Introduction
Thyroid carcinoma is the most common type of primary
endocrine malignancy of the thyroid in adults. The estimated
worldwide incidence rate is ~1.7% of total cancer diagnoses
(1), and the incidence has
increased over the last decades. The mortality rate of thyroid
carcinoma has remained stable for a number of years, with a rate of
0.368 per 100,000 individuals in China (2). Thyroid nodules are diagnosed in >5%
of the adult population and may be benign adenomas or malignant
lesions. There are four types of thyroid cancer: Papillary,
follicular, medullary and anaplastic. Papillary cancer is the most
common type of thyroid cancer, accounting for 80–90% of all cases.
Papillary thyroid carcinoma (PTC) is extremely easy to treat and,
in numerous cases, curable. While papillary thyroid tumors often
spread to the cervical lymph nodes (3), they do not commonly spread to distant
organs, as observed in other types of cancer. If metastasis occurs,
it frequently involves the bones and lungs. The metastasis of
thyroid carcinoma is the major cause of fatal outcome and,
therefore, it is essential to identify metastasis-associated
molecules and to gain an improved understanding of the mechanisms
involved in the metastasis of thyroid carcinoma (4,5).
MicroRNAs (miRNAs) are 22- to 25-nucleotide
non-coding RNA molecules that typically prohibit protein production
by binding to the 3′ untranslated region (UTR) of specific target
mRNAs (6–8). This mutual effect recruits the
RNA-induced silencing complex (RISC) that facilitates nucleolytic
cleavage and/or inhibits the translation of target mRNAs. By
regulating protein yield post-transcriptionally, miRNAs transform
the formation and function of all tissues. Furthermore, certain
miRNAs may serve as oncogenes or tumor suppressor genes in the
development of tumors. However, the regulation of the majority of
miRNAs and their precise mechanisms of action remain unknown for
thyroid carcinoma.
miRNA-101 (miR-101) is a tumor suppressor miRNA.
Existing studies have indicated that miR-101 is markedly
downregulated in a number of carcinomas, including those of the
stomach, liver, breast, prostate and endometrium. Furthermore,
miRNA has been shown to effect tumor cell proliferation, migration
and invasion (9–14). However, little is known with regard
to the expression level and biological role of miR-101 in PTC.
In the current study, the differential expression of
miR-101 in human PTC samples was identified using quantitative
polymerase chain reaction (qPCR), and the function of miR-101 in
migration and invasion of thyroid cancer cells was investigated. In
addition, to understand the molecular mechanism of thyroid cancer
metastasis, the target gene of miR-101 was further investigated. To
the best of our knowledge, this is the first study to investigate
the expression and mechanism of miR-101 in thyroid carcinoma
migration and invasion. This study presents a novel target for
further therapeutic studies of thyroid cancer.
Materials and methods
PTC tissue collection
A total of 16 paired tissue specimens from PTC and
matched normal tissue were obtained from 16 PTC patients (age
range, 40–62 years; nine males and seven females) at the
Departments of Surgery of the Jiangsu Subai and Yangzhou Chinese
Medical Hospitals, affiliated to Yangzhou University (Yangzhou,
China). The absence of tumor cells in the matched normal tissues
was confirmed by a pathologist. All tissues were obtained during
surgery and immediately stored in liquid nitrogen prior to use.
Approval for this study was granted by the Institute Research
Medical Ethics Committee of the Medical College of Yangzhou
University (Yangzhou, China). Patients provided written informed
consent.
Cell line culture
TPC-1 cells were provided by Dr Sissy Jhiang (Ohio
State University, Columbus, OH, USA) and HTH83 cells were provided
by Dr Nils-Erik Heldin (University Hospital, Uppsala, Uppsala,
Sweden). TPC-1, HTH83 and 293T cells (Fengshou, Shanghai, China)
were cultured in Dulbecco’s modified Eagle’s medium, containing 10%
fetal bovine serum (FBS; HyClone, Victoria, Australia), 100 IU/ml
penicillin and 100 mg/ml streptomycin (Beyotime Institute of
Biotechnology, Haimen, China), at 37°C in a 5% CO2
humidified atmosphere.
qPCR
Total RNAs were isolated from cells using TRIzol
reagent (Invitrogen Life Technologies, San Diego, CA, USA) and
reverse transcription was performed using the PrimeScript™ First
Strand cDNA synthesis kit (Takara Bio, Inc., Dalian, Japan)
according to the manufacturer’s instructions. qPCR was performed
using the All-in-One™ miRNA qRT-PCR Detection Kit (GeneCopoeia,
Rockville, MD, USA) on an Applied Biosystems 7500 Real-Time PCR
system (Applied Biosystems, White Plains, NY, USA). The U6 small
RNA and β-actin mRNA were used as internal controls. All the
reactions were run in triplicate and the following primers were
used: Forward, 5′-GAAGATCTCCAGCCACCTGTTTCACA-3′ and reverse,
5′-CCGCTCGAGTAGTCCTTCACTTCATG-3′ for miR-101; forward,
5′-CGCTTCGGCAGCACATATAC-3′ and reverse, 5′-TTCACGAATTTGCGTGTCAT-3′
for U6; forward, 5′-AGGAAGGCGGACATATTAGTCCCT-3′ and
5′-AGACGATAGTTGGGTCCCGGC-3′ for Rac1; and forward,
5′-GTCACCAACTGGGACGACAT-3′ and reverse, 5′-GAGGCGTACAGGGATAGCAC-3′
for β-actin mRNA.
Production of retroviral particles and
infection of thyroid cancer cells
Rac1 shRNA was purchased from Santa Cruz
Biotechnology, Inc. (Santa Cruz, CA, USA). The miR-101-murine stem
cell virus (MSCV) plasmid and pRac-1-shRNA-MSCV were chemically
synthesized at the Department of Pathology, Medical College of
Yangzhou University (Yangzhou, Jiangshu) and sequenced by Sangon
Biotech (Shanghai) Co., Ltd. (Shanghai, China). 293T cells were
seeded on 90-mm dishes one day prior to transfection. Next, 10 μg
of retroviral plasmids together with 15 μg of miR101-MSCV vector
were used to cotransfect 293T cells by Lipofectamine 2000
(Invitrogen Life Technologies), according to the manufacturer’s
instructions. The retroviral supernatants were collected 48 h
following transfection and stored at −80°C. Following this, 5–8 ml
of supernatant containing the miR-101-MSCV virus together with 8
μg/ml polybrene (Sigma-Aldrich, New York, NY, USA) were used to
infect the TPC-1 and HTH83 cells. For the infection of thyroid
cancer cells, the cells were incubated at 37°C in a 5%
CO2 humidified atmosphere (15). TPC-1 and HTH83 cells were also
infected with shRac1-MSCV virus or MSCV empty vector as negative
control (NC). The miR-101 and Rac1 RNA levels in the infected
thyroid cancer cells were identified by qPCR.
Dual-luciferase reporter assay
The full-length 3′-UTR of Rac1 was amplified by PCR
from genomic DNA and cloned into the EcoRI and XhaI
sites of the pGL3-BS vector (Promega Corporation, Madison, WI,
USA). The primer sequences used were as follows: Forward,
5′-GTGAATTCACTGGTTGTTCTGTTAGTCGCT-3′ and reverse,
5′-GTTCTAGACCAGTCGTATGATTCAAGGATTT-3′ for Rac1 3′-UTR. The mutant
construct of Rac1 3′UTR was generated using a Quick Change
mutagenesis kit (Stratagene, Heidelberg, Germany). Cotransfection
of the reporter vectors, and miR-101 mimics or NCs was performed
using Lipofectamine 2000 (Invitrogen Life Technologies). After 48
h, dual-luciferase activity was measured using the
Dual-Luciferase® reporter assay system (Promega
Corporation) according to the manufacturer’s instructions.
Migration and invasion assay
In vitro cell migration and invasion assays
were performed using Transwell chambers. For the migration assays,
5×104 cells were added to the upper chamber of 8-μm pore
size Transwells (BD Biosciences, Franklin Lakes, NJ, USA). For the
invasion assays, 1×105 cells were added to the upper
chamber of 8-μm pore size Tranwells precoated with Matrigel (BD
Biosciences). In these assays, cells were plated in medium without
serum and medium containing 10% FBS in the lower chamber, serving
as a chemoattractant. After 14 h of incubation, the cells that had
not migrated or invaded through the pores were carefully removed.
The filters were then fixed in 90% alcohol, which was followed by
crystal violet staining. Five random fields were counted per
chamber using an inverted microscope (CKX41; Olympus Corporation,
Tokyo, Japan) and each test was performed in triplicate.
Western blot analysis
Proteins were extracted using cell lysis buffer for
western and immunoprecipitation (Beyotime Institute of
Biotechnology) according to the manufacturer’s instructions. The
protein concentration was quantified using the Enhanced BCA Protein
Assay kit (Beyotime Institute of Biotechnology). For western blot
analysis, equal amounts of total protein were boiled and separated
by SDS-PAGE. Following electrophoresis, the protein was blotted
onto a polyvinylidene fluoride membrane and blocked for 2 h at room
temperature. The membranes were then incubated with human
monoclonal anti-rabbit Rac1 antibody (Cell Signaling Technologies,
Boston, MA, USA) at 1:1,000 dilutions overnight at 4°C. The Rac1
protein level was then detected by goat polyclonal anti-mouse
horseradish peroxidase-conjugated secondary antibodies (Beyotime
Institute of Biotechnology) for 2 h at room temperature. The
protein bands were detected using a FluorChem FC2 imaging system
(Alpha Innotech, San Leandro, CA, USA).
Statistical analysis
Statistical analyses were performed using SPSS 16.0
(SPSS Inc., Chicago, IL, USA). All graphs were created using
Microsoft Office Excel 2010 software (Microsoft Corporation,
Redmond, WA, USA). All data from three independent experiments are
presented as the mean ± standard deviation. The differences were
assessed by two-tailed Student’s t-test, while the correlation
between RAC1 and miR-101 expression was investigated using the
two-tailed Pearson’s correlation. P<0.05 was considered to
indicate a statistically significant difference.
Results
Expression of miR-101 is downregulated in
PTC tissues and cell lines
Due to the downregulation of miR-101 in human
melanoma, the downregulation of miR-101 in human thyroid tumors was
investigated for comparison. The endogenous expression of miR-101
in human PTC and the adjacent normal thyroid tissues was compared
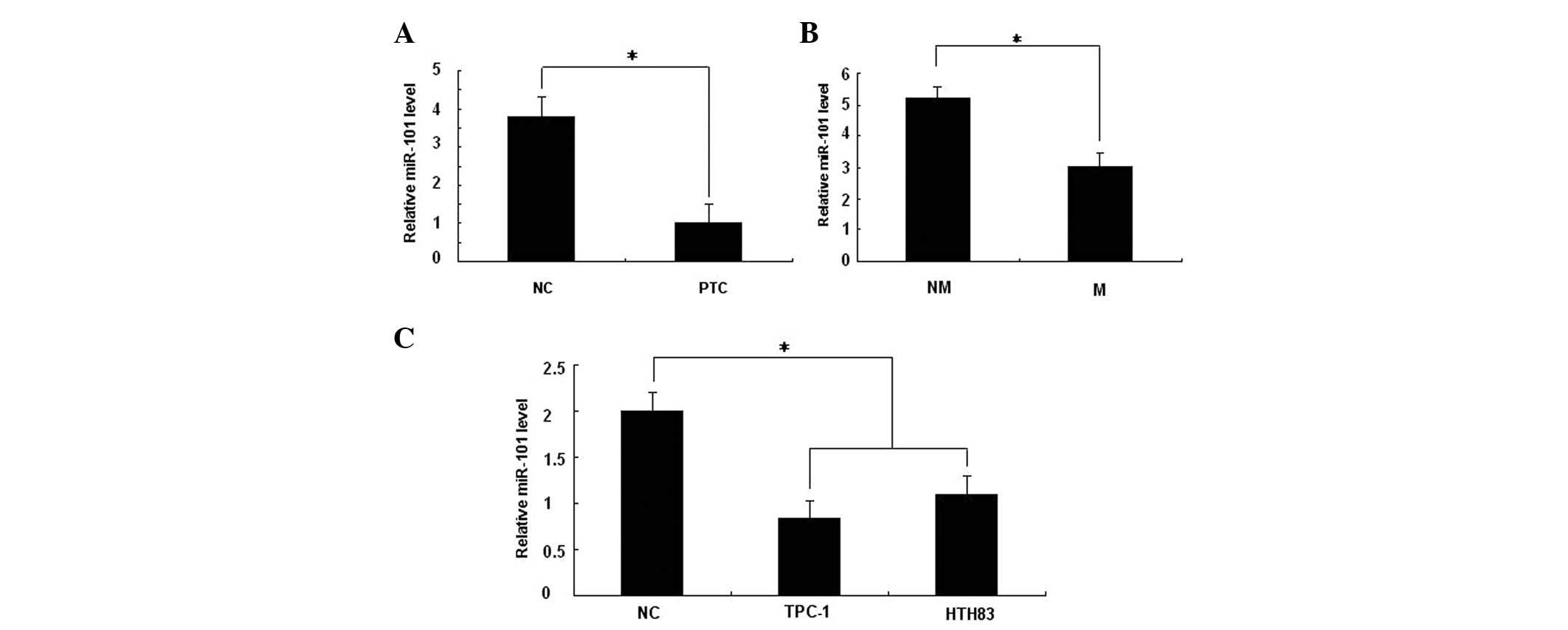
by qPCR. As shown in Fig. 1A, the
expression of miR-101 was downregulated in 93.75% (15 out of 16) of
PTC tissues, compared with the corresponding adjacent normal
thyroid tissues. The expression of miR-101 was also observed to be
further downregulated in 68.75% (11 out of 16) of PTCs with lymph
node metastasis, when compared with those without lymph node
metastasis (P<0.05; Fig. 1B).
Similarly, a decrease in the expression of miR-101 was observed in
two thyroid cancer cell lines, compared with the human thyroid
tissue cells (Fig. 1C).
Collectively, the above findings suggested that the loss of miR-101
expression may be significant in PTC development and
metastasis.
Overexpression of miR-101 inhibits
thyroid cancer cell migration and invasion
Based on the aforementioned results, the possibility
that miR-101 is involved in modulating the migration and invasion
of thyroid cancer cells was investigated. TPC-1 and HTH83 cells
were infected with miR-101-MSCV or the NC and then evaluated by
cell invasion and migration assays. As predicted, infection with
miR-101-MSCV increased miR-101 expression compared with the NC in
TPC-1 and HTH83 cells (Fig. 2A).
Furthermore, the cell migration and invasion assays indicated that
miR-101 overexpression results in a reduced migration and invasion
rate in TPC-1 and HTH83 cells compared with the control (Fig. 2B). The results also indicated that
miR-101 acts as a tumor suppressor miRNA, and contributes to the
inhibition, migration and invasion of thyroid cancer cells.
miR-101 negatively regulates Rac1 gene
expression
miRNAs are known to suppress hundreds of mRNA
targets, resulting in global changes in the cellular phenotype of
cells (16). Initially, potential
targets for miR-101 were identified using the prediction software,
TargetScan (www.targetScan.org). The Rac1 gene was
identified as the putative target gene for miR-101, which mediates
cell migration and invasion. To further confirm that Rac1 was a
target gene for miR-101, qPCR and western blot analysis were used
to detect the expression of Rac1 regulated by miR-101 in TPC-1 and
HTH83 cells. Following the overexpression of miR-101, the
expression of Rac1 was significantly downregulated at the mRNA and
protein level (Fig. 3A and 3B) when
compared with the NC. Furthermore, the mRNA levels of miR-101 and
Rac1 were also detected in PTC and the adjacent normal thyroid
tissues by qPCR. The inverse correlation between miR-101 and Rac1
in PTC was further investigated, and the Rac1 mRNA and miR-101
expression levels were determined in the same PTC specimens by
qPCR. As shown in Fig. 3C, when the
Rac1 mRNA levels were plotted against miR-101 expression, a
significant inverse correlation was observed (two-tailed Pearson’s
correlation analysis; r=−0.750; P<0.05). Similarly, as shown in
Fig. 3D, a significant inverse
correlation was also observed for PTC specimens with lymph node
metastasis (two-tailed Pearson’s correlation analysis; r=−0.820;
P<0.05). Overall, these results suggested that miR-101
negatively regulates the Rac1 gene expression at the transcription
level, and that Rac1 is a potential target gene of miR-101.
Rac1 is a direct target of miR-101
To verify whether miR-101 directly targets Rac1,
dual-luciferase reporter assays were conducted. As shown in
Fig. 4, cotransfection of 293T
cells with Rac1-3′UTR/pGL3-BS and miR-101 mimics caused a
significant decrease in the luciferase activity compared with the
NC (P<0.05). This repressive effect was eliminated by
introducing point mutations into the core binding sites of the Rac1
3′-UTR. This result indicated that miR-101 exerts an inhibitory
effect on Rac1 expression via interactions with the 3′UTR of
Rac1.
Knockdown of Rac1 reduces the migration
and invasion potential of thyroid cancer cells
To confirm the effects of Rac1 on the migration and
invasion of PTC cells, Rac1 expression was knocked down by a Rac1
shRNA. The mRNA and protein expression of Rac1 was significantly
downregulated in TPC-1 and HTH83 cells following Rac1 shRNA
treatment (Fig. 5A and B).
Consistently, the knockdown of Rac1 significantly reduced cell
migration and invasion in TPC-1 and HTH83 cells, which resembled
the inhibitory effects of miR-101 (Fig.
5C and D). These results suggested that the knockdown of Rac1
suppresses the migration and invasion of PTC cells and that Rac1 is
an effective target gene of miR-101.
Discussion
miRNAs are 22- to 25-nucleotide non-coding RNA
molecules that typically prohibit protein production by binding to
the 3′UTR of specific target mRNAs. This mutual effect recruits
RISC, which facilitates nucleolytic cleavage or inhibits
translation of target mRNAs. By regulating the protein production
post-transcriptionally, a number of miRNAs act as oncogenes or
tumor suppressor genes. However, the regulation of miR-101 and its
precise mechanisms of action in thyroid cancers remain unknown.
The results presented in this study indicated that
miR-101 exhibits lower expression in PTC tissues compared with the
matched normal thyroid tissues. miR-101 was also observed to
inhibit the invasion and migration of two thyroid cancer cell
lines. Overall, the results suggested that miR-101, as a newly
identified tumor suppressor, is involved in the metastasis and
infiltration of PTC. Furthermore, Rac1 was identified as a
potential target of miR-101 by theoretical prediction. Rac1 is one
of the most studied Rho GTPase proteins (17). It contributes to cell proliferation,
participates in the signaling pathway promoting cell survival, and
is known for its central role in the control of cell adhesion and
migration (18,19). It has also been reported that Rac1
is overexpressed in colorectal and lung tumors (20,21),
and is associated with metastasis and invasion in breast, upper
urinary tract and oral squamous cell tumors (22–25).
Three pathways have been associated with the
molecular mechanism of the knockdown of Rac1-inhibited migration
and invasion: i) Downregulation of Rac1 targets miR-124, leading to
inactivation of the MKK4/JNK/c-Jun pathway (26); ii) loss of miR-204-targeting Rac1
results in activation of brain-derived neurotrophic factor and
subsequent activation of Rac1 through the AKT/mTOR signaling
pathway, leading to cancer cell migration and invasion (27); and iii) downregulation of diallyl
disulfide-suppresses SW480 cell migration and invasion through the
Rac1-ROCK1/PAK1-LIMK1-ADF/cofilin signaling pathway (28).
In the current study, an inverse correlation was
observed between the miR-101 and Rac1 expression in PTC tissues.
The results also demonstrated that Rac1 is negatively regulated by
miR-101 at the post-transcriptional level via a specific target
site within the 3′UTR, and that miR-101 inhibits thyroid cell
migration and invasion through the Rac1 pathway. Overall, these
results suggested that elevated Rac1, induced by suppressed
miR-101, is involved in the progression of PTC.
This evidence suggests that the dysregulation of the
Rac1 signaling pathway by miRNAs is an important mechanism
underlying cancer metastasis, particularly in cancer cell migration
and cell invasion.
Metastasis is the movement or spread of cancer cells
between one organ or tissue to another. Its sequential events
include detachment, migration, local invasion, formation of tumor
emboli, extravasations and transplantation in various organs
(29,30). Certain miRNAs may regulate the
signaling pathways of tumor metastasis (31), and the identification of miR-101 as
an important regulator of tumor cell migration and invasion in
vitro emphasizes an essential role of this miRNA in mediating
PTC oncogenesis and tumor behavior.
In conclusion, the results presented in the current
study demonstrate that miR-101 is downregulated in PTC and that the
downregulated miR-101 is significantly associated with lymph node
metastasis. Furthermore, miR-101 inhibits the invasion and
migration in thyroid cancer cells; miR-101 directly inhibited Rac1
expression by targeting its 3′UTR. In addition, Rac1 was
downregulated and found to inversely correlate with miR-101 levels
in thyroid carcinoma. These results suggest that miR-101 is an
important tumor suppressor in thyroid carcinoma. This study may
lead to the development of novel therapies for preventing thyroid
cancer metastasis.
Acknowledgements
This study was supported by the China National
Science Foundation (grant no. 81273214) and the Jiangsu Higher
Education Science Foundation (grant no. KYLX_1365, 320007).
References
|
1
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J cancer. 127:2893–2917. 2010.
|
|
2
|
Qian BY, Hem M, Dong SF, Wang JF and Chen
KX: Incidence and mortality of thyroid cancers in Tianjin from 1981
to 2001. Zhonghua Nei Fen Mi Dai Xie Za Zhi. 5:432–434. 2005.(In
Chinese).
|
|
3
|
Perri F, Lorenzo GD, Scarpati GD and
Buonerba C: Anaplastic thyroid carcinoma: A comprehensive review of
current and future therapeutic options. World J Clin Oncol.
2:150–157. 2011.
|
|
4
|
Hunt JP, Buchmann LO, Wang L and Abraham
D: An analysis of factors predicting lateral cervical nodal
metastases in papillary carcinoma of the thyroid. Arch Otolaryngol
Head Neck Surg. 137:1141–1145. 2011.
|
|
5
|
Onoda N, Ishikawa T, Kawajiri H, Takashima
T and Hirakawa K: Pattern of initial metastasis in the cervical
lymph node from papillary thyroid carcinoma. Surg Today.
43:178–184. 2013.
|
|
6
|
Bartel DP: MicroRNAs: genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
|
|
7
|
Ambros V: The evolution of our thinking
about microRNAs. Nat Med. 14:1036–1040. 2008.
|
|
8
|
Carthew RW and Sontheimer EJ: Origins and
mechanisms of miRNAs and siRNAs. Cell. 136:642–655. 2009.
|
|
9
|
Wang HJ, Ruan HJ, He XJ, Ma YY, Jiang XT,
Xia YJ, Ye ZY and Tao HQ: MicroRNA-101 is down-regulated in gastric
cancer and involved in cell migration and invasion. Eur J Cancer.
46:2295–2303. 2010.
|
|
10
|
Su H, Yang JR, Xu T, Huang J, Xu L, Yuan Y
and Zhuang SM: MicroRNA-101, down-regulated in hepatocellular
carcinoma, promotes apoptosis and suppresses tumorigenicity. Cancer
Res. 69:1135–1142. 2009.
|
|
11
|
Li S, Fu H, Wang Y, Tie Y, Xing R, Zhu J,
Sun Z, Wei L and Zheng X: MicroRNA-101 regulates expression of the
v-fos FBJ murine osteosarcoma viral oncogene homolog (FOS) oncogene
in human hepatocellular carcinoma. Hepatology. 49:1194–1202.
2009.
|
|
12
|
Wang R, Wang HB, Hao CJ, Cui Y, Han XC, Hu
Y, Li FF, Xia HF and Ma X: MiR-101 is involved in human breast
carcinogenesis by targeting Stathmin1. PLoS One. 7:e461732012.
|
|
13
|
Hao Y, Gu X, Zhao Y, Greene S, Sha W,
Smoot DT, Califano J, Wu TC and Pang X: Enforced expression of
miR-101 inhibits prostate cancer cell growth by modulating the
COX-2 pathway in vivo. Cancer Prev Res (Phila). 4:1073–1083.
2011.
|
|
14
|
Hiroki E, Akahira J, Suzuki F, Nagase S,
Ito K, Suzuki T, Sasano H and Yaegashi N: Changes in microRNA
expression levels correlate with clinicopathological features and
prognoses in endometrial serous adenocarcinomas. Cancer Sci.
101:241–249. 2010.
|
|
15
|
Zhang J, Socolovsky M, Gross AW and Lodish
HF: Role of Ras signaling in erythroid differentiation of mouse
fetal liver cells: functional analysis by a flow cytometry-based
novel culture system. Blood. 102:3938–3946. 2003.
|
|
16
|
He L and Hannon GJ: MicroRNAs small RNAs
with a big role in gene regulation. Nat Rev Genet. 5:522–531.
2004.
|
|
17
|
Didsbury J, Weber RF, Bokoch GM, Evans T
and Snyderman R: rac, a novel ras-related family of proteins that
are botulinum toxin substrates. J Biol Chem. 264:16378–16382.
1989.
|
|
18
|
del Pozo MA, Price LS, Alderson NB, Ren XD
and Schwartz MA: Adhesion to the extracellular matrix regulates the
coupling of the small GTPase Rac to its effector PAK. EMBO J.
19:2008–2014. 2000.
|
|
19
|
Chung CY, Lee S, Briscoe C, Ellsworth C
and Firtel RA: Role of Rac in controlling the actin cytoskeleton
and chemotaxis in motile cells. Proc Natl Acad Sci USA.
97:5225–5230. 2000.
|
|
20
|
Zhao SY, Sun Y, Lai ZS, Nan QZ, Li K and
Zhang ZS: Inhibition of migration and invasion of colorectal cancer
cells via deletion of Rac1 with RNA interference. Mol Cell Biochem.
322:179–184. 2009.
|
|
21
|
Chen QY, Xu LQ, Jiao DM, Yao QH, Wang YY,
Hu HZ, Wu YQ, Song J, Yan J and Wu LJ: Silencing of Rac1 modifies
lung cancer cell migration, invasion and actin cytoskeleton
rearrangements and enhances chemosensitivity to antitumor drugs.
Int J Mol Med. 28:769–776. 2011.
|
|
22
|
Zhen C, Chen L, Zhao Q, Liang B, Gu YX,
Bai ZF, Wang K, Xu X, Han QY, Fang DF, et al: Gankyrin promotes
breast cancer cell metastasis by regulating Rac1 activity.
Oncogene. 32:3452–3460. 2013.
|
|
23
|
Hernández E, De La Mota-Peynado A,
Dharmawardhane S and Vlaar CP: Novel inhibitors of Rac1 in
metastatic breast cancer. P R Health Sci J. 29:348–356. 2010.
|
|
24
|
Kamai T, Shirataki H, Nakanishi K, Furuya
N, Kambara T, Abe H, Oyama T and Yoshida K: Increased Rac1 activity
and Pak1 overexpression are associated with lymphovascular invasion
and lymph node metastasis of upper urinary tract cancer. BMC
Cancer. 10:1642010.
|
|
25
|
Yap LF, Jenei V, Robinson CM, Moutasim K,
Benn TM, Threadgold SP, Lopes V, Wei W, Thomas GJ and Paterson IC:
Upregulation of Eps8 in oral squamous cell carcinoma promotes cell
migration and invasion through integrin-dependent Rac1 activation.
Oncogene. 28:2524–2534. 2009.
|
|
26
|
Wang P, Chen L, Zhang J, Chen H, Fan J,
Wang K, Luo J, Chen Z, Meng Z and Liu L: Methylation-mediated
silencing of the miR-124 genes facilitates pancreatic cancer
progression and metastasis by targeting Rac1. Oncogene. 23:514–524.
2014.
|
|
27
|
Imam JS, Plyler JR, Bansal H, Prajapati S,
Bansal S, Rebeles J, Chen HI, Chang YF, Panneerdoss S, Zoghi B, et
al: Genomic loss of tumor suppressor miRNA-204 promotes cancer cell
migration and invasion by activating AKT/mTOR/Rac1 signaling and
actin reorganization. PLoS One. 7:e523972012.
|
|
28
|
Zhou Y, Su J, Shi L, Liao Q and Su Q: DADS
downregulates the Rac1-ROCK1/PAK1-LIMK1-ADF/cofilin signaling
pathway, inhibiting cell migration and invasion. Oncol Rep.
29:605–612. 2013.
|
|
29
|
Gupta GP and Massagué J: Cancer
metastasis: building a framework. Cell. 127:679–695. 2006.
|
|
30
|
Klein CA: Cancer. The metastasis cascade.
Science. 321:1785–1787. 2008.
|
|
31
|
Sreekumar R, Sayan BS, Mirnezami AH and
Sayan AE: MicroRNA control of invasion and metastasis pathways.
Front Genet. 2:582011.
|