Introduction
Lung cancer is the leading cause of cancer-related
mortality worldwide (1). Numerous
achievements have been made in chemotherapy, radiotherapy and
surgical techniques. However, the survival rate has not been
improved for many decades. This poor prognosis is mostly due to the
development of drug resistance by cancer cells during treatment and
the likelihood of subsequent metastasis (2). The drug-surviving cells (DSCs) are
responsible for tumor regeneration after chemotherapy, and may be
one of the mechanisms involved in drug resistance (3). Immunoresistance induced by
chemotherapy agents may be another mechanism (4). Am1010, a new DSC line, was established
in our previous study from an arm muscle metastasic tumor of a
patient diagnosed with lung adenocarcinoma (5). The Am1010 cell line demonstrated in
vitro multi-drug-resistance against cisplatin, taxol and
geitinib, as well as in vivo invasion ability (5).
Chemotherapy of lung cancer is targeted not only at
the tumor cells but also at the immune system. The immune system is
a key factor in preventing the development of tumors and in
limiting tumor growth (6). Tumor
infiltration of lymphocytes is frequently found in tumors,
suggesting that tumors trigger an immune response in the host. A
number of studies have reported a survival benefit associated with
the presence of tumor-infiltrating lymphocytes (TILs) (7–9);
although, in some circumstances, it was a paradoxical result
(10,11).
Non-obese diabetic/severe combined immunodeficient
(NOD/SCID) mice have multiple defects in innate and adaptive
immunologic functions (12). They
are absent of T and B lymphocytes and natural killer (NK) cells.
They also exhibit dysfunctional macrophages, dendritic cells and
complement systems. Thus, NOD/SCID mice are an ideal tool for
immunodeficient studies. These mice are also commonly used in
studies involving various types of cancer xenografts (13,14).
Previously, intravenous injection of granulocyte colony-stimulating
factor-induced white blood cells from human peripheral blood into
NOD/SCID mice was shown to produce a 6.5-month detection of human T
lymphocytes (15). Peripheral blood
mononuclear cell (PBMC) injection was reported to inhibit the
growth of various types of tumors in NOD/SCID mice, including
Burkitt’s lymphoma (16) and
neuroblastoma cells (17). However,
the protective role of PBMCs in lung cancer is not yet known to
date.
In the present study, NOD/SCID mice were inoculated
with lung carcinoma fragments (obtained by injecting Am1010 cells
into NOD/SCID mice, allowing the tumors to grow to 150
mm3 and then cutting the tumors into equally sized
fragments) and human PBMCs. An ideal heterotopic lung cancer model
was thus established. Using the model, we observed the protective
effect of PBMCs on tumor growth and the reconstitution of immune
system.
Materials and methods
Reagents
Matrigel and FACS lysing solution were purchased
from BD Biosciences (San Jose, CA, USA). Hydrogen peroxide
(H2O2), 3,3′-diaminobenzidine and
pentobarbital sodium were purchased from Sigma-Aldrich (St. Louis,
MO, USA). RIPA lysis buffer and enhanced chemiluminescence (ECL)
reagent were purchased from Pierce Biotechnology, Inc. (Rockford,
IL, USA). Rabbit polyclonal antibody against PC5-CD3 was purchased
from Beckman Coulter, Inc. (Brea, CA, USA). Rabbit monoclonal
antibody against CD3 (ab109531), and rabbit polyclonal antibodies
against CD4 (ab70951), CD8 (ab85792) and FoxP3 (ab10563) were
purchased from Abcam (Cambridge, MA, USA). Rabbit polyclonal
antibody against GAPDH was purchased from Cell Signaling
Technology, Inc. (Danvers, MA, USA). Horseradish peroxidase
(HRP)-coupled goat polyclonal anti-rabbit secondary IgG (sc-2004)
and biotinylated goat polyclonal anti-rabbit IgG (sc-2040)
secondary antibodies, as well as the avidin-biotin-HRP complex were
purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA).
RPMI-1640 medium and fetal bovine serum (FBS) were purchased from
Life Technologies (Grand Island, NY, USA).
Animals
Male NOD/SCID mice were provided by Experimental
Animal Center, Sun Yat-Sen University (Guangzhou, China). The mice
were kept in separate cages in a room with specific pathogen-free
standards at a constant humidity and temperature, with food and
water available ad libitum. The animal room was on a 12/12-h
light/dark cycle. At the age of 4 weeks, blood samples were taken
from the tail vein for determination of immunoglobulin (Ig) levels.
Only the mice with IgM levels <1 μg/ml were used for further
study. The experiments were performed at the 6th week. Tumor
fragments were obtained by injecting Am1010 cells into six NOD/SCID
mice, allowing the tumors to grow to 150 mm3 and then
cutting the tumors into equally sized fragments. For the
inoculation with tumor fragments and/or PBMCs, the mice were
divided randomly into three groups. The Am1010+PBMC group was
inoculated with a tumor fragment and PBMCs (n=10); the Am1010 group
was inoculated with a tumor fragment (n=10); and the PBMC group was
only injected with PBMCs (n=5). This study was approved by
Guangzhou Medical College (Guangzhou, China).
Establishment of xenografts
The green fluorescent protein (GFP)-Am1010 cell line
was established in our previous study (5). The cells were stored in liquid
nitrogen until use. A total of 2×107 cells in a 200 μl
volume of 50% Matrigel were injected subcutaneously into the dorsal
surface of the right lower quadrant of the six NOD/SCID mice. Tumor
growth was assessed by palpation every 3 days. The two bisecting
diameters were measured with calipers, and the volume was
calculated using the formula 0.4xab2, where
a represents the longer diameter and b the shorter
perpendicular diameter (18). When
the tumors grew up to a size of 150 mm3, they were
removed and cut into 1×3×3 mm fragments. Twenty recipient mice were
anesthetized with pentobarbital sodium (10 mg/kg). A 3-mm skin
incision was cut at the sixth rib, left midaxillary line. A
fragment of tumor was put into the incision, and the incision was
sutured. The five other mice in the PBMC group underwent the same
surgical procedure, but without insertion of a tumor fragment.
Human PBMC preparation and
transplantation
On the day of inoculation with tumor segments, human
PBMCs were prepared according to a previous study (19). Fresh peripheral venous blood from
healthy adult volunteers was collected at The First Affiliated
Hospital of Guangzhou Medical College (Guangzhou, China) in
heparinized tubes. For the isolation of PBMCs, leucosep tubes
(Greiner Bio-One, Wemmel, Belgium) were used, and blood was diluted
1:1 with RPMI-1640 medium (vol/vol) prior to transferring into the
leucosep tube. Following centrifugation (10 min, 1000 × g), the
PBMC layer was pooled and transferred into a 15-ml falcon tube. The
sample was washed with 10 ml phosphate-buffered saline (PBS) and
centrifuged again for 10 min at 250 × g. The obtained cell pellet
was resuspended in PBS. A total of 1×108 PBMCs per mouse
were intraperitoneally injected into NOD/SCID mice of the
Am1010+PBMC and PBMC groups, for the reconstitution of immune
system. The same volume of PBS was injected into the mice of the
Am1010 group.
Flow cytometry
To examine reconstitution of the immune system due
to PBMC transplantation in the recipient NOD/SCID mice,
CD3+ cells were analyzed by flow cytometry. In brief,
peripheral blood (100 μl) was collected into EDTA-coated tubes from
the tail vein at every week following the transplantation for four
weeks. Red blood cells were first lysed with FACS lysing solution
and then washed twice with PBS containing 2% FBS. The leukocytes
were then incubated with PC5-labeled anti-CD3 antibody for 30 min
at 4°C. The staining was assessed by flow cytometry. PBMCs obtained
from normal human volunteers and normal mice were used as positive
and negative controls, respectively. Results are expressed as the
percentage of positive cells gated in the human lymphocyte
population in the scatter plot.
Whole-body fluorescence imaging
Whole-body fluorescence imaging was performed to
examine the growth of the inoculated tumor fragment every seven
days. The mice were anesthetized with 1% pentobarbital sodium (0.2
ml/20 g body weight), and were placed in a NightOWLII LB 983
molecular light imager (Berthold Technologies, Bad Wildbad,
Germany). The images were photographed for 1 sec using a GFP filter
(GFP Ex480/20 and Em520/10; Berthold Technologies). Data were
processed with WinLight software (Winlight System, Pertuis, France)
and the fluorescent area was recorded.
Histological examination and
immunohistochemistry
At four weeks frollowing tumor fragment and/or PBMC
administration, all mice were anesthetized with 10% chloral hydrate
and perfused intracardially with normal saline and 4%
paraformaldehyde in 0.1 M phosphate buffer (pH 7.4). The spleens
and tumors were fixed in 10% formalin solution, embedded in
paraffin and cut serially into 4-μm sections. Some of the sections
were stained with hematoxylin and eosin (H&E), while others
were used for immunohistochemistry.
Immunohistochemistry was performed to detect CD3,
CD4, CD8 and FoxP3 expression according to a previous study
(20). Briefly, endogenous
peroxidase was first quenched with 0.3% H2O2.
Prior to the application of primary antibody, nonspecific binding
was blocked with normal non-immune serum, and tissue sections were
incubated with primary antibody (1:500 each) at 4°C overnight.
Sections were then incubated with biotinylated secondary antibody
(1:200) for 2 h at room temperature, followed by avidin-biotin-HRP
complex (1:150) for 1 h. Immunohistochemical reactions were
revealed by using 0.05% 3,3′-diaminobenzidine and 0.03%
H2O2 as chromogen. Following each incubation,
sections were thoroughly washed with PBS. Negative controls were
performed by omitting the primary antibody, and showed no positive
staining.
Western blotting
Total tissue proteins were extracted with RIPA lysis
buffer, quantified using the Bradford method and separated by
SDS-PAGE (12%). A total of 40 μg of protein was used to test for
CD3, CD4, CD8 and FoxP3. Proteins were transferred to
polyvinylidene fluoride membranes (Millipore, Billerica, MA, USA),
and membranes were incubated overnight at 4°C with antibody against
CD3, CD4, CD8, FoxP3 (1:1000 each) or GAPDH (1:10,000). The
membranes were incubated with HRP-coupled secondary IgG for 1 h.
The bound proteins were then visualized using ECL and analyzed
using BioImaging Systems (UVP, Upland, CA, USA).
Statistics
Data are presented as the mean ± standard error of
the mean. Statistical analysis was performed using one-way analysis
of variance followed by Dunnett’s test for multiple comparisons.
P<0.05 was considered to indicate a statistically significant
difference.
Results
PBMC transplantation inhibited tumor
growth
The inoculation of tumor segments produced 100%
tumor growth in the NOD/SCID mice in the Am1010 and Am1010+PBMC
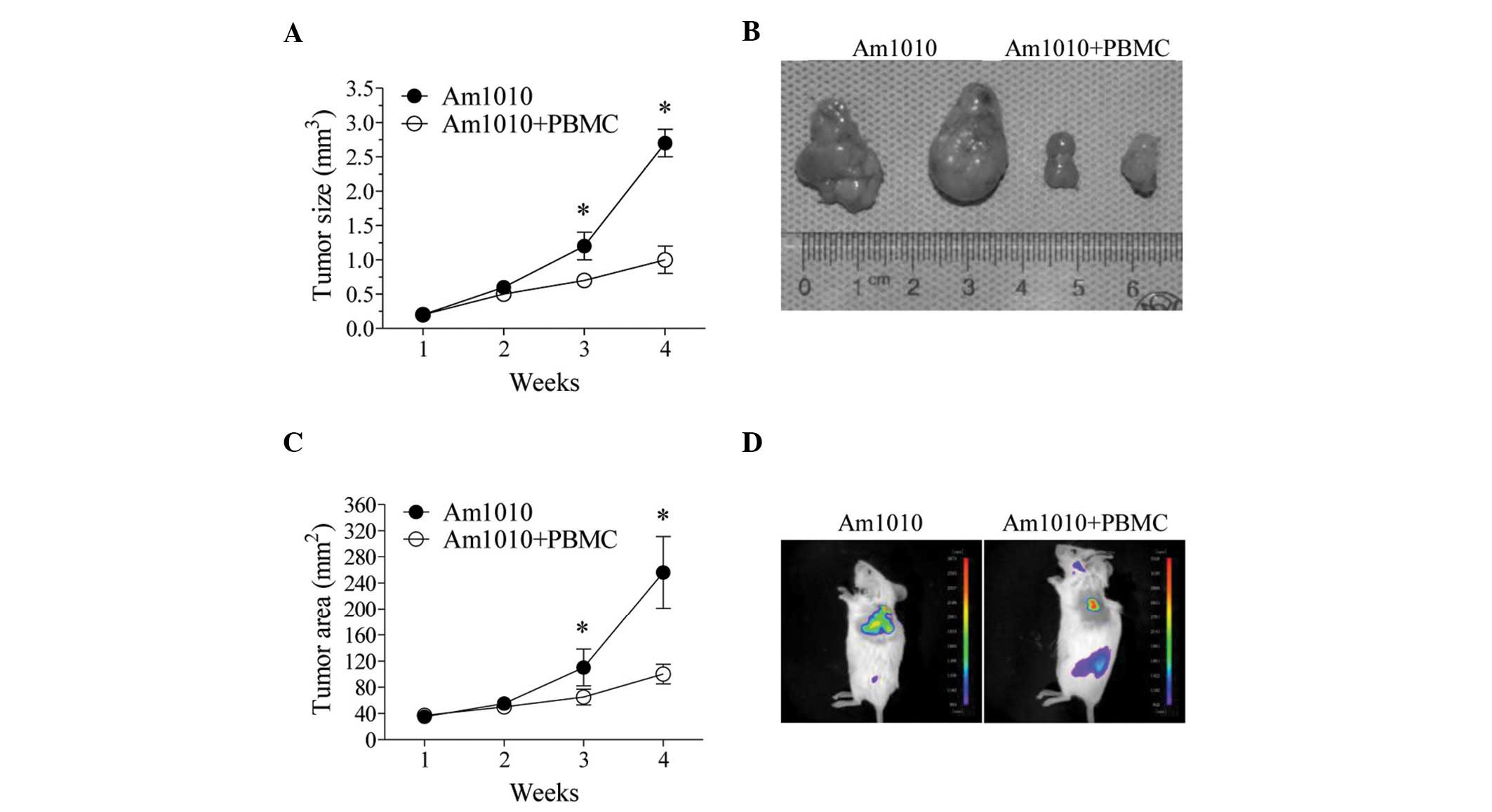
groups. The tumor size increased with time (Fig. 1A), and when comparing tumor growth
in the two groups, it was faster in the Am1010 group compared with
the Am1010+PBMC group. The difference in tumor size between the two
groups became significant from the third week after inoculation
(P<0.05) (Fig. 1A,B).
We further examined the tumor growth with
fluorescence imaging in the Am1010 and Am1010+PBMC groups. Under
fluorescence imaging, the GFP protein of the tumors was expressed
stably. The tumors appeared green-yellow at the inoculation sites
(Fig. 1D). The fluorescence area in
the two groups increased with time (Fig. 1C). It was significantly larger in
the Am1010 group than in the Am1010+PBMC group at the third and
fourth week (P<0.05, Fig. 1C,D).
These results showed that PBMC transplantation exhibited a marked
inhibitory effect on tumor growth.
T-lymphocyte reconstitution in the
peripheral blood
SCID/NOD mice are absent from T and B lymphocytes
and NK cells. Previously, it has been shown that PBMC
transplantation can reconstitute the immune system (15). To confirm this, we tested
T-lymphocyte marker CD3 in the peripheral blood with flow
cytometry. CD3+ cells were detected early at the first
week after PBMC injection in the PBMC and Am1010+PBMC groups
(Fig. 2). The ratio of
CD3+ cells to normal human nucleated cells was more than
1% at the 1st week, and increased with time. The increase/trend was
similar between the two groups. At the fourth week, the ratio of
CD3+ cells was increased to 70%. Thus, the T-lymphocyte
reconstitution was successfully carried out in this study.
T-lymphocyte recruitment in the
spleen
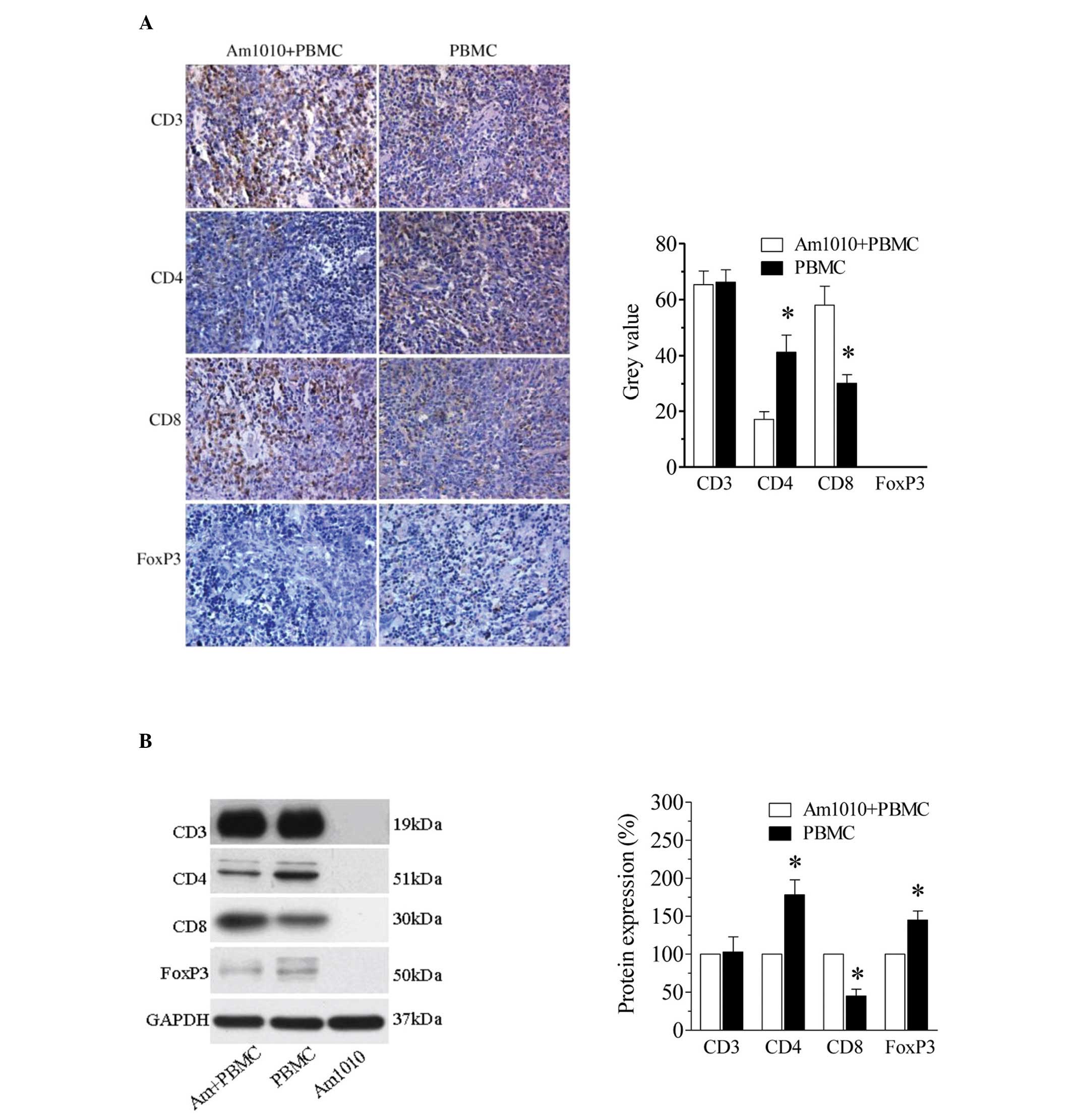
To further examine the reconstitution of the immune
system, we examined T-lymphocyte recruitment in the spleen at the
fourth week following PBMC transplantation using
immunohistochemistry (Fig. 3A).
Numerous CD3+ cells were seen in the spleens in the
Am1010+PBMC and PBMC groups. The number of CD3+ cells
appeared similar between the two groups. The subtypes of T
lymphocytes were further evaluated by examining CD4, CD8 and FoxP3
immunoreactivity in the spleens. Among these subtypes in the
Am1010+PBMC group, there were more CD8+ cells and fewer
CD4+ cells. By contrast, there were more CD4+
cells and fewer CD8+ cells in the PBMC group. FoxP3, a
specific marker of regulatory T-lymphocytes, was not detected in
either of the two groups.
These T-lymphocyte subtypes were further examined
using western blotting (Fig. 3B).
Consistent with the immunohistochemistry results, the expression of
CD3 was similar between the Am1010+PBMC and PBMC groups. In the
Am1010+PBMC group, the CD8 levels were higher (P<0.05), but the
CD4 levels were lower (P<0.05), compared with those in the PBMC
group. Notably, FoxP3 protein expression was observed in the two
groups. The levels of FoxP3 were lower in the Am1010+PBMC group
than in the PBMC group. No CD3, CD4, CD8 or FoxP3 protein
expression was observed in the Am1010 group, which served as
negative control to prove the successful reconstitution of the
immune system.
T-lymphocyte recruitment in tumors
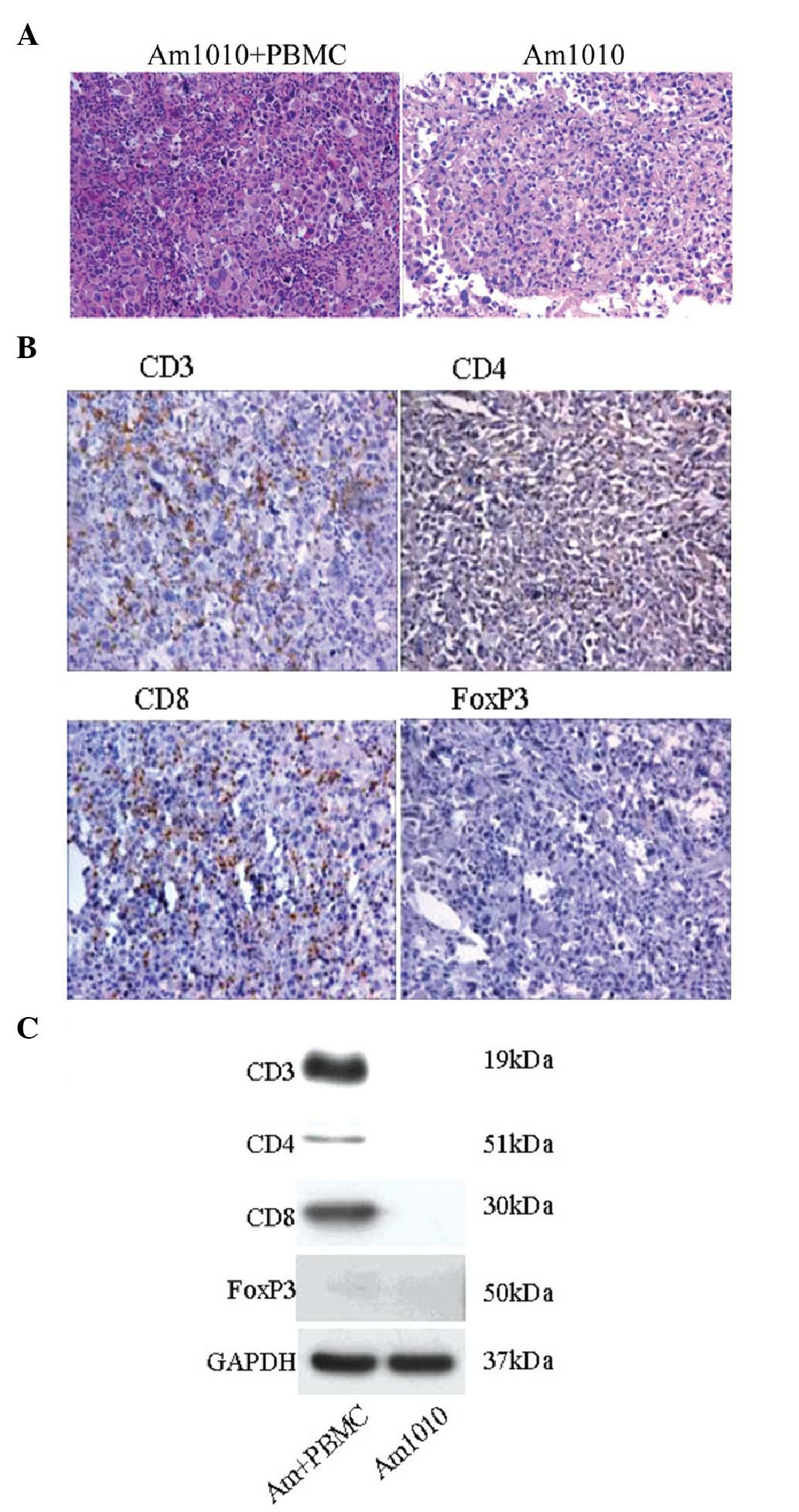
We examined whether T lymphocytes were recruited in
the tumor tissues. Firstly, the tumor tissues were examined using
H&E staining (Fig. 4A). In the
Am1010 group, the tumor cells had similar size and regular
arrangement. In the Am1010+PBMC group, the tumor cells were of
different sizes. Notably, there were numerous smaller cells
surrounding them. The infiltrated smaller cells were analyzed using
immunohistochemistry (Fig. 4B); a
large quantity of CD3+ and CD8+ cells, and a
small quantity of CD4+ cells were identified. No
FoxP3+ cells were observed. The four T-lymphocyte
markers in the tumor tissues were then evaluated using western
blotting (Fig. 4C). The results
showed that CD3, CD4 and CD8 were present in cells of the
Am1010+PBMC group, but not in cells of the Am1010 group. No FoxP3
expression was identified in either of the two groups.
Discussion
The poor prognosis of lung cancer is largely due to
the fact that lung cancer cells are resistant to chemotherapy drugs
and metastasis. The underlying mechanisms have been presumed to be
closely associated with dysfunction of immune system, although they
are yet not elucidated to date (21,22).
In the present study, we established a heterotopic lung carcinoma
model in NOD/SCID mice using multi-drug-resistant Am1010 cells,
which were prepared from human lung adenocarcinoma. In the model,
human PBMC transplantation produced a significant inhibitory effect
on tumor growth, and was accompanied by numerous T lymphocytes,
particularly CD8+ cells, in the peripheral blood, spleen
and tumor tissue. Our results provide direct evidence for the
important role of immune reconstitution from PBMCs in lung cancer
regression.
The Am1010 lung carcinoma cell line is resistant to
cisplatin, taxol, and gefitinib. The cancer cells grow and form
tumors in vivo, when injected into nude mice (5). In the present study, Am1010 lung
carcinoma cells were injected into subcutaneous tissues in NOD/SCID
mice, successfully inducing heterotopic tumor growth. When
fragments of these tumors were inoculated into recipient NOD/SCID
mice, they grew rapidly. The results support our hypothesis that
Am1010 lung carcinoma cells and tumor fragments are a useful tool
for studying lung carcinoma.
The NOD/SCID mouse strain was developed by crossing
SCID mice with NOD mice (12). It
has been reported that the engraftment levels of human splenocytes
and PBMCs in NOD/SCID mice are 5- to 10-fold higher than those in
the classical SCID mice (23,24).
Consistent with these reports, the present study showed that
intraperitoneal injection of human PBMCs into the NOD/SCID mice
produced a gradual increase of human CD3 lymphocytes in the
peripheral blood, and the ratio of CD3 lymphocytes was ≤70% of the
original amount at the fourth week. In addition,
immunohistochemistry and western blotting revealed the presence of
numerous CD3 cells in the spleen. Thus, the data demonstrate that
PBMC transplantation successfully induces T-lymphocyte
reconstitution in NOD/SCID, not only in the peripheral blood, but
also in a lymphoid organ.
Numerous studies have analyzed the association
between tumors and immunity. TILs have been widely reported to have
protective functions against tumors. TILs have been identified in
various types of tumors, including lung cancer. Their existence has
been found to be associated with decreased tumor progression
(25), induction of responses to
chemotherapy (26,27) and increased lifetime of patients
(28). TILs derived from neonatal
cord blood mononuclear cells induced marked human lung and cervical
tumor remission in NOD/SCID mice, and the antitumor effect was
accompanied with a high infiltration of CD3+ T cells in
tumors and a marked induction of apoptotic cell death (25). In the present study, PBMC
engraftment by intraperitoneal injection significantly slowed the
lung tumor growth and induced CD3+ T-lymphocyte
recruitment in tumors. Therefore, the TILs may be an important
factor for lung cancer remission in this study.
T lymphocytes are mainly composed of helper
lymphocytes (CD4+ T cells), cytotoxic T lymphocytes
(CTLs or CD8+ T cells) and regulatory T cells
(FoxP3+ T cells) (29).
A great quantity of animal studies and clinical data has revealed
the important role of T-lymphocyte subtypes in antitumor effects
(30,31). An investigation of 335 cases of
non-small cell lung cancer (NSCLC) showed that an increased number
of epithelial CD8+, stromal CD8+ and stromal
CD4+ lymphocytes was significantly correlated with
improved disease-specific survival. In particular, a low level of
stromal CD8+ lymphocyte infiltration was associated with
an increased incidence of angiolymphatic invasion (32).
CD8+ T cells have been found to be
associated with improved outcome in the majority of human tumors
(33), including lung cancer
(32,34). In the present study, we observed
that T lymphocytes infiltrated into tumors. The T-lymphocyte
subtypes were mainly CD8+ lymphocytes. Thus, the
CD8+ lymphocytes from PBMCs may underlie the key
protective mechanism against lung cancer progression in this
study.
In contrast to CD8+ lymphocytes,
infiltration of tumors by regulatory T cells is instead associated
with poor prognosis in NSCLC and other carcinomas (35). The regulatory T cells are thought to
function primarily in cancers by repressing CD8+ T-cell
function. The regulatory T-cell depletion may be therapeutically
beneficial (35). In the present
study, FoxP3, the marker of regulatory T cells, was not observed in
tumors in immunohistochemistry and western blotting, although FoxP3
protein expression was observed in the spleen. The results suggest
that PBMC-inoculated mice may have intact CD8+ T-cell
function, which exerts potent tumor toxicity effect.
In conclusion, in the present study, we applied
multi-drug-resistant lung carcinoma cells to explore the protective
effect of immune reconstitution from PBMC transplantation. The
infiltration of a high proportion of CD8+ T lymphocytes,
but not regulatory T lymphocytes, into tumors may underlie the
mechanism. To the best of our knowledge, this is the first study
involving immunoprotection and multi-drug-resistant lung carcinoma
in general. This study may provide a basis for immunotherapy in
drug-resistant lung carcinoma.
Acknowledgements
This study was supported by the National Natural
Science Foundation (81000951), the China Postdoctoral Science
Foundation (684750) and initial PhD funding by Guangzhou Medical
College (2008C3).
References
|
1
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J,
Murray T and Thun MJ: Cancer statistics, 2008. CA Cancer J Clin.
58:71–96. 2008.
|
|
2
|
Nadkar A, Pungaliya C, Drake K, Zajac E,
Singhal SS and Awasthi S: Therapeutic resistance in lung cancer.
Expert Opin Drug Metab Toxicol. 2:753–777. 2006.
|
|
3
|
Levina V, Marrangoni AM, DeMarco R,
Gorelik E and Lokshin AE: Drug-selected human lung cancer stem
cells: cytokine network, tumorigenic and metastatic properties.
PLoS One. 3:e30772008.
|
|
4
|
Wang X, Long M, Dong K, Lin F, Weng Y,
Ouyang Y, Liu L, Wei J, Chen X, He T and Zhang HZ: Chemotherapy
agents-induced immunoresistance in lung cancer cells could be
reversed by trop-2 inhibition in vitro and in vivo by
interaction with MAPK signaling pathway. Cancer Biol Ther.
14:1123–1132. 2013.
|
|
5
|
Li HL, Xie SM, Zhang L, Cai CJ, Wang W,
Huang J, Wang DY, Wen DP, Deng QH, Zhong NS and He JX:
Establishment and characterization of a new drug surviving cell
line Am1010, derived directly from muscle metastases of a human
lung adenocarcinoma patient with multi-drug-resistance to
cisplatin, taxol, and gefitinib. Acta Pharmacol Sin. 31:601–608.
2010.
|
|
6
|
Prestwich RJ, Errington F, Hatfield P,
Merrick AE, Ilett EJ, Selby PJ and Melcher AA: The immune system -
is it relevant to cancer development, progression and treatment?
Clin Oncol (R Coll Radiol). 20:101–112. 2008.
|
|
7
|
Gooden MJ, de Bock GH, Leffers N, Daemen T
and Nijman HW: The prognostic influence of tumour-infiltrating
lymphocytes in cancer: a systematic review with meta-analysis. Br J
Cancer. 105:93–103. 2011.
|
|
8
|
Sharma P, Shen Y, Wen S, Yamada S,
Jungbluth AA, Gnjatic S, Bajorin DF, Reuter VE, Herr H, Old LJ and
Sato E: CD8 tumor-infiltrating lymphocytes are predictive of
survival in muscle-invasive urothelial carcinoma. Proc Natl Acad
Sci USA. 104:3967–3972. 2007.
|
|
9
|
Staibano S, Mascolo M, Tranfa F, Salvatore
G, Mignogna C, Bufo P, Nugnes L, Bonavolontà G and De Rosa G: Tumor
infiltrating lymphocytes in uveal melanoma: a link with clinical
behavior? Int J Immunopathol Pharmacol. 19:171–179. 2006.
|
|
10
|
Nakamura H, Saji H, Ogata A, Hosaka M,
Hagiwara M, Kawasaki N, Konaka C and Kato H: Immunologic parameters
as significant prognostic factors in lung cancer. Lung Cancer.
37:161–169. 2002.
|
|
11
|
Pelletier MP, Edwardes MD, Michel RP,
Halwani F and Morin JE: Prognostic markers in resectable non-small
cell lung cancer: a multivariate analysis. Can J Surg. 44:180–188.
2001.
|
|
12
|
Shultz LD, Schweitzer PA, Christianson SW,
Gott B, Schweitzer IB, Tennent B, McKenna S, Mobraaten L, Rajan TV,
Greiner DL, et al: Multiple defects in innate and adaptive
immunologic function in NOD/LtSz-scid mice. J Immunol. 154:180–191.
1995.
|
|
13
|
Smit JK, Faber H, Niemantsverdriet M,
Baanstra M, Bussink J, Hollema H, van Os RP, Plukker JT and Coppes
RP: Prediction of response to radiotherapy in the treatment of
esophageal cancer using stem cell markers. Radiother Oncol.
107:434–441. 2013.
|
|
14
|
Yamauchi Y, Izumi Y, Asakura K, Kawai K,
Wakui M, Ohmura M, Suematsu M and Nomori H: Lewis lung carcinoma
progression is facilitated by TIG-3 fibroblast cells. Anticancer
Res. 33:3791–3798. 2013.
|
|
15
|
van der Loo JC, Hanenberg H, Cooper RJ,
Luo FY, Lazaridis EN and Williams DA: Nonobese diabetic/severe
combined immunodeficiency (NOD/SCID) mouse as a model system to
study the engraftment and mobilization of human peripheral blood
stem cells. Blood. 92:2556–2570. 1998.
|
|
16
|
Kretz-Rommel A, Qin F, Dakappagari N,
Cofiell R, Faas SJ and Bowdish KS: Blockade of CD200 in the
presence or absence of antibody effector function: implications for
anti-CD200 therapy. J Immunol. 180:699–705. 2008.
|
|
17
|
Xu Y, Sun J, Sheard MA, Tran HC, Wan Z,
Liu WY, Asgharzadeh S, Sposto R, Wu HW and Seeger RC: Lenalidomide
overcomes suppression of human natural killer cell anti-tumor
functions by neuroblastoma microenvironment-associated IL-6 and
TGFβ1. Cancer Immunol Immunother. 62:1637–1648. 2013.
|
|
18
|
Perdomo C, Campbell JD, Gerrein J, Tellez
CS, Garrison CB, Walser TC, Drizik E, Si H, Gower AC, Vick J,
Anderlind C, Jackson GR, Mankus C, Schembri F, O’Hara C, Gomperts
BN, Dubinett SM, Hayden P, Belinsky SA, Lenburg ME and Spira A:
MicroRNA 4423 is a primate-specific regulator of airway epithelial
cell differentiation and lung carcinogenesis. Proc Natl Acad Sci
USA. 110:18946–18951. 2013.
|
|
19
|
Maes E, Landuyt B, Mertens I and Schoofs
L: Interindividual variation in the proteome of human peripheral
blood mononuclear cells. PLoS One. 8:e619332013.
|
|
20
|
Dai YQ, Jin DZ, Zhu XZ and Lei DL:
Triptolide inhibits COX-2 expression via NF-kappa B pathway in
astrocytes. Neurosci Res. 55:154–160. 2006.
|
|
21
|
D’Antonio C, Passaro A, Gori B, Del
Signore E, Migliorino MR, Ricciardi S, Fulvi A and de Marinis F:
Bone and brain metastasis in lung cancer: recent advances in
therapeutic strategies. Ther Adv Med Oncol. 6:101–114. 2014.
|
|
22
|
Wang C, Xiao Q, Li YW, Zhao C, Jia N, Li
RL, Cao SS, Cui J, Wang L, Wu Y and Wen AD: Regulatory mechanisms
of annexin-induced chemotherapy resistance in cisplatin resistant
lung adenocarcinoma. Asian Pac J Cancer Prev. 15:3191–3194.
2014.
|
|
23
|
Greiner DL, Shultz LD, Yates J, Appel MC,
Perdrizet G, Hesselton RM, Schweitzer I, Beamer WG, Shultz KL,
Pelsue SC, et al: Improved engraftment of human spleen cells in
NOD/LtSz-scid/scid mice as compared with C.B-17-scid/scid mice. Am
J Pathol. 146:888–902. 1995.
|
|
24
|
Hesselton RM, Greiner DL, Mordes JP, Rajan
TV, Sullivan JL and Shultz LD: High levels of human peripheral
blood mononuclear cell engraftment and enhanced susceptibility to
human immunodeficiency virus type 1 infection in NOD/LtSz-scid/scid
mice. J Infect Dis. 172:974–982. 1995.
|
|
25
|
Lee YS, Kim TS and Kim DK: T lymphocytes
derived from human cord blood provide effective antitumor
immunotherapy against a human tumor. BMC Cancer. 11:2252011.
|
|
26
|
Liu H, Zhang T, Ye J, Li H, Huang J, Li X,
Wu B, Huang X and Hou J: Tumor-infiltrating lymphocytes predict
response to chemotherapy in patients with advance non-small cell
lung cancer. Cancer Immunol Immunother. 61:1849–1856. 2012.
|
|
27
|
Morris M, Platell C and Iacopetta B:
Tumor-infiltrating lymphocytes and perforation in colon cancer
predict positive response to 5-fluorouracil chemotherapy. Clin
Cancer Res. 14:1413–1417. 2008.
|
|
28
|
Lee WS, Kang M, Baek JH, Lee JI and Ha SY:
Clinical impact of tumor-infiltrating lymphocytes for survival in
curatively resected stage IV colon cancer with isolated liver or
lung metastasis. Ann Surg Oncol. 20:697–702. 2013.
|
|
29
|
Batchelder CA, Duru N, Lee CI, Baker CA,
Swainson L, Mccune JM and Tarantal AF: Myeloid-lymphoid ontogeny in
the rhesus monkey (Macaca mulatta). Anat Rec (Hoboken). May
28–2014.(Epub ahead of print).
|
|
30
|
Fearon ER, Pardoll DM, Itaya T, Golumbek
P, Levitsky HI, Simons JW, Karasuyama H, Vogelstein B and Frost P:
Interleukin-2 production by tumor cells bypasses T helper function
in the generation of an antitumor response. Cell. 60:397–403.
1990.
|
|
31
|
Rosenberg SA: A new era for cancer
immunotherapy based on the genes that encode cancer antigens.
Immunity. 10:281–287. 1999.
|
|
32
|
Al-Shibli KI, Donnem T, Al-Saad S, Persson
M, Bremnes RM and Busund LT: Prognostic effect of epithelial and
stromal lymphocyte infiltration in non-small cell lung cancer. Clin
Cancer Res. 14:5220–5227. 2008.
|
|
33
|
Fridman WH, Pagès F, Sautès-Fridman C and
Galon J: The immune contexture in human tumours: impact on clinical
outcome. Nat Rev Cancer. 12:298–306. 2012.
|
|
34
|
Donnem T, Al-Shibli K, Andersen S, Al-Saad
S, Busund LT and Bremnes RM: Combination of low vascular
endothelial growth factor A (VEGF-A)/VEGF receptor 2 expression and
high lymphocyte infiltration is a strong and independent favorable
prognostic factor in patients with nonsmall cell lung cancer.
Cancer. 116:4318–4325. 2010.
|
|
35
|
Ganesan AP, Johansson M, Ruffell B,
Beltran A, Lau J, Jablons DM and Coussens LM: Tumor-infiltrating
regulatory T cells inhibit endogenous cytotoxic T cell responses to
lung adenocarcinoma. J Immunol. 191:2009–2017. 2013.
|