Introduction
Lung cancer is the most common cause of
cancer-related mortality worldwide, for which non-small cell lung
cancer (NSCLC) is the most common type of lung cancer. Although
there has been a degree of progress in understanding the biology of
lung cancer and in the generation of novel therapeutic agents, the
five-year overall survival (OS) rate for patients with lung cancer
is <15% (1). The low survival
rate indicates that novel effective treatment options are required.
Identification of molecular pathways involved in lung
carcinogenesis may lead to improvements in the management of the
disease and the generation of novel-targeted therapies.
Prostaglandin E2 (PGE2) has a predominant function
in promoting carcinogenesis and cancer progression, including tumor
cell proliferation, invasion, immunosuppression and angiogenesis
(2). The key enzymes involved in
PGE2 catabolism and the modulation of tumorigenic processes are
topics of interest. Cyclooxygenase-2 (COX-2), the rate-limiting
enzyme in the synthesis of PGE2, has been regarded as an
oncogenesis factor through the accumulation of PGE2 (3). Another central enzyme is
15-hydroxyprostaglandin dehydrogenase (15-PGDH). 15-PGDH oxidizes
the 15(S)-hydroxyl group of PGE2 to generate
15-keto-prostaglandin, which exhibits greatly reduced biological
activity (4). 15-PGDH therefore
functions as a COX-2 antagonist in mammalian tissues, including the
lung, breast, prostate, placenta and gut (5).
Previous studies have identified the tumor
suppressor activity of 15-PGDH in solid tumors. Downregulation of
15-PGDH at the mRNA and protein levels has been found in various
malignancies (4,6–10). The
genetic ablation of 15-PGDH has been shown to increase the
proliferation and motility of human cancer cells (7,9,11). In
addition, 15-PGDH overexpression has been observed to lead to the
inhibition of tumor angiogenesis by modulating PGE2 and vascular
endothelial growth factor expression in NSCLC cells (12). This anti-angiogenic mechanism has a
critical function in tumor suppression and provides a novel
potential target for anticancer therapy in NSCLC. Various
compounds, including COX-2 and histone deacetylation inhibitors,
have been identified to induce 15-PGDH as part of their
chemo-preventative activities (13,14);
however, the clinical studies of 15-PGDH on lung cancer are
limited. It is therefore important to explore additional clinical
data on the function of 15-PGDH to devise more efficacious
treatment strategies for NSCLC.
The present study used an immunohistochemical
staining method to analyze the expression of 15-PGDH and COX-2 in
NSCLC human specimens and evaluate their clinical prognostic value
in patients with NSCLC. Furthermore, the reciprocal regulation of
15-PGDH and COX-2 and their association with angiogenesis was
investigated.
Materials and methods
Samples from patients
Formalin-fixed, paraffin-embedded tissue specimens
from 35 surgically resected primary NSCLC cases, during the period
between January 2001 and December 2004, were acquired from the
Department of Pathology, Peking University First Hospital (China).
These patients did not receive radiotherapy or chemotherapy prior
to surgery, and clinical follow up data was available.
Paracancerous normal lung tissues from six patients were collected
for use as control specimens. The clinical data included age (mean,
60±18 years; range, 41–81 years), gender, smoking history, TNM
staging (according to the American Joint Committee on Cancer
staging system, 7th edition) (15),
histological type and differentiation grading (according to the
World Health Organization criteria) (16) and tumor size. All patients were
monitored by follow-up until December 2009 where possible. The
median follow-up time for survivors was 34 months (range, 3–86
months). The study protocol was approved by the Clinical Research
Ethics Committee of the Peking University First Hospital (Beijing,
China). Patients provided written informed consent.
Immunohistochemical staining
Paraffin-embedded tissue blocks for all the cases
were consecutively cut into 4-μM sections, deparaffinized in xylene
and rehydrated in graded alcohol. Antigen retrieval was performed
by boiling the sections for 10 min in a 0.01 M sodium citrate
buffer (pH 6.0) in a water bath. Following natural cooling,
blocking for endogenous peroxides was performed for 10 min in 3%
H2O2 in methanol. The sections were blocked
with 3% bovine serum albumin for 30 min at 37°C and then incubated
with rabbit polyclonal antibody directed against human 15-PGDH
(1:50 dilution; 160615; Cayman Chemical Co., Ann Arbor, MI, USA),
rabbit monoclonal antibody directed against human COX-2 (1:80
dilution; ZA-0515), mouse monoclonal antibody directed against
human CD34 (1:100 dilution; ZM-0046) or 0.01 M phosphate-buffered
saline (ZLI-9062) (all Zhongshan Golden Bridge Biotechnology Co.
Ltd., Beijing, China) for the negative control at 4°C overnight and
the next day at 37°C for 1 h. The sections were then incubated with
biotinylated goat anti-rabbit immunoglobulin G (SP-9000 Reagent B)
for 30 min at 37°C and subsequently incubated with horseradish
peroxidase-labeled streptavidin (SP-9000 Reagent C; both Zhongshan
Golden Bridge Biotechnology Co. Ltd.) for 20 min at 37°C. Finally,
3,3′-diaminobenzidine (ZLI-9032; Zhongshan Golden Bridge
Biotechnology Co. Ltd.) was used as a chromogen and hematoxylin
(ZLI-9609; Zhongshan Golden Bridge Biotechnology Co. Ltd.) was used
as a counterstain.
Evaluation of immunohistochemical
staining
Evaluation of the stained sections was performed by
two pathologists blinded to the clinical information. Each section
was scanned at ×100 and ×200 magnification. The expression of
15-PGDH and COX-2 was assessed by determining the
immunohistochemistry score (IHS) as follows: IHS = intensity score
(absent, 0; weak, 1; moderate, 2; strong, 3) × percentage score
(<5%, 0; 5–25%, 1; 25–50%, 2; 50–75%, 3; >75% of total tumor
area, 4) (10). The scores ranged
from 0 to 12. An IHS of 9–12 was considered strong
immunoreactivity, 5–8 was moderate, 1–4 was weak and 0 was
negative. For statistical analysis, 15-PGDH and COX-2 scores were
divided into a high expression group (strong and moderate
immunoreactivity) and a low expression group (weak and negative
immunoreactivity) (17).
Assessment of angiogenesis
An assessment of the microvessel density (MVD) was
performed by anti-CD34 staining. This was used to provide an
assessment of angiogenesis according to the criteria of Weidner
et al (18). Tumor sections
were scanned at a low magnification (x100) to identify vessels
highly positive in CD34 expression, known as ‘hot spots’. The
number of CD34-positive cells and cell clusters was counted in five
×200 magnification fields for microvessel counting. The presence of
a visible blood vessel lumen was not required for a positive
definition. The mean value of the five hot spots was calculated as
the MVD for each section. To assess the impact of MVD on prognosis,
35 tumor cases were divided into high and low MVD groups, according
to the mean value of the MVD.
Statistical analysis
χ2 or Fisher’s tests were used to compare
the statistical differences between the groups. Spearman’s rank
test was used to assess the correlation between 15-PGDH, COX-2 and
MVD. The Kaplan-Meier method was used to estimate the survival
curves for each group, and the differences between the curves were
analyzed according to the log-rank test. OS was measured from the
date the surgery was carried out until mortality (from any cause)
or the end of the observation period. The groups were classified by
clinicopathological features, including gender (male vs. female),
age (<70 vs. ≥70 years old), smoking history (never smoked vs.
smoked), histological type (squamous cell carcinoma vs.
adenocarcinoma), histological differentiation (poor vs. moderate or
well), tumor size (≤3 vs. >3 cm), lymph node invasion (no
invasion vs. invasion of ≥1 node) and TNM stage (I + II vs. III +
IV). P<0.05 (two-sided) was considered to indicate a
statistically significant difference. Statistical analyses were
conducted using SPSS version 17.0 (SPSS, Inc., Chicago, IL,
USA).
Results
Expression of 15-PGDH and COX-2 in the
NSCLC and normal control group
The predominant pattern of 15-PGDH and COX-2
staining was a yellow or brownish-yellow stain in the cytoplasm.
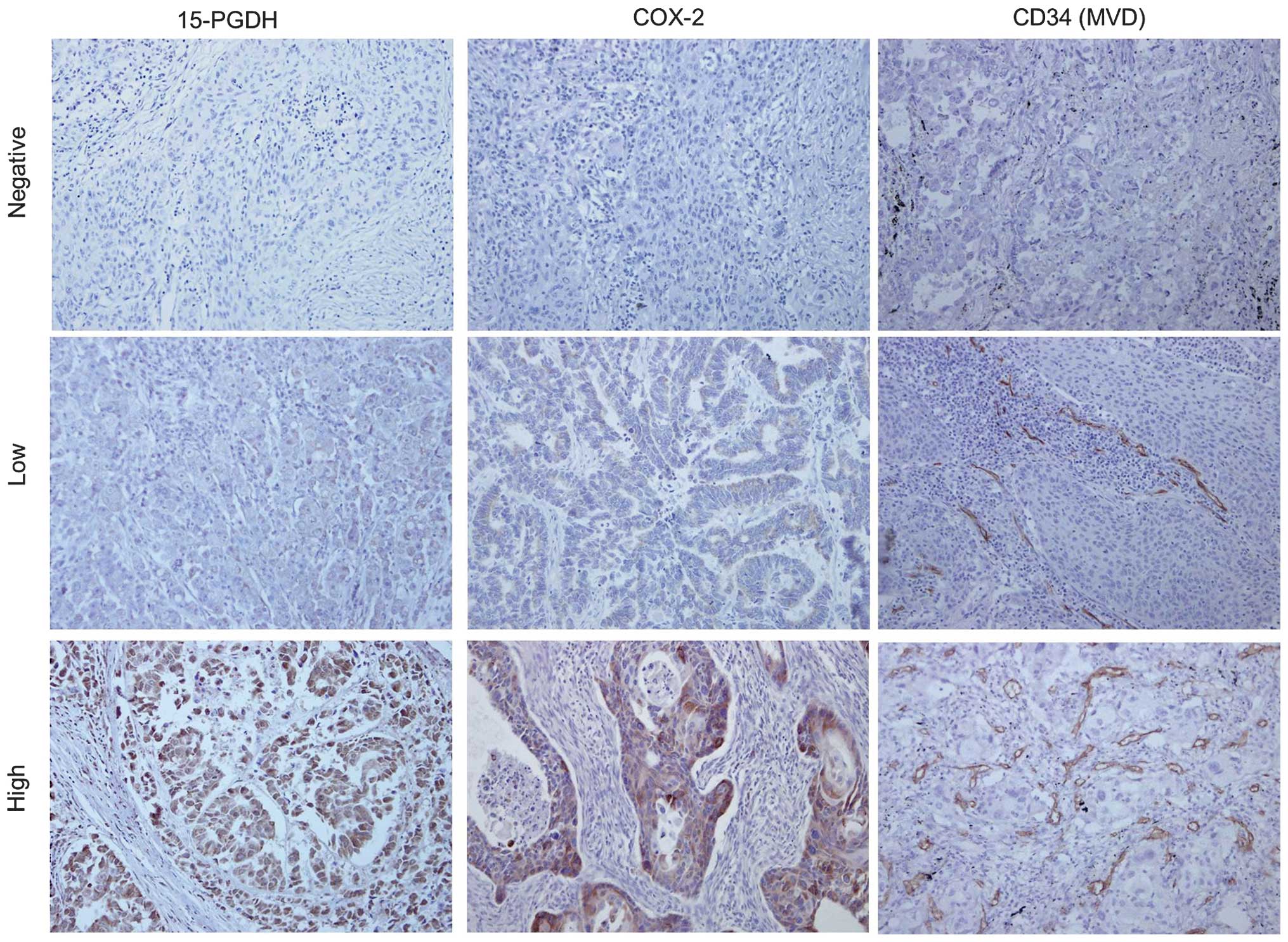
The different intensities of the staining are shown in Fig. 1. The high expression rate of 15-PGDH
was 40% (14/35) in the NSCLC group and 100% (6/6) in the normal
group (Table I), while the high
expression rate of COX-2 was 65.7% (23/35) in NSCLC group and 0%
(0/6) in normal group. The NSCLC tissue showed a significantly
lower expression level of 15-PGDH (P=0.009) and a higher expression
level of COX-2 (P=0.004) compared with normal lung tissue.
 | Table IExpression of 15-PGDH and COX-2 in
NSCLC tissues and matched normal lung tissues. |
Table I
Expression of 15-PGDH and COX-2 in
NSCLC tissues and matched normal lung tissues.
| 15-PGDH
expression | | COX-2 expression | |
|---|
|
| |
| |
|---|
| Groups | High, n (%) | Low, n (%) | P-value | High, n (%) | Low, n (%) | P-value |
|---|
| NSCLC tissue
(n=35) | 14 (40.0) | 21 (60.0) | 0.009a | 23 (65.7) | 12 (34.3) | 0.004a |
| Normal lung tissue
(n=6) | 6 (100.0) | 0 (0.0) | | 0 (0.0) | 6 (100.0) | |
Correlation of 15-PGDH, COX-2 and MVD
with clinicopathological characteristics
Table II summarizes
the association between 15-PGDH, COX-2 and MVD, and the
clinicopathological parameters of NSCLC patients. There was no
significant correlation between 15-PGDH and any of the assessed
clinicopathological characteristics, including gender, age,
smoking, histological type, histological differentiation, tumor
size, lymph node invasion and TNM stage (P>0.05). In addition,
COX-2 expression and MVD were not correlated with any
clinicopathological characteristics (P>0.05).
 | Table IICorrelation between 15-PGDH, COX-2,
MVD and clinicopathological features. |
Table II
Correlation between 15-PGDH, COX-2,
MVD and clinicopathological features.
| | 15-PGDH
expression | COX-2 expression | MVD |
|---|
| |
|
|
|
|---|
| Characteristics | No. | High, n | Low, n | P-value | High, n | Low, n | P-value | Mean ± SD | P-value |
|---|
| Patients
included | 35 | 14 | 21 | | 23 | 12 | | 30.01±13.97 | |
| Gender | | | | 0.392 | | | 1.000 | | 0.246 |
| Male | 22 | 10 | 12 | | 14 | 8 | | 32.14±15.65 | |
| Female | 13 | 4 | 9 | | 9 | 4 | | 26.40±10.08 | |
| Age, years | | | | 1.000 | | | 0.918 | | 0.475 |
| <70 | 15 | 6 | 9 | | 10 | 5 | | 31.99±13.74 | |
| ≥70 | 20 | 8 | 12 | | 13 | 7 | | 28.52±14.31 | |
| Smoking | | | | 0.324 | | | 0.827 | | 0.655 |
| No | 14 | 7 | 7 | | 10 | 4 | | 31.33±14.04 | |
| Yes | 21 | 7 | 14 | | 13 | 8 | | 29.13±14.20 | |
| Histological
type | | | | 0.486 | | | 0.537 | | 0.806 |
| Squamous cell
carcinoma | 20 | 7 | 13 | | 14 | 6 | | 29.50±13.87 | |
| Adenocarcinoma | 15 | 7 | 8 | | 9 | 6 | | 30.69±14.57 | |
| Histological
differentiation | | | | 1.000 | | | 0.576 | | 0.579 |
| Moderate/Well | 24 | 10 | 14 | | 17 | 7 | | 30.92±15.24 | |
| Poor | 11 | 4 | 7 | | 6 | 5 | | 28.04±11.10 | |
| Tumor size | | | | 0.056 | | | 1.000 | | 0.969 |
| ≤3 cm | 10 | 7 | 3 | | 7 | 3 | | 29.86±16.32 | |
| >3 cm | 25 | 7 | 18 | | 16 | 9 | | 30.07±13.29 | |
| Lymph node
invasion | | | | 0.407 | | | 0.903 | | 0.518 |
| No | 17 | 8 | 9 | | 11 | 6 | | 28.41±15.12 | |
| Yes | 18 | 6 | 12 | | 12 | 6 | | 31.52±13.04 | |
| TNM stage | | | | 0.158 | | | 0.329 | | 0.532 |
| I+II | 24 | 12 | 12 | | 14 | 10 | | 30.51±15.40 | |
| III+IV | 11 | 2 | 9 | | 9 | 2 | | 28.92±10.79 | |
Correlation between 15-PGDH, COX-2 and
MVD
The mean MVD for all 35 cases was 30.01 (range,
10.40–74.20). Representative immunohistochemical staining of CD34
(MVD staining) is shown in Fig. 1.
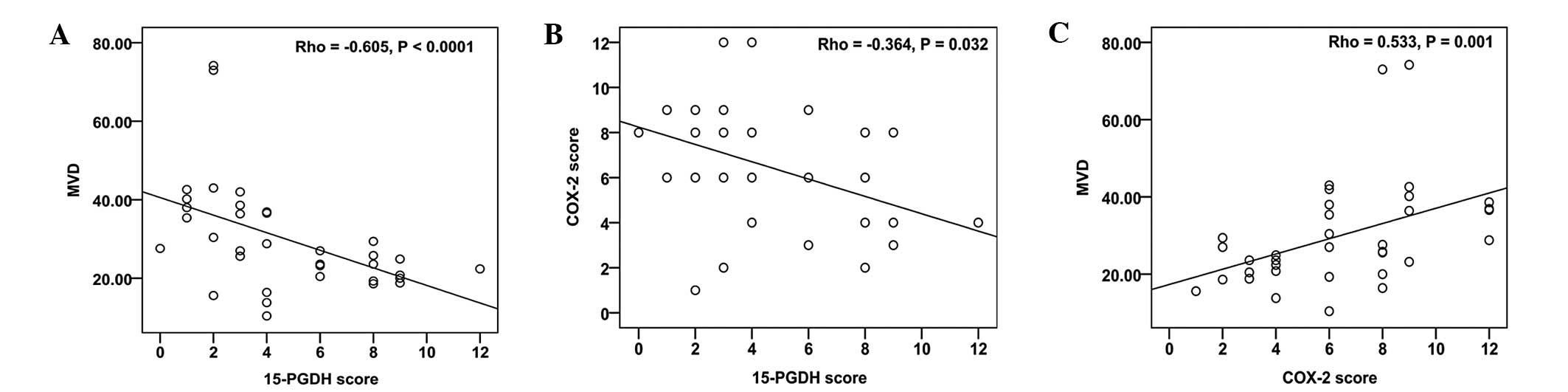
The expression of 15-PGDH was negatively correlated with MVD
(Fig. 2A; correlation coefficient
(ρ) = −0.605, P<0.001) and COX-2 expression (Fig. 2B; ρ = −0.364, P=0.032). In addition,
the MVD was significantly associated with COX-2 (Fig. 2C; ρ = 0.533, P=0.001).
Survival analysis
The prognostic value of 15-PGDH, COX-2 and MVD on OS
was evaluated in all NSCLC patients. The median survival time for
the 35 patients was 34 months. The five-year OS rate for all
patients was 42.9% (15/35). A strong prognostic impact of the three
predictor variables on the survival curve was identified (Fig. 3). Patients with a low 15-PGDH
expression level had a significantly poorer prognosis compared with
patients with a high 15-PGDH expression level (Fig. 3A; log-rank, P<0.0001). Patients
with a high COX-2 expression level (Fig. 3B) or a high MVD (Fig. 3C) had a shorter survival time
(log-rank, P=0.038 and P<0.0001, respectively).
Discussion
15-PGDH is regarded as a tumor suppressor, and has
been shown to be overexpressed in cancer cells, including breast,
colon, lung and glioma cells, resulting in reduced cellular
proliferation (4). Furthermore,
mice inoculated with cancer cells that were transfected with
15-PGDH showed a marked retardation of xenograft tumor growth
(3,4). The present study found that the
expression of 15-PGDH was low in the human NSCLC tissues compared
with the normal lung tissues, which is consistent with the
downregulation of 15-PGDH in numerous human cancer specimens,
including those of lung (19),
colorectal (20,21), gastric (10,22),
breast (7), bladder (11) and pancreatic (8) cancers. These findings indicate that
the reduction of 15-PGDH may be associated with the occurrence of
NSCLC. Furthermore, it was identified that there was no significant
association between the expression of 15-PGDH and tumor
differentiation, node invasion and TNM stage. Lim et al
(21) reported similar data in
colorectal cancer. However, a loss of 15-PGDH has been positively
correlated with the differentiation, distant metastasis and TNM
stage of gastric cancer (10,22).
These discrepancies are likely affected by intratumoral
heterogeneity and the sample size. The loss of 15-PGDH may have a
role in the occurrence, but not the progression of lung cancer.
15-PGDH is known to be an endogenous COX-2
antagonist (23), and the increased
expression level of COX-2 has been observed to be inversely
associated with the expression of 15-PGDH in various cancer tissues
(18,22,24–27).
Based on these studies, the association of 15-PGDH and COX-2 was
investigated in the present study. The expression of 15-PGDH was
shown to be negatively correlated with the expression of COX-2 in
the human NSCLC specimens. This result supports the hypothesis that
15-PGDH and COX-2 are reciprocally regulated in cancer. In the
present study, overexpression of COX-2 and low levels of 15-PGDH
were simultaneously detected in the lung cancer tissues.
Stimulation of A549 human lung adenocarcinoma cells with IL-1β
revealed an increase in COX-2 expression and endogenous synthesis
of PGE2, accompanied by the downregulation of 15-PGDH (24). Overexpression of COX-2 and
repression of 15-PGDH may coordinately increase the level of PGE2
in the tumor microenvironment and exacerbate the carcinogenic
process (24).
Angiogenesis is important for cancer progression and
prognosis (28). MVD, as a standard
quantification of tumor angiogenesis, is considered to be a
prognostic indicator of solid tumors (29–32),
and high levels of MVD indicate a short survival time in lung
cancer patients (33). The data
from the present study are consistent with these results. Previous
studies have demonstrated that COX-2-mediated PGE2 promotes
neovascularization through the upregulation of pro-angiogenic
factors, such as vascular endothelial growth factor (34,35).
However, few studies have reported the function of 15-PGDH in tumor
angiogenesis. Huang et al (12) observed that the MVD was
significantly reduced in xenografts derived from
15-PGDH-overexpressing H358 lung cancer cells, and that the
expression of 15-PGDH could inhibit capillary tube formation of
human umbilical endothelial cells and VEGF production. Consistent
with these results, the present study showed that the expression of
15-PGDH was inversely associated with MVD in human lung cancer
tissues. Additionally, it has been reported that apoptotic cells,
which originated from stressed or damaged tissues, were found to
induce PGE2-modulated angiogenic properties in human macrophages,
accompanied by COX-2 upregulation and repression of 15-PGDH
(36). A previous study showed that
the combinatory treatment of 15-PGDH gene therapy and COX-2
inhibitor significantly inhibits murine breast tumor growth and
tumor angiogenesis compared with monotherapy or controls (37). The effect of this combination
treatment was associated with a significant reduction of PGE2 in
the serum, which resulted from increased 15-PGDH and decreased
COX-2 expression in tumor tissues (37). An anti-angiogenic mechanism may
contribute to the tumor suppressor function of 15-PGDH, and it has
been shown that administration with a COX-2 inhibitor enhances the
efficacy of 15-PGDH (12,37). When considering the relevance of
15-PGDH and COX-2 to neovascularization, the present study found
that the low 15-PGDH and high COX-2 expression levels were
associated with an unfavorable prognosis in the NSCLC patients.
These findings indicate that 15-PGDH and COX-2 may affect prognosis
by regulating the angiogenic activity of lung cancer cells.
Although the present study demonstrated that 15-PGDH
and COX-2 reciprocally regulated angiogenesis and were prognostic
predictive factors of patients with NSCLC, there are several
limitations to consider. Firstly, this study had a relatively small
sample size, which could affect the statistical significance.
Furthermore, limited to the small number of patients, a
multivariate survival analysis could not be conducted to determine
the independent prognostic predictors. Thirdly, the present study
only included two main histological types of NSCLC specimens.
Therefore, the data regarding the expression of markers may not
reflect the comprehensive status of NSCLC. These results may only
apply to squamous cell carcinoma and adenocarcinoma rather than to
all NSCLC histological types.
In conclusion, the present respective study found
downregulated 15-PGDH expression in human NSCLC tissues. The
correlations between 15-PGDH and COX-2 expression, and their
association with angiogenesis and prognosis, was evaluated. To the
best of our knowledge, this is the first study to determine that a
loss of 15-PGDH is associated with a poor prognosis in patients
with NSCLC. These results provide preliminary evidence for further
studies in NSCLC, including those with a larger sample size,
prospective clinical studies and those with therapies targeting
15-PGDH and COX-2, such as the use of COX-2 inhibitors.
References
|
1
|
Jemal A, Siegel R, Ward E, et al: Cancer
statistics, 2009. CA Cancer J Clin. 59:225–249. 2009.
|
|
2
|
Wang D and Dubois RN: Prostaglandins and
cancer. Gut. 55:115–122. 2006.
|
|
3
|
Tai HH: Prostaglandin catabolic enzymes as
tumor suppressors. Cancer Metastasis Rev. 30:409–417. 2011.
|
|
4
|
Na HK, Park JM, Lee HG, et al:
15-Hydroxyprostaglandin dehydrogenase as a novel molecular target
for cancer chemoprevention and therapy. Biochem Pharmacol.
82:1352–1360. 2011.
|
|
5
|
Tai HH, Cho H, Tong M and Ding Y:
NAD+-linked 15-hydroxyprostaglandin dehydrogenase: structure and
biological functions. Curr Pharm Des. 12:955–962. 2006.
|
|
6
|
Tatsuwaki H, Tanigawa T, Watanabe T, et
al: Reduction of 15-hydroxyprostaglandin dehydrogenase expression
is an independent predictor of poor survival associated with
enhanced cell proliferation in gastric adenocarcinoma. Cancer Sci.
101:550–558. 2010.
|
|
7
|
Wolf I, O’Kelly J, Rubinek T, et al:
15-hydroxyprostaglandin dehydrogenase is a tumor suppressor of
human breast cancer. Cancer Res. 66:7818–7823. 2006.
|
|
8
|
Pham H, Chen M, Li A, et al: Loss of
15-hydroxyprostaglandin dehydrogenase increases prostaglandin E2 in
pancreatic tumors. Pancreas. 39:332–339. 2010.
|
|
9
|
Quidville V, Segond N, Lausson S, et al:
15-Hydroxyprostaglan- din-dehydrogenase is involved in
anti-proliferative effect of non-steroidal anti-inflammatory drugs
COX-1 inhibitors on a human medullary thyroid carcinoma cell line.
Prostaglandins Other Lipid Mediat. 81:14–30. 2006.
|
|
10
|
Lou LH, Jing DD, Lai YX, et al: 15-PGDH is
reduced and induces apoptosis and cell cycle arrest in gastric
carcinoma. World J Gastroenterol. 18:1028–1037. 2012.
|
|
11
|
Tseng-Rogenski S, Gee J, Ignatoski KW, et
al: Loss of 15-hydroxyprostaglandin dehydrogenase expression
contributes to bladder cancer progression. Am J Pathol.
176:1462–1468. 2010.
|
|
12
|
Huang G, Eisenberg R, Yan M, et al:
15-Hydroxyprostaglandin dehydrogenase is a target of hepatocyte
nuclear factor 3beta and a tumor suppressor in lung cancer. Cancer
Res. 68:5040–5048. 2008.
|
|
13
|
Wakimoto N, Wolf I, Yin D, et al:
Nonsteroidal anti-inflammatory drugs suppress glioma via
15-hydroxyprostaglandin dehydrogenase. Cancer Res. 68:6978–6986.
2008.
|
|
14
|
Backlund MG, Mann JR, Holla VR, et al:
Repression of 15-hydroxyprostaglandin dehydrogenase involves
histone deacetylase 2 and snail in colorectal cancer. Cancer Res.
68:9331–9337. 2008.
|
|
15
|
Edge SB and Compton CC: The American Joint
Committee on Cancer: the 7th edition of the AJCC cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474.
2010.
|
|
16
|
Travis WD, Brambilla E, Müller-Hermelink
HK and Harris CC: Pathology & Genetics: Tumours of the Lung,
Pleura, Thymus and Heart. IARC Press; Lyon: pp. 26–44. 2004
|
|
17
|
Gou HF, Chen XC, Zhu J, et al: Expressions
of COX-2 and VEGF-C in gastric cancer: correlations with
lymphangiogenesis and prognostic implications. J Exp Clin Cancer
Res. 30:142011.
|
|
18
|
Weidner N, Semple JP, Welch WR and Folkman
J: Tumor angiogenesis and metastasis - correlation in invasive
breast carcinoma. N Engl J Med. 324:1–8. 1991.
|
|
19
|
Ding Y, Tong M, Liu S, Moscow JA and Tai
HH: NAD+-linked 15-hydroxyprostaglandin dehydrogenase (15-PGDH)
behaves as a tumor suppressor in lung cancer. Carcinogenesis.
26:65–72. 2005.
|
|
20
|
Backlund MG, Mann JR, Holla VR, et al:
15-Hydroxyprostaglandin dehydrogenase is down-regulated in
colorectal cancer. J Biol Chem. 280:3217–3223. 2005.
|
|
21
|
Lim SC, Cho H, Lee TB, et al: Impacts of
cytosolic phospholipase A2, 15-prostaglandin dehydrogenase, and
cyclooxygenase-2 expressions on tumor progression in colorectal
cancer. Yonsei Med J. 51:692–699. 2010.
|
|
22
|
Liu Z, Wang X, Lu Y, et al: Expression of
15-PGDH is downregulated by COX-2 in gastric cancer.
Carcinogenesis. 29:1219–1227. 2008.
|
|
23
|
Tennis MA, Vanscoyk M, Keith RL and Winn
RA: The role of prostacyclin in lung cancer. Transl Res. 155:57–61.
2010.
|
|
24
|
Tong M, Ding Y and Tai HH: Reciprocal
regulation of cyclooxygenase-2 and 15-hydroxyprostaglandin
dehydrogenase expression in A549 human lung adenocarcinoma cells.
Carcinogenesis. 27:2170–2179. 2006.
|
|
25
|
Krishnan AV, Swami S and Feldman D:
Vitamin D and breast cancer: inhibition of estrogen synthesis and
signaling. J Steroid Biochem Mol Biol. 121:343–348. 2010.
|
|
26
|
Lim K, Han C, Xu L, et al:
Cyclooxygenase-2-derived prostaglandin E2 activates beta-catenin in
human cholangiocarcinoma cells: evidence for inhibition of these
signaling pathways by omega 3 polyunsaturated fatty acids. Cancer
Res. 68:553–560. 2008.
|
|
27
|
Lim K, Han C, Dai Y, Shen M and Wu T:
Omega-3 polyunsaturated fatty acids inhibit hepatocellular
carcinoma cell growth through blocking beta-catenin and
cyclooxygenase-2. Mol Cancer Ther. 8:3046–3055. 2009.
|
|
28
|
Sahin M1, Sahin E and Gümüslü S:
Cyclooxygenase-2 in cancer and angiogenesis. Angiology. 60:242–253.
2009.
|
|
29
|
Zhou D, Cheng SQ, Ji HF, et al: Evaluation
of protein pigment epithelium-derived factor (PEDF) and microvessel
density (MVD) as prognostic indicators in breast cancer. J Cancer
Res Clin Oncol. 136:1719–1727. 2010.
|
|
30
|
Qin LX and Tang ZY: Recent progress in
predictive biomarkers for metastatic recurrence of human
hepatocellular carcinoma: a review of the literature. J Cancer Res
Clin Oncol. 130:497–513. 2004.
|
|
31
|
Gulubova M and Vlaykova T: Prognostic
significance of mast cell number and microvascular density for the
survival of patients with primary colorectal cancer. J
Gastroenterol Hepatol. 24:1265–1275. 2009.
|
|
32
|
Aurello P, Bellagamba R, Rossi Del Monte
S, et al: Apoptosis and microvessel density in gastric cancer:
correlation with tumor stage and prognosis. Am Surg. 75:1183–1188.
2009.
|
|
33
|
Meert AP, Paesmans M, Martin B, et al: The
role of microvessel density on the survival of patients with lung
cancer: a systematic review of the literature with meta-analysis.
Br J Cancer. 87:694–701. 2002.
|
|
34
|
Luo H, Chen Z, Jin H, et al:
Cyclooxygenase-2 up-regulates vascular endothelial growth factor
via a protein kinase C pathway in non-small cell lung cancer. J Exp
Clin Cancer Res. 30:62011.
|
|
35
|
Huang SP, Wu MS, Shun CT, et al:
Cyclooxygenase-2 increases hypoxia-inducible factor-1 and vascular
endothelial growth factor to promote angiogenesis in gastric
carcinoma. J Biomed Sci. 12:229–241. 2005.
|
|
36
|
Brecht K, Weigert A, Hu J, et al:
Macrophages programmed by apoptotic cells promote angiogenesis via
prostaglandin E2. FASEB J. 25:2408–2417. 2011.
|
|
37
|
Zhang B, Ma X, Li Z, et al: Celecoxib
enhances the efficacy of 15-hydroxyprostaglandin dehydrogenase gene
therapy in treating murine breast cancer. J Cancer Res Clin Oncol.
139:797–807. 2013.
|