Introduction
Gastric cancer is the fourth most common type of
malignant tumor worldwide (1) and
the annual number of novel cases is ~95 million. Each year ~70
million individuals succumb to gastric cancer, which makes it the
second most common cause of cancer-related mortality worldwide
(2). Since the SWOG/INT-0116 trial
(3) in 2001, adjuvant
chemoradiotherapy has become an established standard treatment for
gastric cancer. In contrast to the INT-0116 trial, which included
D0- or D1-resected gastric cancer patients, Kim et al
(4) studied D2-resected
participants using the same chemoradiotherapy regimens, and also
demonstrated that concurrent chemotherapy increased survival and
reduced recurrence.
However, compared with surgery alone, postoperative
chemoradiotherapy significantly increased toxicity in patients. In
the INT-0116 study, 57% of patients experienced grade 3 or 4
toxicity (3). Ringash et al
(5) found that the application of
three-dimensional conformal radiotherapy (3D-CRT) in patients with
gastric cancer, which is different from the 2D radiotherapy used in
the INT-0116 trial, decreased the incidence of grade 2 or higher
toxicity to 25%. Similar studies have shown that conformal
intensity-modulated radiation therapy (IMRT) achieves superior
planning tumor volume (PTV) target coverage and improved normal
tissue sparing (6–8). Furthermore, although the National
Comprehensive Cancer Network Guidelines recommend either 3D-CRT or
IMRT, it is now widely accepted in the medical profession that IMRT
is superior to 3D-CRT in terms of tumor coverage, increased local
tumor control probability and dose reduction to certain organs at
risk (OARs).
Volumetric modulated arc therapy (VMAT), as a
modified version of IMRT, employs the linear accelerators Elekta
Synergy VMAT and Elekta Precise (Elekta Oncology Systems, Crawley,
UK) to conduct dynamic modulation rotation radiotherapy. The
advantages of VMAT when compared with IMRT, include a reduction in
the number of monitor units (MUs), shorter delivery times and lower
exposure of OARs. In practice, the VMAT optimization depends on the
number of arcs and the gantry angle spacing between subsequent
control points. At present, controversy exists as to whether a
single arc VMAT can achieve dose distributions comparable to IMRT
plans. Bertelsen et al (9)
demonstrated that single arc is sufficient to achieve a plan
quality similar to IMRT, however, Guckenberger et al
(10) have reported that it is
dependent on the complexity of the target volume.
VMAT is considered to be equivalent or superior to
IMRT for certain malignancies, including head and neck, prostate,
lung, cervical and pancreatic cancer (11), however, a lack of comprehensive
comparison between IMRT to VMAT exists with regards to gastric
cancer treatment. Therefore, the present study aimed to elucidate
the dosimetric quality of single-arc (SA)/double-arc (DA)-VMAT for
gastric cancer, compared with 5-field IMRT (5F-IMRT) and 7-field
IMRT (7F-IMRT).
Patients and methods
Patient samples
A total of 29 patients with nonmetastatic gastric or
gastroesophageal (GE) junction cancer who received radiotherapy
treatment at the West China Hospital (Chengdu, China) between
February 2012 and August 2012 were included in the study. All
patients were confirmed by pathology and disease was limited to the
stomach or GE junction and regional lymph nodes. Patients were
staged according to the American Joint Committee on Cancer staging
system (7th edition) (12). Each
patient was rescheduled retrospectively via inverse planning
5F-IMRT, 7F-IMRT, SA-VMAT and DA-VMAT techniques using Pinnacle
treatment planning systems (TPS; Philips Medical Systems,
Fitchburg, WI, USA). Patient characteristics are summarized in
Table I. Patients provided written
informed consent.
 | Table IPatient characteristics. |
Table I
Patient characteristics.
| Parameters | Patients, n (%) |
|---|
| Gender |
| Male | 24 (83) |
| Female | 5 (17) |
| Grade |
|
Well-differentiated | 0 (0) |
| Moderately
differentiated | 3 (10) |
| Poorly
differentiated | 26 (90) |
| Clinical T
Classification |
| T1 | 1 (3) |
| T2 | 3 (10) |
| T3 | 15 (52) |
| T4 | 10 (35) |
| Clinical N
Classification |
| N0 | 1 (3) |
| Nl | 5 (17) |
| N2 | 11 (38) |
| N3 | 12 (42) |
| Location |
| GE junction | 0 (0) |
| Cardia/proximal
one-third | 10 (34.5) |
| Body/middle
one-third | 10 (34.5) |
| Antrum/distal
one-third | 9 (31) |
| Surgery |
| Total
gastrectomy | 13 (45) |
| Subtotal
gastrectomy | 13 (45) |
| Proximal
gastrectomy | 3 (10) |
| Ivor-Lewis
esophagectomy and proximal gastrectomy | 0 (0) |
| Total
esophagogastrectomy | 0 (0) |
Immobilization, simulation and target
delineation
All patients were immobilized in a supine position,
with arms crossed above the head using a thermoplastic shell.
Intravenous contrast-enhanced computed tomography (CT)-simulation
was performed at 3 mm intervals of abdomen using Gemini GXL
positron emission tomography/CT (Philips Medical Systems).
Respiratory control and abdominal compression were not used.
Following simulation, the CT images were transferred to the
Pinnacle3 version 9.2 radiation treatment planning system (Philips
Medical Systems). The clinical target volume (CTV) included tumor
bed and perigastric lymph nodes, following the recommendations
outlined in the INT-0116 trial (3).
Paracardial, splentichilum, paraaortic, celiac, paraesophageal,
hepatoduodenal and pancreaticoduodenal cancer celiac, as well as
paracardial, paraaortic, celiac, paraesophageal, hepatic portal,
pancreaticoduodenal and splenic hilum lymph nodes were included if
deemed as high risk, based on the pathologically involved regional
lymph nodes and the primary tumor location. The CTV to PTV
expansion was typically 5–10 mm to account for daily setup error
and organ motion. Normal structures, including the spinal cord,
liver, colon, duodenum, small intestine and kidneys were also
contoured. All the contours were drawn by the same physician. Each
patient had one 5F-IMRT, one 7F-IMRT, one SA-VMAT and one DA-VMAT
plan created by the same radiation therapist. The same dose
constraints were used for creation of 5F-IMRT, 7F-IMRT, SA-VMAT and
DA-VMAT plans (Table II).
 | Table IIOARs dose constraints. |
Table II
OARs dose constraints.
| OARs | Prescribed dose
limit |
|---|
| Spinal Cord | Dmax<40
Gy |
| Liver | V30<30% |
| Kidney | V13<50% |
| V18<33% |
| Small intestine | Dmax<50
Gy |
| V50<10% |
| V45<15% |
| Duodenum | Dmax<50
Gy |
| V50<10% |
| V45<15% |
All generated plans for each patient consisted of
50.4 Gy to be delivered to PTV in 28 fractions. The objective of
planning was to deliver the prescribed dose to ≥95% of the PTV with
a dose range that did not exceed −10 and +15% of the prescribed
dose. All plans were generated for the Elekta Beam Modulator
(Elekta Oncology Systems).
Treatment planning and optimization;
5F-IMRT and 7F-IMRT
The IMRT optimization was performed using the direct
machine parameter optimization algorithm in the treatment planning
system (Pinnacle3; Philips Radiation Oncology Systems). IMRT uses
five and seven coplanar beams; five beam beam irradiation, angles
of 25, 60, 95, 180 and 315°; or seven bean irradiation, angles of
0, 51, 102, 153, 204, 255 and 306°. In the plan generation, the
maximum iterations in the plan optimization were 80. There were no
limitations with regard to the MUs per segment. Plans were
generated for the Elekta Beam Modulator with 6-MV.
SA-VMAT
The single arc VMAT planning was performed using the
SmartArc planning algorithm in Pinnacle3 version 9.2 (Philips
Radiation Oncology Systems). The single arc VMAT was planned with a
beam delivery time ≤240 sec, and with an arc from 181–180° (a
control point every 4°). The accelerator used automatic dose rate
selection, which ensured that the maximal possible dose rate was
selected for each individual segment of the arc. The initial step
was performed using the SmartArc algorithm to obtain the optimal
modulated fluency. In the second step, the segments were optimized
based on the small target areas receiving insufficient irradiation
dose, using the same algorithm. Plans were generated with 6-MV.
DA-VMAT
The plans were optimized in the same planning system
as mentioned previously. The double arc VMAT was planned with a
beam delivery time of ≤120 sec x2, and with a gantry rotation of
181-180-181° (a control point every 4°). Plans were generated with
6-MV and all the objective parameters and algorithm used were the
same as that for the single arc VMAT. All the plans were repeatedly
optimized until the objectives were met.
Evaluation of the dose-volumetric
histogram (DVH)-based parameters
For the PTV, D98, D95,
D50 and D2%, where D is the accepting dose
and n is the percentage of the PTV, were selected to comply with
the International Commission on Radiation Units and Measurements
Report No. 83 (13). The conformal
index (CI) and homogeneity index (HI) for PTV were calculated. The
CI was defined as follows: CI = cover factor (the percentage of the
PTV volume receiving 50.4 Gy) × spill factor (the volume of the PTV
receiving the 50.4 Gy relative to the total prescription
dose-volume). The HI was defined as follows: HI = the minimum dose
in 5% of the PTV (D5)/minimum dose in 95% of the PTV
(D95). The following dosimetric parameters were
retrospectively analyzed: Volumes of kidney receiving a dose of ≥13
and 18 Gy (V13 and V18); volumes of liver receiving a dose of ≥30
Gy; D2 of the spinal cord; volumes of small intestine
and colon receiving a dose of ≥50 Gy (V50); the mean dose to OARs
and remaining volume at risk; the maximum dose to 1, 5 and 10
cm3 of the pancreas and duodenum; and the volume of
pancreas and duodenum receiving 5, 10, 15, 20, 25, 30, 35, 40, 45,
and 50 Gy.
Statistical analysis
The data were analyzed using SPSS software, version
16.0 (SPSS, Inc., Chicago, IL, USA) and all data are presented as
the mean ± standard deviation. The Wilcoxon’s signed rank test was
performed and P<0.05 was considered to indicate a statistically
significant difference.
Results
PTV coverage
The evaluation of the DVH-based parameters of the
PTV is shown in Table III. The
D98 and D95 of the PTV were similar among the
5F-IMRT, 7F-IMRT, SA-VMAT and DA-VMAT plans, respectively, and no
significant differences were identified (P>0.05). For the PTV
coverage, the mean CI of the DA-VMAT plans (0.87±0.03) was
significantly higher than that of 5-IMRT (0.86±0.02), 7-IMRT
(0.86±0.02) and SA-VMAT (0.83±0.03), respectively (P<0.05).
Additionally, the mean HI of the DA-VMAT plans (0.10±0.01) was
found to be significantly improved when compared with those in
5-IMRT (0.13±0.17), 7-IMRT (0.10±0.02) and SA-VMAT (0.12±0.02),
respectively (P<0.05). DA-VMAT plans also exhibited a lower D2
(54.21±49.92) when compared with the 5-IMRT (54.52±43.27), 7-IMRT
(54.54±57.63) and SA-VMAT (55.33±109.69) plans (P<0.05). A
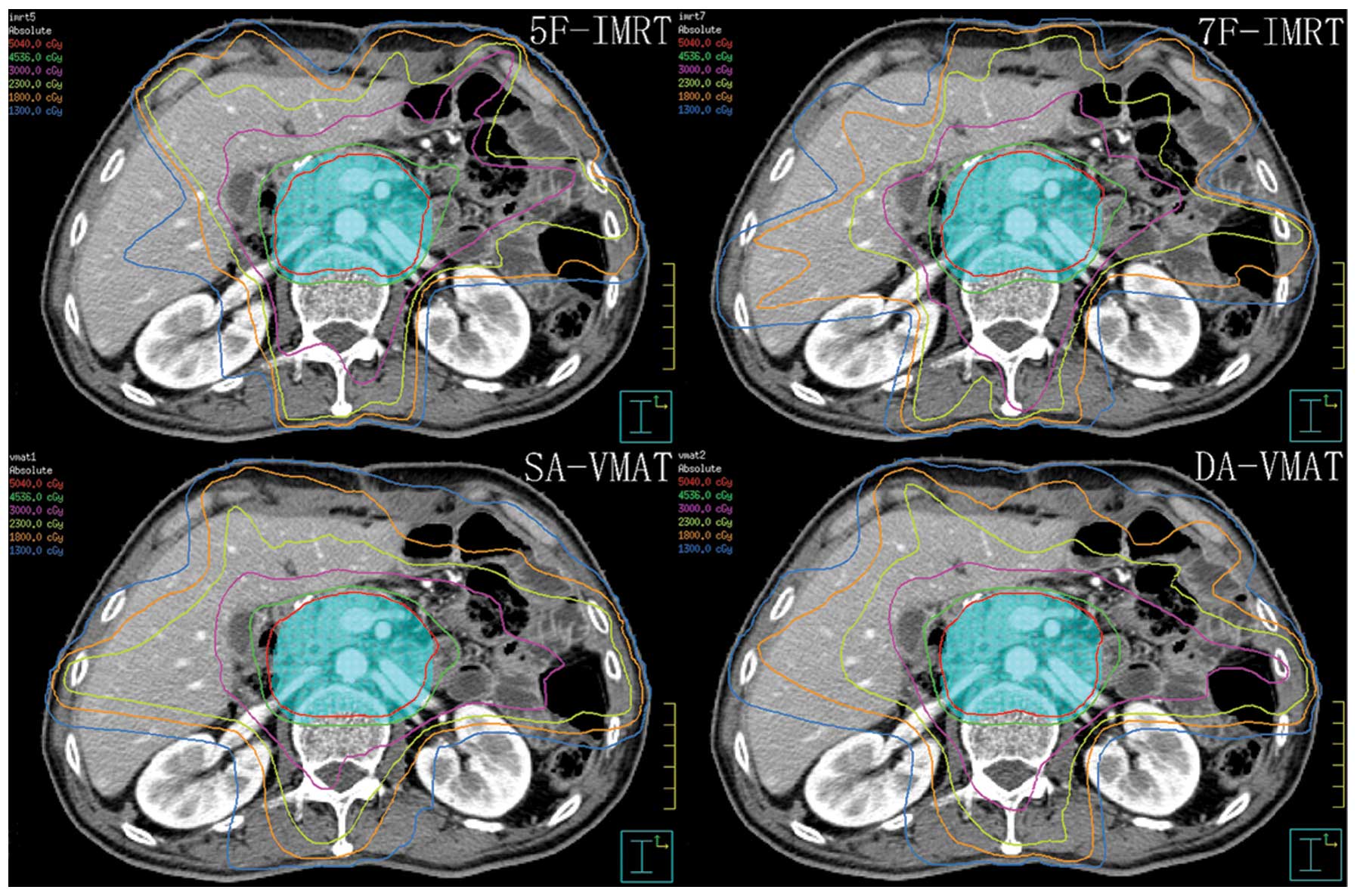
typical dose distribution in the transverse section is shown in
Fig. 1.
 | Table IIIComparisons of the dose-volume
histogram-based parameters of the planning tumor volume. |
Table III
Comparisons of the dose-volume
histogram-based parameters of the planning tumor volume.
| Radiotherapy | P-value |
|---|
|
|
|
|---|
| Prameters | 5F-IMRT | 7F-IMRT | SA-VMAT | DA-VMAT | 5F-IMRT vs.
7F-IMRT | 5F-IMRT vs.
SA-VMAT | 5F-IMRT vs.
DA-VMAT | 7F-IMRT vs.
SA-VMAT | 7F-IMRT vs.
DA-VMAT | SA-VMAT vs.
DA-VMAT |
|---|
| D98,
Gy | 49.10±0.57 | 49.12±0.59 | 49.06±0.51 | 49.11±0.46 | 0.680 | 0.363 | 0.624 | 0.180 | 0.280 | 0.524 |
| D95,
Gy | 50.35±0.44 | 50.34±0.41 | 50.41±0.38 | 54.21±0.50 | 0.870 | 0.104 | 0.446 | 0.070 | 0.480 | 0.380 |
| D50,
Gy | 51.10±8.81 | 52.60±0.50 | 53.43±0.54 | 52.81±0.43 | 0.219 | <0.001 | 0.944 | <0.001 | 0.380 | <0.001 |
| D2,
Gy | 54.52±0.43 | 54.54±0.57 | 55.33±1.10 | 54.21±0.50 | 0.834 | <0.001 | 0.001 | <0.001 | <0.001 | <0.001 |
| CI | 0.86±0.02 | 0.86±0.02 | 0.83±0.03 | 0.87±0.03 | 0.994 | <0.001 | 0.012 | <0.001 | 0.010 | <0.001 |
| HI | 0.13±0.17 | 0.10±0.02 | 0.12±0.02 | 0.10±0.01 | 0.715 | 0.003 | 0.013 | <0.001 | 0.030 | <0.001 |
OARs
DA-VMAT significantly decreased the mean dose
(14.44±157.59 Gy), V13 (0.36±0.04 Gy) and V18 (0.26±0.03 Gy) of the
left kidney. Similarly, a lower mean dose (11.23±188.43 Gy), V13
(0.27±0.06 Gy) and V18 (0.17±0.05 Gy) were observed in the
contralateral kidney with DA-VMAT. The mean doses to the normal
liver for each method were 21.90±138.97 Gy (DA-VMAT), 23.42±194.66
Gy (SA-VMAT), 21.91±147.73 Gy (7F-IMRT) and 19.82±196.08 Gy
(5F-IMRT), with the mean dose to the normal liver with 5F-IMRT
found to be the lowest. Furthermore, the V30 Gy (%) with SA-VMAT
(0.22±0.05) was higher than that with 5F-IMRT (0.19± 0.03)
(P<0.05), 7F-IMRT (0.19±0.03)(P<0.05) and DA-VMAT
(0.19±0.03)(P<0.05). The results are shown in Table IV.
 | Table IVComparisons of the dose-volume
hisotgram-based parameters of the kidneys and liver in present
study. |
Table IV
Comparisons of the dose-volume
hisotgram-based parameters of the kidneys and liver in present
study.
| | | | | P-value |
|---|
| | | | |
|
|---|
| OARs | 5F-IMRT | 7F-IMRT | SA-VMAT | DA-VMAT | 5F-IMRT vs.
7F-IMRT | 5F-IMRT vs.
SA-VMAT | 5F-IMRT vs.
DA-VMAT | 7F-IMRT vs.
SA-VMAT | 7F-IMRT vs.
DA-VMAT | SA-VMAT vs.
DA-VMAT |
|---|
| Left kidney |
| V13 | 0.38±0.05 | 0.39±0.04 | 0.41±0.04 | 0.36±0.04 | 0.539 | 0.039 | 0.010 | 0.075 | 0.001 | <0.001 |
| V18 | 0.27±0.04 | 0.28±0.04 | 0.29±0.05 | 0.26±0.03 | 0.586 | 0.038 | 0.211 | 0.068 | 0.104 | 0.001 |
| Mean dose, Gy | 15.42±1.93 | 14.96±1.91 | 15.68±1.98 | 14.44±1.58 | 0.159 | 0.680 | 0.003 | 0.153 | 0.080 | 0.010 |
| Right kidney |
| V13 | 0.36±0.06 | 0.34±0.05 | 0.33±0.05 | 0.27±0.06 | 0.053 | 0.016 | <0.001 | 0.479 | <0.001 | <0.001 |
| V18 | 0.19±0.06 | 0.23±0.04 | 0.22±0.06 | 0.17±0.05 | 0.005 | 0.046 | 0.179 | 0.549 | <0.001 | 0.002 |
| Mean dose, Gy | 12.83±2.15 | 12.83±2.03 | 12.62±2.10 | 11.23±1.88 | 0.680 | 0.529 | 0.001 | 0.780 | 0.001 | 0.008 |
| Liver |
| V30 | 0.19±0.03 | 0.19±0.03 | 0.22±0.05 | 0.19±0.03 | 0.592 | 0.013 | 0.895 | 0.038 | 0.762 | 0.018 |
| Mean dose, Gy | 19.82±1.96 | 21.92±1.48 | 23.42±1.95 | 21.90±1.39 | <0.001 | <0.001 | <0.001 | 0.002 | 0.721 | 0.001 |
For the other OARs (Table V), no significant differences in
dose were identified among the four methods, with the exception of
the marginal edge in D2 for the duodenum with DA-VMAT and D1, D5
and D10 cm3 for the pancreas with DA-VMAT. The maximum
dose to the spinal cord (D2) was equal for all four methods.
 | Table VComparisons of the dose-volume
historgram-based parameters of the other organs at risk and MUs in
present study. |
Table V
Comparisons of the dose-volume
historgram-based parameters of the other organs at risk and MUs in
present study.
| | | | | P-value |
|---|
| | | | |
|
|---|
| OARs | 5F-IMRT | 7F-IMRT | SA-VMAT | DA-VMAT | 5F-IMRT vs.
7F-IMRT | 5F-IMRT vs.
SA-VMAT | 5F-IMRT vs.
DA-VMAT | 7F-IMRT vs.
SA-VMAT | 7F-IMRT vs.
DA-VMAT | SA-VMAT vs.
DA-VMAT |
|---|
| Small
intestine |
| D2,
Gy | 48.22±9.46 | 48.67±9.43 | 49.55±9.53 | 48.58±9.38 | 0.499 | 0.044 | 0.686 | 0.137 | 0.846 | 0.107 |
| V50% | 0.04±0.03 | 0.04±0.03 | 0.05±0.03 | 0.04±0.03 | 0.721 | 0.237 | 0.750 | 0.312 | 0.889 | 0.327 |
| Mean dose, Gy | 17.19±6.26 | 16.50±5.76 | 18.01±6.34 | 16.90±5.92 | 0.658 | 0.613 | 0.858 | 0.301 | 0.762 | 0.388 |
| Colon |
| D2,
Gy | 51.38±2.87 | 51.54±2.94 | 52.51± 2.47 | 51.46±2.93 | 0.703 | 0.018 | 0.715 | 0.040 | 0.932 | 0.042 |
| V50% | 0.09±0.07 | 0.09±0.07 | 0.11±0.08 | 0.09±0.07 | 0.926 | 0.316 | 0.895 | 0.423 | 0.883 | 0.347 |
| Mean dose, Gy | 22.39±5.07 | 21.47±4.42 | 23.50±5.66 | 22.57±5.19 | 0.539 | 0.397 | 0.950 | 0.159 | 0.451 | 0.451 |
| Spinal cord |
| D2,
Gy | 38.98±2.41 | 38.90±2.29 | 39.08± 4.44 | 38.50± 3.18 | 0.950 | 0.432 | 0.686 | 0.410 | 0.762 | 0.347 |
| Pancreas |
| D1cm3,
Gy | 54.52±0.70 | 54.51±1.03 | 55.38± 1.16 | 54.13± 0.57 | 0.834 | 0.002 | 0.004 | 0.005 | 0.003 | <0.001 |
| D5cm3,
Gy | 53.92±0.47 | 54.02±0.60 | 54.84±0.99 | 53.73±0.54 | 0.646 | <0.001 | 0.059 | <0.001 | 0.006 | <0.001 |
| D10cm3,
Gy | 53.53±0.48 | 53.70±0.56 | 54.50±0.94 | 53.47±0.54 | 0.312 | <0.001 | 0.280 | <0.001 | 0.024 | <0.001 |
| V5% | 0.99±0.01 | 0.99±0.02 | 0.97±0.17 | 1.00±0.01 | 0.601 | 0.975 | 0.992 | 0.601 | 0.580 | 0.983 |
| V10% | 0.98±0.05 | 0.98±0.06 | 0.98±0.06 | 0.98±0.06 | 0.885 | 0.680 | 0.687 | 0.809 | 0.830 | 0.970 |
| V15% | 0.97±0.08 | 0.97±0.08 | 0.98±0.08 | 0.97±0.09 | 0.639 | 0.664 | 0.841 | 0.986 | 0.782 | 0.795 |
| V20% | 0.97±0.09 | 0.97±0.09 | 0.97±0.09 | 0.97±0.09 | 0.696 | 0.935 | 0.872 | 0.626 | 0.845 | 0.788 |
| V25% | 0.96±0.10 | 0.94±0.13 | 0.96±0.09 | 0.96±0.10 | 0.738 | 0.961 | 1.000 | 0.816 | 0.713 | 0.852 |
| V30% | 0.94±0.10 | 0.94±0.10 | 0.95±0.10 | 0.95±0.10 | 0.797 | 0.845 | 0.743 | 0.685 | 0.477 | 0.814 |
| V35% | 0.92±0.11 | 0.92±0.11 | 0.93±0.11 | 0.93±0.11 | 0.907 | 0.785 | 0.618 | 0.767 | 0.613 | 0.767 |
| V40% | 0.89±0.12 | 0.89±0.12 | 0.90±0.12 | 0.90±0.12 | 0.907 | 0.709 | 0.756 | 0.658 | 0.785 | 0.919 |
| V45% | 0.85±0.13 | 0.85±0.13 | 0.86±0.13 | 0.86±0.13 | 0.969 | 0.504 | 0.803 | 0.608 | 0.876 | 0.810 |
| V50% | 0.76±0.16 | 0.77±0.15 | 0.78±0.15 | 0.78±0.15 | 0.870 | 0.570 | 0.774 | 0.703 | 0.797 | 0.810 |
| Mean dose, Gy | 48.02±7.12 | 48.15±6.82 | 49.93±4.52 | 49.27±4.42 | 0.969 | 0.138 | 0.602 | 0.146 | 0.750 | 0.216 |
| Duodenum |
| D1cm3,
Gy | 53.11±1.59 | 53.15± 1.36 | 53.93±1.77 | 53.02± 1.41 | 0.762 | 0.036 | 0.534 | 0.036 | 0.786 | 0.017 |
| D5cm3,
Gy | 50.84±4.20 | 51.03±3.30 | 51.79±4.36 | 50.83±4.41 | 0.703 | 0.041 | 0.994 | 0.020 | 0.738 | 0.039 |
| D10cm3,
Gy | 46.97±9.57 | 46.87±9.19 | 48.05±9.49 | 46.78±9.97 | 0.715 | 0.174 | 0.994 | 0.107 | 0.663 | 0.225 |
| V5% | 0.88±0.16 | 0.88±0.16 | 0.89±0.15 | 0.89±0.16 | 0.890 | 0.942 | 0.961 | 0.821 | 0.885 | 0.859 |
| V10% | 0.83±0.19 | 0.83±0.19 | 0.84±0.18 | 0.83±0.19 | 0.912 | 0.869 | 0.950 | 0.832 | 0.912 | 0.826 |
| V15% | 0.81±0.21 | 0.81±0.20 | 0.82±0.20 | 0.81±0.20 | 0.895 | 0.761 | 0.839 | 0.679 | 0.851 | 0.778 |
| V20% | 0.76±0.22 | 0.76±0.21 | 0.78±0.22 | 0.77±0.23 | 0.926 | 0.554 | 0.697 | 0.427 | 0.709 | 0.634 |
| V25% | 0.72±0.23 | 0.73±0.22 | 0.75±0.23 | 0.73±0.24 | 0.864 | 0.437 | 0.726 | 0.470 | 0.870 | 0.619 |
| V30% | 0.67±0.24 | 0.68±0.23 | 0.70±0.24 | 0.67±0.24 | 0.858 | 0.524 | 0.994 | 0.646 | 0.846 | 0.565 |
| V35% | 0.61±0.24 | 0.61±0.24 | 0.65±0.25 | 0.62±0.25 | 0.932 | 0.570 | 0.981 | 0.581 | 0.969 | 0.504 |
| V40% | 0.55±0.24 | 0.55±0.24 | 0.58±0.25 | 0.56±0.25 | 0.981 | 0.669 | 0.957 | 0.658 | 0.907 | 0.646 |
| V45% | 0.48±0.24 | 0.48±0.24 | 0.50±0.24 | 0.49±0.24 | 0.969 | 0.680 | 0.932 | 0.680 | 0.889 | 0.797 |
| V50% | 0.37±0.23 | 0.38±0.23 | 0.40±0.25 | 0.38±0.23 | 0.944 | 0.560 | 0.726 | 0.635 | 0.944 | 0.750 |
| Mean dose, Gy | 36.12±10.39 | 36.18±10.15 | 37.53±10.66 | 36.41±10.56 | 0.994 | 0.451 | 0.907 | 0.423 | 0.883 | 0.539 |
| MUs | 4.56±0.90 | 5.79±0.98 | 3.46±0.45 | 4.37±0.63 | <0.001 | <0.001 | 0.721 | <0.001 | <0.001 | <0.001 |
| RVR, Gy | 20.69±1.51 | 21.04±2.11 | 21.18±1.90 | 20.51±1.57 | 0.339 | 0.414 | 0.613 | 0.944 | 0.148 | 0.222 |
The VMAT plans were applied with fewer MUs
(346.10±44.94 MUs for SA-VMAT and 437.66±62.69 MUs for DA-VMAT)
than the efficient 5F-IMRT plans (456.41±89.50 MUs), while the
7F-IMRT required more MUs (578.55±97.98 MUs).
Discussion
As mentioned previously, adjuvant chemoradiotherapy
for resectable gastric adenocarcinoma has become the standard
treatment for D0 and D2 gastrectomy. However, due to the
combination of radiotherapy and chemotherapy, treatment-associated
toxicities are enhanced, which often leads to relinquishment of
treatment among patients. A number of studies on dosimetric
comparison of 3D-CRT and IMRT have shown that IMRT exhibits
improved OAR sparing. Few studies have investigated the application
of VMAT in treating postoperative gastric cancer patients (14).
It is known that the complexity of the target volume
and the number of VMAT arcs are major determinants of whether VMAT
is advantageous when compared with IMRT (11). In contrast to the studies on head
and neck cancer mentioned previously (9,15),
certain studies on cervical cancer (16) and benign intra-cranial tumors
(17) have demonstrated that
SA-VMAT is superior or equivalent to IMRT. However, in contrast to
these studies, less complexity was identified in target volume for
gastric cancer with one dose level than that for head and neck
cancer with two or three dose levels (15,18).
In addition, the OARs in gastric cancer radiotherapy were found to
be more radiosensitive than that in cervix uteri radiotherapy
(18,19). As expected, the data in the present
study indicated that the treatment planning for gastric cancer
DA-VMAT plans achieved superior dose coverage for PTV (CI and HI
were improved; P<0.05). Regarding HI, SA-VMAT exhibited an
advantage when compared with 5F-IMRT, but not 7F-IMRT. For the CI,
the SA-VMAT exhibited no advantage when compared with IMRT.
It is known that the kidney is a radiosensitive
organ and that damage to the kidneys is an inevitable side effect
of pelvic or abdominal radiotherapy. Previous studies (20,21)
have suggested that total doses of 18–23 Gy and 28 Gy in 0.5–1.25
Gy/fractions may be associated with a 5 and 50% risk of injury in
five years, respectively. Jansen et al (22) conducted a prospective study
analyzing kidney function in 44 gastric cancer patients following
abdominal irradiation and observed an 11 and 52% decrease in left
renal function after six months and 18 months, respectively. The
V20 (left kidney) and mean left kidney dose were identified as
parameters associated with decreased kidney function. Therefore, in
the present study V13 and V18 Gy were selected as indicators. The
doses to the kidneys were significantly decreased in DA-VMAT plans;
however, the V13 Gy, V18 Gy and Dmean in the left kidney
were generally higher than those of the right kidney. One reason
for this may be that the majority of the left kidney is located in
the superior section of the target volume. In order to optimize
dose distribution in the tumor bed, which is anterior to the left
kidney, it is difficult for TPS to reduce the irradiation dose to
the left kidney. By contrast, the right kidney is located in the
lower section which is the paraaortic lymph node region. Since it
is much smaller and more regular than the upper section, it is
easier to complete dose computation.
For patients in China, radiation-induced liver
disease (RILD) must be considered. As a parallel organ, the
radiation injury to the liver is found to positively correlate with
the volume and dosage of radiation to the normal hepatic tissues.
Emami et al (23) reported
that TD5/5 (the tolerance dose leading to a 5% complication rate at
five years) for one-third, two-thirds and the whole liver at one
dose of 8–2 Gy/day were 50, 35 and 30 Gy, respectively. However,
these data were predominantly obtained from clinical practice in
North America and may not apply to the situation in China. Based on
a national seroepidemiological survey, the carrier rate of HBsAg in
China among 1- to 59-year-olds is 7.18% and among the medically
examined individuals in Chengdu, the HBsAg positive rate is 6.1%
(24). At present, there is no
constraint on the standard dose for those vulnerable patients.
The incidence of RILD is significantly associated
with mean dose to normal liver (MDTNL) which may be a predictor of
RILD. In a study that investigated the dose-volume tolerance for
RILD using the Lyman-Kutcher-Burman normal tissue complication
probability model, it was found that no cases of RILD were
identified when the mean liver dose was <31 Gy (25). Each 1 Gy increase in MDTNL exhibited
a 4% increase in the incidence of RILD. Furthermore, at an MDTNL of
43 Gy the incidence was as high as 50%. Liang et al
(26) demonstrated that when the
MDTNL was 23 and 31 Gy, the RILD occurrence rate was 6 and 69%,
respectively. In addition, a MDTNL of 23 Gy may be used as a
predictor of RILD. Furthermore, the risk of hepatitis B virus (HBV)
radiotherapy reactivation has been identified, which must be
considered. Previous study has revealed that radiotherapy is a
significant risk factor to RILD in patients with postgastrectomy
adenocarcinoma carrying HBV (27).
For those patients, a reduction in volume and dosage of radiation
to the normal hepatic tissues, as well as frequent monitoring of
liver function and routine detection of the HBV-DNA copy numbers,
are required. If necessary, regular antiviral treatment should be
provided. In the present study, only the MDTNL of SA-VMAT plans
exceeded 23 Gy and 5F-IMRT plans provided improved sparing of the
liver with a marginal advantage when compared with SA-VMAT.
In addition, the dosimetric parameters of the
duodenum and pancreas were compared among the four technologies.
Anatomically, the duodenum is the first section of the small
intestine. However, in the practice of radiotherapy, they are
different with regard to dose and volume limit. As the duodenum
adjoins the stomach, the majority of it is located within the
target volume. Additionally, as the duodenum is fixed by the
ligament of Treitz, the motion of the duodenum is more limited than
that of the rest of the small bowel. In a dose escalation trial of
pancreatic cancer, Singh et al (28) revealed that the volume of duodenum
receiving a dose of >80% of the prescribed dose was greater than
the remaining small bowel; however, individual variations were
significant. Therefore, reducing the dose received by the duodenum
is an important issue. Severe gastrointestinal (GI) toxicity
appears to be the main dose-limiting factor in abdominal
radiotherapy and it may be one of the reasons why the IMRT is
superior to 3D-CRT in terms of OARs sparing. However, in practice,
there is no difference in acute GI toxicity grade 2 between IMRT
and 3D-CRT. In a previous study, the acute grade 2 or greater GI
toxicity was found to be 61.5 and 61.2% for 3D-CRT and IMRT,
respectively (7). According to Liu
et al (14), the acute
toxicity was 56 and 54% for the IMRT and 3D-CRT groups,
respectively. At present, studies investigating dose constraints of
the duodenum are rare. Huang et al (29) have suggested that the V25 Gy of the
duodenum is the best predictor for GI toxicity in pancreatic cancer
patients with concurrent gemcitabine-erlotinib and radiotherapy.
The 12-month GI toxicity rates were found to be 8 and 48% for V25
Gy ≤45% and V25 Gy≥ 45%, respectively (P=0.03). Excluding the
erlotinib group, the V35 Gy was the best predictor and the 12-month
GI toxicity rates were 0 and 41% for V35 Gy ≤20% and V35 Gy ≥20%,
respectively (P=0.04). Although chemotherapeutics are different in
the treatment of gastric cancer and pancreatic cancer, the
indicators remain useful. In conclusion, in the present study, all
four technologies reached the standard for the indicator of V25 Gy
≤45%, however, for V35 Gy≤0%, all technologies failed. This
analysis is only a preliminary step and, thus, further study is
required to improve the sparing of the duodenum and identify
dosimetric predictors for GI toxicity.
The manner in which the pancreas may be protected
during abdominal radiotherapy is another issue which remains
unclear. Similar to the duodenum, the motion of the pancreas is
limited and the majority of it is within the target volume.
Radiation induced damage of the pancreas predominantly decreases
the endocrine and exocrine functions of the pancreas (30,31).
In the present study, DA-VMAT was found to be marginally more
effective than the other three technologies in D1, 5 and 10
cm3.
Regarding the issue of how to improve the sparing of
OARs, other options are available. Hu et al (32) reported that the dose for
postoperative gastric cancer patients may be increased to 54 Gy
without increasing the toxicity to critical organs, by using a
combination of breath-holding techniques and online image-guided
IMRT. However, the problem of controlling gastric emptying remains;
patients do not always follow doctor’s advice to ensure the GI
tract is empty during the course of radiotherapy.
In conclusion, although VMAT has been demonstrated
to exhibit advantages in the treatment of other kinds of
malignancies, the dosimetric advantage of VMAT in this study was
not always evident when compared with IMRT. In addition, it is
unclear whether IMRT should be replaced by VMAT. Considering the
lower MUs, shorter delivery times and reduced low-dose exposure of
OARs, the use of VMAT in postoperative radiotherapy remains
suitable for gastric carcinoma; however, the clinical implications
and outcome require further study.
References
|
1
|
Kamangar F, Dores GM and Anderson WF:
Patterns of cancer incidence, mortality, and prevalence across five
continents: defining priorities to reduce cancer disparities in
different geographic regions of the world. J Clin Oncol.
24:2137–2150. 2006.
|
|
2
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108.
2005.
|
|
3
|
Macdonald JS, Smalley SR, Benedetti J, et
al: Chemoradiotherapy after surgery compared with surgery alone for
adenocarcinoma of the stomach or gastroesophageal junction. N Engl
J Med. 345:725–730. 2001.
|
|
4
|
Kim S, Lim DH, Lee J, et al: An
observational study suggesting clinical benefit for adjuvant
postoperative chemoradiation in a population of over 500 cases
after gastric resection with D2 nodal dissection for adenocarcinoma
of the stomach. Int J Radiat Oncol Biol Phys. 63:1279–1285.
2005.
|
|
5
|
Ringash J, Khaksart SJ, Oza A, et al:
Post-operative radiochemotherapy for gastric cancer: adoption and
adaptation. Clin Oncol (R Coll Radiol). 17:91–95. 2005.
|
|
6
|
Alani S, Soyfer V, Strauss N, Schifter D
and Corn BW: Limited advantages of intensity-modulated radiotherapy
over 3D conformal radiation therapy in the adjuvant management of
gastric cancer. Int J Radiat Oncol Biol Phys. 74:562–566. 2009.
|
|
7
|
Minn AY, Hsu A, La T, et al: Comparison of
intensity-modulated radiotherapy and 3-dimensional conformal
radiotherapy as adjuvant therapy for gastric cancer. Cancer.
116:3943–3952. 2010.
|
|
8
|
Dahele M, Skinner M, Schultz B, Cardoso M,
Bell C and Ung YC: Adjuvant radiotherapy for gastric cancer: A
dosimetric comparison of 3-dimensional conformal radiotherapy,
tomotherapy and conventional intensity modulated radiotherapy
treatment plans. Med Dosim. 35:115–121. 2010.
|
|
9
|
Bertelsen A, Hansen CR, Johansen J and
Brink C: Single arc volumetric modulated arc therapy of head and
neck cancer. Radiother Oncol. 95:142–148. 2010.
|
|
10
|
Guckenberger M, Richter A, Krieger T,
Wilbert J, Baier K and Flentje M: Is a single arc sufficient in
volumetric-modulated arc therapy (VMAT) for complex-shaped target
volumes? Radiother Oncol. 93:259–265. 2009.
|
|
11
|
Alvarez-Moret J, Pohl F, Koelbl O and
Dobler B: Evaluation of volumetric modulated arc therapy (VMAT)
with Oncentra MasterPlan® for the treatment of head and
neck cancer. Radiat Oncol. 5:1102010.
|
|
12
|
Edge SB and Compton CC: The American Joint
Committee on Cancer: the 7th edition of the AJCC cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474.
2010.
|
|
13
|
International Commission of Radiation
Units and Measurements. ICRU report 83: Prescribing, Recording, and
Reporting Photon-Beam Intensity-Modulated Radiation Therapy (IMRT).
Journal of the ICRU. 10:12010.
|
|
14
|
Liu GF, Bair RJ, Bair E, Liauw SL and
Koshy M: Clinical outcomes for gastric cancer following adjuvant
chemoradiation utilizing intensity modulated versus
three-dimensional conformal radiotherapy. PLoS One.
9:e826422014.
|
|
15
|
Stieler F, Wolff D, Schmid H, et al: A
comparison of several modulated radiotherapy techniques for head
and neck cancer and dosimetric validation of VMAT. Radiother Oncol.
101:388–393. 2011.
|
|
16
|
Cozzi L, Dinshaw KA, Shrivastava SK, et
al: A treatment planning study comparing volumetric arc modulation
with RapidArc and fixed field IMRT for cervix uteri radiotherapy.
Radiother Oncol. 89:180–191. 2008.
|
|
17
|
Fogliata A, Clivio A, Nicolini G, Vanetti
E and Cozzi L: Intensity modulation with photons for benign
intracranial tumours: a planning comparison of volumetric single
arc, helical arc and fixed gantry techniques. Radiother Oncol.
89:254–262. 2008.
|
|
18
|
Wang X, Li G, Zhang Y, et al: Single-arc
volumetric-modulated arc therapy (sVMAT) as adjuvant treatment for
gastric cancer: Dosimetric comparisons with three-dimensional
conformal radiotherapy (3D-CRT) and intensity-modulated
radiotherapy (IMRT). Med Dosim. 38:395–400. 2013.
|
|
19
|
Murofushi K, Kitamura N, Machida H, et al:
The impact of vicryl mesh sheet placed on pelvic wall for reducing
the irradiated bowel volume in VMAT of cervical cancer: Planning
study. Int J Radiat Oncol Biol Phys. 84:S430–S431. 2012.
|
|
20
|
Peschel RE, Chen M and Seashore J: The
treatment of massive hepatomegaly in stage IV-S neuroblastoma. Int
J Radiat Oncol Biol Phys. 7:549–553. 1981.
|
|
21
|
Cassady JR: Clinical radiation
nephropathy. Int J Radiat Oncol Biol Phys. 31:1249–1256. 1995.
|
|
22
|
Jansen EPM, Saunders MP, Boot H, et al:
Prospective study on late renal toxicity following postoperative
chemoradiotherapy in gastric cancer. Int J Radiat Oncol Biol Phys.
67:781–785. 2007.
|
|
23
|
Emami B, Lyman J, Brown A, et al:
Tolerance of normal tissue to therapeutic irradiation. Int J Radiat
Oncol Biol Phys. 21:109–122. 1991.
|
|
24
|
Wang YB, Chen EQ, Cui YL, Zeng L, Wang YJ
and Tang H: Seroprevalence of hepatitis B virus markers in
individuals for physical examination in West China Hospital, China.
Eur Rev Med Pharmacol Sci. 15:592–596. 2011.
|
|
25
|
Dawson LA, Normolle D, Balter JM, McGinn
CJ, Lawrence TS and Ten Haken RK: Analysis of radiation-induced
liver disease using the Lyman NTCP model. Int J Radiat Oncol Biol
Phys. 53:810–821. 2002.
|
|
26
|
Liang SX, Zhu XD, Xu ZY, et al:
Radiation-induced liver disease in three-dimensional conformal
radiation therapy for primary liver carcinoma: the risk factors and
hepatic radiation tolerance. Int J Radiat Oncol Biol Phys.
65:426–434. 2006.
|
|
27
|
Xu J, Zhu H, Zhao Y, et al: Factors
associated with hepatic dysfunction in hepatitis B-positive
patients with postgastrectomy adenocarcinoma. Oncol Lett.
4:471–476. 2012.
|
|
28
|
Singh AK, Tierney RM, Low DA, et al: A
prospective study of differences in duodenum compared to remaining
small bowel motion between radiation treatments: Implications for
radiation dose escalation in carcinoma of the pancreas. Radiat
Oncol. 1:332006.
|
|
29
|
Huang J, Robertson JM, Ye H, Margolis J,
Nadeau L and Yan D: Dose-volume analysis of predictors for
gastrointestinal toxicity after concurrent full-dose gemcitabine
and radiotherapy for locally advanced pancreatic adenocarcinoma.
Int J Radiat Oncol Biol Phys. 83:1120–1125. 2012.
|
|
30
|
Ahmadu-Suka F, Gillette EL, Withrow SJ,
Husted PW, Nelson AW and Whiteman CE: Exocrine pancreatic function
following intraoperative irradiation of the canine pancreas.
Cancer. 62:1091–1095. 1988.
|
|
31
|
Yamaguchi K, Nakamura K, Kimura M, et al:
Intraoperative radiation enhances decline of pancreatic exocrine
function after pancreatic head resection. Dig Dis Sci.
45:1084–1090. 2000.
|
|
32
|
Hu W, Ye J, Wang J, Xu Q and Zhang Z:
Incorporating breath holding and image guidance in the adjuvant
gastric cancer radiotherapy: a dosimetric study. Radiat Oncol.
7:982012.
|