not altered
significantly (P>0.05; Fig.
4E).
Discussion
ENKL is characterized by a highly aggressive
clinical course, poor prognosis and is analogous to ANKL (16). Generally, ENKL patients have
immunosuppression or immune disorders and, therefore, we suspect
that the expression of immune inhibitory molecules in ENKL tumor
cells impedes or paralyzes the antitumoral immune responses. It was
indicated that using tumor antigen-specific CTLs against tumor
cells is likely to have broad application prospects in the
treatment of malignancies. However, the functional activity of
tumor-specific T cells is likely to be attenuated by its receptor
(CTLA-4 and PD-1) interactions with its ligands, which transfer
negative regulation signals and ultimately prevent an effective
antitumor immune response (10).
Thus, the manner in which to improve tumor cell immunogenicity or
interfere with the biological signal for immune evasion is likely
to be via hotspots in ENKL immunotherapy studies.
The B7 immunoglobulin superfamily are the sole
costimulatory molecules which deliver regulatory signals from the
antigen-presenting cells (APCs) to the T cells. At present, the B7
family comprises eight members: CD80 (B7-1), CD86 (B7-2), CD274
(PD-L1 or B7-H1), CD273 (PD-L2 or B7-DC), CD275 (B7-H2 or ICOS-L),
CD276 (B7-H3), B7-H4 (B7S1 or B7x) and B7-H6 (17). PD-L1 is predominantly expressed in
activated T and B cells, APCs, (dendritic cells) DCs, macrophages
and human tumor cells (16). PD-L2
is not only expressed in DCs and macrophages, but also in
immunocytes, including Th2. The gene structure, gene sequence and
function of PD-L2 are similar to that of PD-L1 (18), and the extracellular region of PD-1
is 28% identical to that of CTLA-4 (19). In addition, PD-1 is expressed in
activated T and B lymphocytes, myeloid cells and thymocytes
(16).
PD-L1 and PD-L2, but particularly PD-L1, are
extensively expressed in entity tumors and hematopoietic
malignancies (9). Although PD-L1
and PD-L2 molecules share 34% identity of amino acids, their
expression has been suggested to be differentially regulated
(20). Furthermore, PD-L1 exhibits
a more extensive expression pattern and higher expression intensity
than PD-L2 in Hodgkin’s lymphoma (HL) and NHL (21–23).
However, the expression of PD-1, PD-L1 and PD-L2 in ENKL has never
been shown. The current study detected the expression and
distribution of PD-1, PD-L1 and PD-L2 in ENKL. The results showed
that PD-L1 and PD-L2 are aberrantly expressed at a high level in
the cell lines compared with normal NK cells. In addition, PD-L1
and PD-L2 were found to simultaneously exhibit higher expression
levels in T-cell lymphoma than B-cell lymphoma. By contrast, the
ENKL cell lines, SNK-6 and YTS, were found to lack expression of
PD-1. In the IHC analysis, the PD-L1 and PD-L2 proteins were found
to be located in the cytoplasm and cell membrane of tumor and
stromal cells. However, PD-1 was predominantly expressed in tumor
stromal lymphocytes. PD-L1 and PD-L2 expression in ENKL tumor cells
was significantly increased in contrast to that in rhinitis
tissues. In human pancreatic cancer, tumor PD-L1 but not PD-L2
expression showed a poorer postoperative prognosis and PD-L1
expression was found to inversely correlate with tumor
antigen-specific CD8+ T cells (24). In human esophageal cancer, no
correlation was identified between PD-L1 expression and TILs
(25). However, no correlation was
identified between PD-1 and PD-L1 expression and the stage of
disease or LDH in chronic lymphocytic leukemia (26). The results of the current study
found that PD-L1 and PD-L2 expression in tumor cells positively
correlates with clinical stage. In addition, PD-L1 but not PD-L2
expression was found to positively correlate with LDH and Ki-67
levels, as well as negatively correlate with IPI score. However, no
significant correlation was identified between PD-L1 and PD-L2
expression, and the other clinical histopathological parameters.
Notably, Greene et al (27)
using EBV-transformed B cells demonstrated that the EBV-encoded
latent membrane protein 1 through activator protein 1 and JAK-STAT
signaling can upregulate PD-L1 expression. The aberrant signaling
through EBV-encoded gene products provides alternative mechanisms
to promote PD-L1 expression in EBV-positive classical Hodgkin
lymphoma and post-transplant lymphoproliferative disorders
(13). However, the results of the
current study showed no correlation between EBV-encoded small RNA
and PD-L1 and PD-L2 expression in ENKL. However, the reason for
this may be due to the limited number of cases. In the present
study, PD-L1 and PD-L2 expression were found to negatively
correlate with PD-1+ TILs. The results of the current
study markedly indicated that ENKL tumor cells inhibit TIL activity
or promote TIL apoptosis, ultimately promoting immune evasion via
the PD-1/PD-L pathway. The Th1 cytokine (IL-2 and IFN-γ) levels
were also detected in the serum and found to be significantly
decreased. In addition, the immune inhibitory receptor PD-1
expression in CD4+ and CD8+ T cells was
significantly upregulated in the 20 ENKL patients in contrast to
that in the 10 HVs. These observations not only indicated that the
immune function in ENKL patients is suppressed, but were also in
favor of the theory that cellular immunity deficiency frequently
occurs in cancer patients. PD-1 has been identified as a prognostic
risk factor in follicular lymphoma and a marker of
angioimmunoblastic T-cell lymphoma (28,29).
However, the current study found that PD-1 expression levels are
attenuated with chemotherapy. This phenomenon may be explained by
the robust increase in the PD-1 expression of T lymphocytes in ENKL
patients when the cells are activated by specific antigens. In
addition, we suspect that chemotherapy may alter the tumor
antigen-specific response.
PD-L1 has been proposed as a bridge to connect the
innate and adaptive immunity in combating leukemia (30). In ovarian cancer, the lysis of tumor
cells with PD-L1 overexpression in CTLs is attenuated, however,
when PD-L1 is silenced, the lysis is promoted. In mouse models of
ovarian cancer following peritoneal dissemination, PD-L1 depletion
has been found to inhibit tumor growth and prolong survival
(31). Andorsky et al
(21) established a coculture
system of ALCL cell lines and allogeneic T cells, and it was
observed that T-cell proliferation and IFN-γ secretion was
increased in the presence of the anti-PD-L1 blocking antibody. In
conclusion, the activity of CTL was inhibited by tumor cells via
the PD-L1/PD-1 negative regulation pathway. However, antitumor
immune response was restored using a recombinant soluble PD-L1 or
blocking antibodies to interfere with the PD-L1/PD-1 pathway in
specific types of tumors. The current study simulated the in
vivo tumor environment and CD8+ T cells were
activated as effective cells by incubation with PHA for 48 h, and
PD-1 expression was significantly elevated compared with the
unactivated CD8+ T cells. Subsequently, SNK-6 cells and
purified activated CD8+ T cells were cocultured for 72 h
in the presence or absence of the PD-L1 blocking antibody.
Consequently, no significant change in CD8+ T cell
apoptosis and SNK-6 proliferation with or without PD-L1 blocking
antibody was identified. However, a significant inhibitory effect
of PD-L1 for allogeneic CD8+ T-cytokine secretion (IL-2
and IFN-γ) was identified and this inhibitory effect was restored
with the PD-L1 blocking antibody. The results of the current study
showed that PD-L1 expression in SNK-6 cells inhibits the Th1
cytokine secretion of CD8+ T cells in vivo.
Although PD-L1 is frequently expressed in malignant cells, the
regulatory mechanisms are not uniform and a number of complex
signaling pathways are involved in its regulation in HL and NHL
(with the exception of ENKL), such as MEK/ERK, NF-jB, PI3K/Akt,
JAK/STAT and p38 MAPK (32,33). Furthermore, two major regulatory
pathways of PD-1/PD-L2 are the NF κB and STAT6 pathways (18). However, the regulatory mechanisms of
PD-1/PD-L in ENKL are less well known; therefore, future studies
are required to delineate the role of PD-L interaction with tumor T
cells in vivo.
Currently, bioimmunotherapy is a pivotal study field
in the therapy of tumors. However, the immunotherapy of ENKL
remains immature. The results of the current study revealed that
PD-Ls and PD-1 are aberrantly expressed in ENKL and may prevent
effective antitumor immunity in vivo by interacting with
tumor T cells, which provides important information to reveal the
cellular immune deficiency mechanism in ENKL. Future studies in
animal models and patients are required to fully delineate the
immune regulation functions of PD-Ls as well as molecules involved
in the mediation mechanism of ENKL. Thus, the manner in which to
selectively block these inhibitory molecules is likely to present
an attractive approach to ENKL immunotherapy.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant no. 81172118). The authors would
like to thank the Institute of Clinical Medicine, the Biotherapy
Center and the First Affiliated Hospital of Zhengzhou University
(Zhengzhou, China) for their assistance.
References
|
1
|
Harabuchi Y, Takahara M, Kishibe K, et al:
Nasal natural killer (NK)/T-cell lymphoma: clinical, histological,
virological, and genetic features. Int J Clin Oncol. 14:181–190.
2009.
|
|
2
|
Jaffe ES: The 2008 WHO classification of
lymphomas: implications for clinical practice and translational
research. Hematology Am Soc Hematol Educ Program. 2009:523–531.
2009.
|
|
3
|
Mao Y, Zhang DW, Zhu H, et al: LMP1 and
LMP2A are potential prognostic markers of extranodal NK/T-cell
lymphoma, nasal type (ENKTL). Diag Pathol. 7:1782012.
|
|
4
|
Roma E and Smith AG: Epidemiology of
lymphoma. Histopathology. 58:4–14. 2011.
|
|
5
|
Suzuki R: NK/T-Cell Lymphomas:
pathobiology, prognosis and treatment paradigm. Curr Oncol Rep.
14:395–402. 2012.
|
|
6
|
Chen L: Co-inhibitory molecules of the
B7-CD28 family in the control of T-cell immunity. Nat Rev Immunol.
4:336–347. 2004.
|
|
7
|
Fife BT, Panken KE, Eagar TN, et al:
Interractions between PD-1 and PD-L1 promote tolerance by blocking
the TCR-induced stop signal. Nat Immunol. 10:1185–1192. 2009.
|
|
8
|
Atanackovic D, Luetkens T and Kröger N:
Coinhibitory molecule PD-1 as a potential target for the
immunotherapy of multiple myeloma. Leukemia. Oct 23–2013.(Epub
ahead of print).
|
|
9
|
Zitvogel L and Kroemer G: Targeting
PD-1/PD-L1 interactions for cancer immunotherapy. Oncoimmunology.
1:1223–1225. 2012.
|
|
10
|
Iwamura K, Kato T, Miyahara Y, et al:
siRNA-mediated silencing of PD-1 ligands enhances tumor-specific
human T-cell effector functions. Gene Ther. 19:959–966. 2012.
|
|
11
|
Brahmer JR, Tykodi SS, Chow LQ, et al:
Safety and activity of anti-PD-L1 antibody in patients with
advanced cancer. N Engl J Med. 366:2455–2465. 2012.
|
|
12
|
Topalian SL, Hodi FS, Brahmer JR, et al:
Safety, activity, and immune correlates of anti-PD-1 antibody in
cancer. N Engl J Med. 366:2443–2454. 2012.
|
|
13
|
Chen BJ, Chapuy B, Ouyang J, et al: PD-L1
expression is characteristic of a subset of aggressive B-cell
lymphomas and virus-associated malignancies. Clin Cancer Res.
19:3462–3473. 2013.
|
|
14
|
Beahrs OH, Henson DE and Hutter RVP:
Handbook for staging of cancer, from the manual for staging of
cancer. 4th ed. American joint committee on cancer (AJCC). JB
Lippincott Co; 257. pp. 1992
|
|
15
|
Taube JM, Anders RA, Young GD, et al:
Colocalization of inflammatory response with B7-h1 expression in
human melanocytic lesions supports an adaptive resistance mechanism
of immune escape. Sci Transl Med. 4:127ra372012.
|
|
16
|
Li Y, Wang J, Li C and Ke XY: Contribution
of PD-L1 to oncogenesis of lymphoma and its RNAi-based targeting
therapy. Leuk Lymph. 53:2015–2023. 2012.
|
|
17
|
Greaves P and Gribben JG: The role of B7
family molecules in hematological malignancy. Blood. 121:734–744.
2013.
|
|
18
|
Rozali EN, Hato SV, Robinson BW, et al:
Programmed death ligand 2 in cancer-induced immune suppression.
Clin Dev Immunol. 2012:6563402012.
|
|
19
|
Ishida Y, Agata Y, Shibahara K and Honjo
T: Induced expression of PD-1, a novel member of the immunoglobulin
gene superfamily, upon programmed cell death. EMBO J. 11:3887–3895.
1992.
|
|
20
|
Loke P and Allison JP: PD-L1 and PD-L2 are
differentially regulated by Th1 and Th2 cells. Proc Natl Acad Sci
USA. 100:5336–5341. 2003.
|
|
21
|
Andorsky DJ, Yamada RE, Said J, et al:
Programmed death ligand 1 is expressed by non-hodgkin lymphomas and
inhibits the activity of tumor-associated T cells. Clin Cancer Res.
17:4232–4244. 2011.
|
|
22
|
Yamamoto R, Nishikori M, Kitawaki T, et
al: PD-1-PD-1 ligand interaction contributes to immunosuppressive
microenvironment of Hodgkin lymphoma. Blood. 111:3220–3224.
2008.
|
|
23
|
Brusa D, Serra S, Coscia M, et al: The
PD-1/PD-L1 axis contributes to T-cell dysfunction in chronic
lymphocytic leukemia. Haematologica. 98:953–963. 2013.
|
|
24
|
Nomi T, Sho M, Akahori T, et al: Clinical
significance and therapeutic potential of the programmed death-1
ligand/programmed death-1 pathway in human pancreatic cancer. Clin
Cancer Res. 13:2151–2157. 2007.
|
|
25
|
Ohigashi Y, Sho M, Yamada Y, et al:
Clinical significance of programmed death-1 ligand-1 and programmed
death-1 ligand-2 expression in human esophageal cancer. Clin Cancer
Res. 11:2947–2953. 2005.
|
|
26
|
Grzywnowicz M, Zaleska J, Mertens D, et
al: Programmed death-1 and its ligand are novel immunotolerant
molecules expressed on leukemic B cells in chronic lymphocytic
leukemia. PLoS One. 7:e351782012.
|
|
27
|
Green MR, Rodig S, Juszczynski P, et al:
Constitutive AP-1 activity and EBV infection induce PD-L1 in
Hodgkin lymphomas and posttransplant lymphoproliferative disorders:
implications for targeted therapy. Clin Cancer Res. 18:1611–1618.
2012.
|
|
28
|
Takahashi H, Tomita N, Sakata S, et al:
Prognostic significance of programmed cell death-1-positive cells
in follicular lymphoma patients may alter in the rituximab era. Eur
J Haematol. 90:286–290. 2013.
|
|
29
|
Ohmatsu H, Suqaya M, Fujita H, et al:
Primary cutaneous follicular helper T-cell lymphoma treated with
allogeneic bone marrow transplantation: immunohistochemical
comparison with angioimmunoblastic T-cell lymphoma. Acta Derm
Venereol. Jan;2014.(Epub ahead of print).
|
|
30
|
Chen L: B7-H1 connection of innate and
adaptive immunity against tumor dormancy. Blood. 105:2242–2243.
2005.
|
|
31
|
Abiko A, Mandai M, Hamanishi J, et al:
PD-L1 on tumor cells is induced in ascites and promotes peritoneal
dissemination of ovarian cancer through CTL dysfunction. Clin
Cancer Res. 19:1363–1374. 2013.
|
|
32
|
Yamamoto R, Nishikori M, Tashima M, et al:
B7-H1 expression is regulated by MEK/ERK signaling pathway in
anaplastic large cell lymphoma and Hodgkin lymphoma. Cancer Sci.
100:2093–2100. 2009.
|
|
33
|
Küppers R: The biology of Hodgkin’s
lymphoma. Nat Rev Cancer. 9:15–27. 2009.
|