Introduction
Surgery for digestive tract disease predominantly
consists of reconstruction and anastomosis (1). The methods of anastomosis influence
the outcome of surgery, postoperative quality of life and
complications.
Traditional manual anastomosis is a complex surgery,
consisting of double-layer interrupted suture, which requires an
experienced surgeon, and has a higher incidence of postoperative
complication. The incidence of stricture and leakage in traditional
colorectal anastomosis is 4.5 and 1.8%, respectively (2). Furthermore, in certain types of
surgery, such as esophagogastrostomy and colorectostomy, the
surgery is more difficult due to special anatomic location and poor
exposure of the back wall of the anastomosis, increasing the risk
of complication accordingly.
In 1909, the first surgical stapler was developed
and primarily used for dividing and stapling bowel segments
(3). With the general application
of the stapling device, an increasing number of proximal gastric
resections via the abdomen have been performed for cardiac cancer.
The stapling device has contributed to reduced surgery times in
difficult locations (4) and
decreased the rates of edema (5),
infection (6), leakage and
stricture of anastomosis (7), as
well as morbidity of pulmonary complication (8). However, limitations of this method
exist, and improper manipulation may cause partial incisions and
tearing, which result in leakage and bleeding of the anastomosis
(9,10). In addition, irregular suture of the
mucosa may cause hyperplasia of granulation tissue and scar
formation, which further increase the risk of stricture (11,12).
In certain cases, with preoperative obstruction of the digestive
tract, mucosal edema, thickened muscle layer and dysfunctional
healing, the application of stapling device is constricted. The
stapling techniques have been criticized in view of their apparent
expense. Therefore, the identification of a novel manual method
with simple and convenient characteristics for reconstruction in
difficult locations is beneficial.
Single-layer continuous suture is a common method of
blood vessel surgery (13). The
advantage of this approach is its simplicity, and is particularly
suited for vessel anastomosis in deep tissues. Single-layer suture
has been confirmed to be tight and safe, similar to double-layer
suturing in gastrointestinal anastomosis (14,15),
and superior to conventional suturing in the colon (4). However, the efficacy of the method
combined with continuous suture in difficult anastomotic locations
remains unknown.
In the present study, prospective and controlled
experiments were performed, including single-layer continuous
suture of the posterior wall, and double-layer interrupted suture
of the anterior wall. The aim was to investigate the efficacy of
the novel manual method in difficult anastomotic locations, and the
method was found to be feasible and safe. This novel method may
simplify the approach in complex surgery as a result of the special
anatomic sites, and reduce expenditure.
Materials and methods
Animals
In total, 15 beagle dogs, including 10 females and
five males, with a median weight of 9 kg (range, 7–12 kg), were
included in the study. The dogs were fed with specialized dog food
and supplied with an appropriate amount water. The dogs were
divided into two groups: A manual group and a staple group. The
manual group underwent single-layer continuous suture, while the
staple group underwent suture with a stapler device. The study was
approved by the Xi’an Medical Experimental Animal Care Commission
(Xi’an, China).
Procedures
The dogs were anesthetized by an intravenous
injection of pentobarbital sodium (30 mg/kg; Sigma-Aldrich China,
Inc., Shanghai, China), and an intratracheal tube was inserted and
connected to a respirator during the surgery. A total of 800,000 IU
of penicillin and 250 mg of metronidazole (both North China
Pharmaceutical Group Corporation, Shanghai, China) were
administered intravenously at the initiation and end of the
anesthesia.
Esophagogastric anastomosis
All animals were fasted for one day prior to the
surgery. Following disinfection, a laparotomy was performed through
an upper midline abdominal incision. The distal esophagus and
gastric fundus were subsequently disassociated and removed. The
approach of the staple suture was similar to that performed on
human patients. Briefly, a purse-string suture was inserted and the
anvil of a circular staple was introduced into the distal
esophageal end. Next, the central shaft of the stapler
(Q/CYAE561-2001, Shanghai Medical Instruments (Group) Ltd.,
Shanghai, China) was inserted through the anterior wall of the
gastric fundus and the anvil (outer ring) was refitted onto the
shaft. An end-to-side esophagogastric anastomosis was created with
the button, the stapler was withdrawn and the residual end of the
gastric fundus was closed with sutures. For the manual suture, the
lesser curvature of the proximal stomach was closed with silk
stitch, and the greater curvature was prepared for end-to-end
anastomosis. The posterior wall of the anastomosis was then closed
by single-layer continuous suture using 4-0 Prolene threads
(Ethicon, Inc., Somerville, NJ, USA), and the priority wall was
conventionally sutured by double-layer interrupted suture using
silk thread (total layer suture combined with embedding of the
serosa and muscle tissue).
Colon-to-rectum anastomosis
Bowel preparation consisted of fluids by mouth for
three days prior to surgery. Laparotomy was performed through a
midline incision, and the distal colon and rectum were
disassociated. A circumferential dissection of the rectum was
undertaken to the level of 6 cm from the dentate line, and ~5 cm of
the rectum was resected. For the manual suture, the posterior wall
of the anastomosis was sutured by single-layer continuous suture
with 4-0 Prolene thread, and the priority wall was sutured by
double-layer interrupted suture with silk thread as usual. For the
staple suture, a purse-string suture was inserted and the anvil was
placed on the proximal end of the rectum, while the distal colon
end was used to introduce the staple. The central shaft of the
stapler was then inserted through the antimesenteric border of the
colon and the anvil was refitted. An end-to-side esophagogastric
anastomosis was created with the button, the stapler was withdrawn
and the distal colon end was closed with suture, while the
abdominal wound was sutured in layers.
Following surgery, the dogs received intravenous
injections of electrolyte solution and antibiotics for two days. On
the third postoperative day, the dogs were allowed to drink water
and a fluid meal.
Observations
Prior to and for 0.5 months following surgery, the
anal temperature of each dog was measured every two days, and prior
to and for three months following surgery, the feeding amount and
weights were measured once a week. The complications of surgery,
including wound infection, stricture and leakage of the
anastomosis, were observed according to symptoms and signs.
Blood samples
Prior to and one month following surgery, blood
samples were obtained from a rear-leg vein and drawn into
ice-chilled glass tubes containing EDTA-2Na (1.25 mg/ml blood).
Regular blood and liver function tests were then performed under
the appropriate circumstances.
Specimen collection
At three months following surgery, each dog was
anesthetized with pentobarbital sodium and laparotomized on the day
following a 24-h fast. Next, full-thickness tissue specimens were
obtained from the anastomotic port. The diameter of the anastomotic
port and thickness of the wall were measured. In total, four tissue
specimens were dissected from each anastomotic port symmetrically,
and then dipped into 10% formalin solution. The tissues were
embedded in paraffin, sectioned (3 μm thick) along the longitudinal
axis of the intestine and stained with hematoxylin and eosin
(H&E).
Morphological examinations of the
anastomotic port
All slices with H&E staining were evaluated for
the severity of inflammation, stricture and fibrosis by two
independent pathologists under a light microscope (x100
magnification; Q550cw, Leica, Mannheim, Germany) of 10 fields. The
standard scores were determined as follows: i) inflammation: 1,
small amount of lymphocytes or granulocytes observed in <4
fields; 2, medium amount of lymphocytes or granulocytes observed in
4–7 fields; and 3, large amount of lymphocytes or granulocytes
observed in >7 fields; ii) fibrosis: 1, small amount of
fibrocytes; 2, medium amount of fibrocytes; and 3, large amount of
fibrocytes; iv) irregular structure: 1, slight; 2, moderate; and 3,
severe.
Statistical methods
Data were analyzed using SPSS 11.5 software (SPSS
Inc., Chicago, IL, USA). Student’s t-test was used to analyze the
differences between weight, temperature, feeding amount, surgery
time and amount of bleeding. Fisher’s exact test was used for the
comparison between the incidence rate and complications. All
statistical tests were two-sided and P<0.05 was considered to
indicate a statistically significant difference.
Results
Total status of animal
A total of 15 dogs underwent surgery successfully.
In the manual group, eight dogs underwent single-layer continuous
suture of the posterior wall in esophagogastric anastomosis or
colon rectum anastomosis, while in the staple group, seven dogs
underwent staple anastomosis as controls. Following three months of
observation, intra- and postoperative complications were
identified, including bleeding, shock, leakage or stricture of
anastomosis and infection (Table
I).
 | Table ISurgical methods and survival of total
animals. |
Table I
Surgical methods and survival of total
animals.
| n | Group | Anastomosis | Complication
(yes/no) | Postoperative
complication |
|---|
| 1 | Staple | EG | Yes | Infection and
jaundice |
| 2 | Staple | EG | Yes | Bleeding and
shock |
| 3 | Staple | EG | Yes | Stricture of
anastomosis |
| 4 | Staple | EG | Yes | Leakage and
infection |
| 5 | Staple | CR | No | |
| 6 | Staple | CR | No | |
| 7 | Staple | CR | No | |
| 8 | Manual | CR | No | |
| 9 | Manual | CR | No | |
| 10 | Manual | CR | Yes | Infection |
| 11 | Manual | EG | Yes | Bleeding and
shock |
| 12 | Manual | EG | Yes | Infection |
| 13 | Manual | EG | No | |
| 14 | Manual | EG | No | |
| 15 | Manual | EG | Yes | Infection |
Weight, temperature and diet
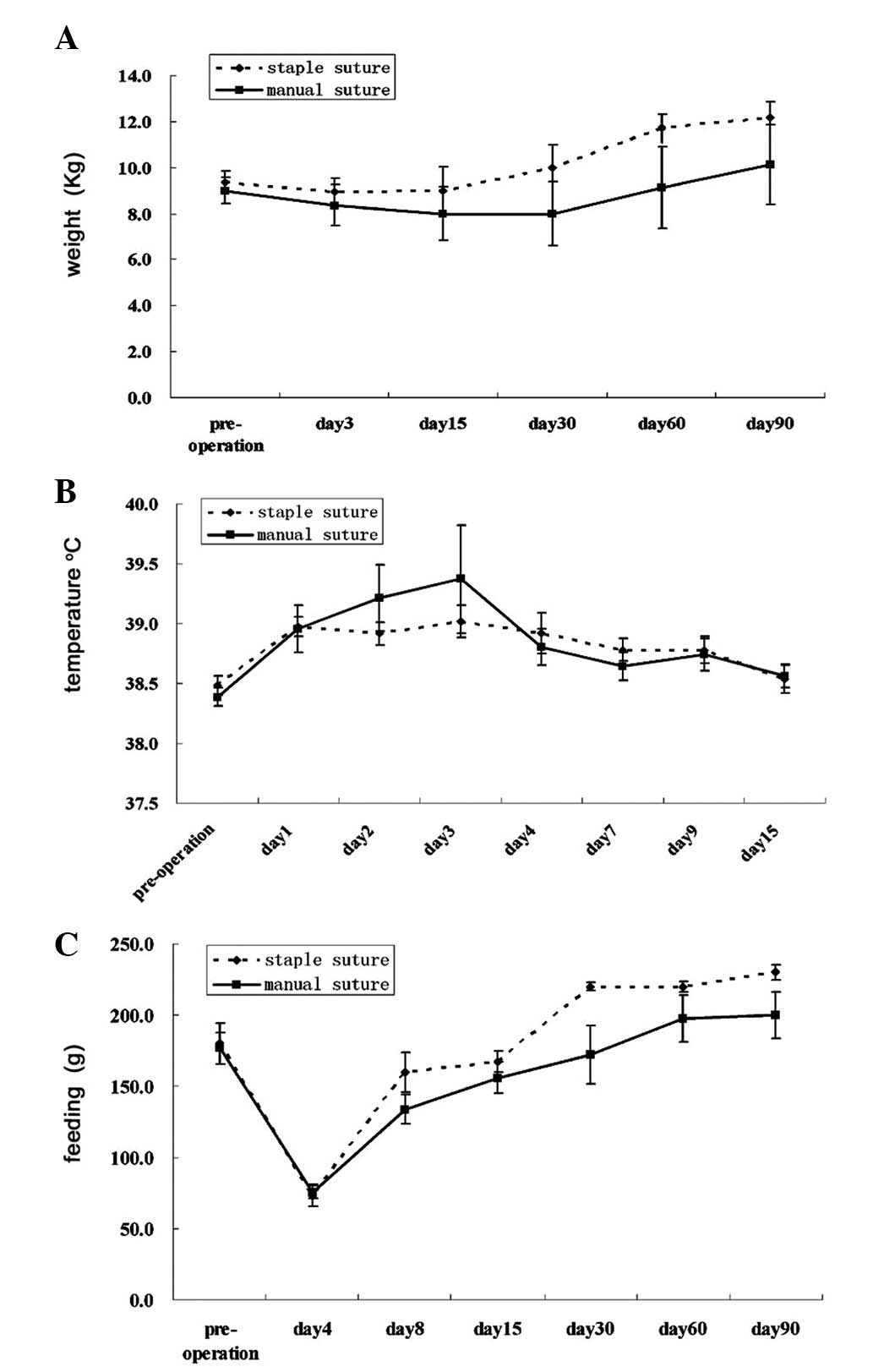
Prior to surgery, the mean weight of animals in the
manual group was 9.0±0.6 kg, while this was 9.4±0.5 kg (P=0.658) in
the staple group. At three months following surgery, the mean
weight of the animals in the manual and suture groups was 10.1±1.7
and 12.2±0.7 kg, respectively (P=0.367; Table II). Similarly, no significant
difference was identified between the mean weights of the two
groups at other observation time points (Fig. 1A). Prior to surgery, the mean
temperature of the animals in the manual group was 38.4±0.1°C,
while this was 38.5±0.1°C (P=0.392) in the staple group. At three
days following surgery, the mean temperature of the manual and
suture groups was 38.8±0.2 and 38.9±0.2, respectively (P=0.628). No
significant difference was identified between the mean temperature
in the two groups at other observation time points (Fig. 1B). Prior to surgery, the mean
feeding amount of animals in the manual group was 176.7±10.9 g, and
180±14.1 g in the staple group (P=0.852). At one month following
surgery, the mean feeding amount of the animals in the two groups
was 172.0±20.3 versus 220±3.0 g (P=0.127). No significant
difference was identified between the feeding amount of the two
groups at other observation time points. (Fig. 1C).
 | Table IIPreoperative, operative and
postoperative observations. |
Table II
Preoperative, operative and
postoperative observations.
| Variables | Manual suture (mean ±
SD) | Staple suture (mean ±
SD) | P-value |
|---|
| n | 8 | 7 | |
| Weight, g |
| Preoperative | 9.0±0.6 | 9.4±0.5 | 0.658 |
| Postoperative (3
months) | 10.1±1.7 | 12.2±0.7 | 0.367 |
| Temperature |
| Preoperative | 38.4±0.1 | 38.5±0.1 | 0.392 |
| Postoperative (third
day) | 38.8±0.2 | 38.9±0.2 | 0.628 |
| Feeding, g |
| Preoperative | 176.7±10.9 | 180±14.1 | 0.852 |
| Postoperative (1
month) | 172.0±20.3 | 220±3.0 | 0.127 |
| Bleeding, ml | 132.2±23.9 | 151.4±36.7 | 0.655 |
| Surgery time, h | 2.0±0.1 | 1.9±0.2 | 0.915 |
| Expenditure
(RMB) | 1726.7±33.5 | 2135.7±43.1 | 0.001 |
Surgery time, amount of bleeding and
expenditure
The mean surgical time in the manual group was
2.0±0.1 h, while this was 1.9±0.2 h in the staple group (P=0.915).
The mean volume of blood lost during surgery was 132.2±23.9 ml in
the manual group and 151.4±36.7 ml in the staple group (P=0.655)
(Table II). The surgical
expenditure included the apparatus, stapler, thread and drugs. The
total surgical expenditure in the manual group was significantly
lower than that of the control group; 1726.7±33.5 versus
2135.7±43.1 renminbi (P=0.001) (Table
II).
Blood test
Blood tests were performed prior to and one month
following surgery. In the manual and staple groups, no significant
difference was identified between the routine blood and liver
function tests (Table III).
 | Table IIIComparison between laboratory tests in
the staple and manual groups. |
Table III
Comparison between laboratory tests in
the staple and manual groups.
| Preoperative (mean ±
SD) | Postoperative (mean ±
SD) |
|---|
|
|
|
|---|
| Variables | Staple | Manual | P-value | Staple | Manual | P-value |
|---|
| Blood routine
examination |
| WBC | 6.99±2.40 | 6.44±2.99 | 0.70 | 7.07±2.11 | 7.44±4.09 | 0.89 |
| HCT | 40.77±7.80 | 39.59±7.44 | 0.76 | 38.07±7.70 | 37.68±9.55 | 0.95 |
| RBC | 5.94±1.10 | 5.69±1.01 | 0.64 | 6.29±1.37 | 6.59±0.95 | 0.72 |
| Hb | 143.00±26.94 | 136.67±24.49 | 0.63 | 133.67±23.86 | 148.40±21.31 | 0.40 |
| PLT | 160.00±139.05 | 245.11±130.54 | 0.23 | 226.00±87.07 | 446.0±249.51 | 0.20 |
| Liver function |
| T.BIL | 2.42±0.50 | 2.09±0.74 | 0.40 | 2.33±0.59 | 2.14±0.74 | 0.71 |
| ALT | 48.40±15.60 | 40.13±7.22 | 0.22 | 45.67±9.87 | 52.00±12.63 | 0.49 |
| AST | 56.60±14.66 | 52.25±20.94 | 0.69 | 59.33±26.08 | 56.60±15.66 | 0.86 |
| TBA | 60.68±4.62 | 59.65±7.40 | 0.79 | 65.47±3.18 | 60.92±8.86 | 0.44 |
| ALB | 29.76±2.82 | 28.88±3.36 | 0.64 | 22.10±5.63 | 23.26±2.72 | 0.70 |
| GLB | 30.92±3.73 | 30.78±5.36 | 0.96 | 43.37±7.14 | 37.66±6.82 | 0.30 |
| A/G | 0.98±0.15 | 0.95±0.14 | 0.72 | 0.53±0.21 | 0.64±0.09 | 0.34 |
Complications of anastomosis
The incidence rate of the total complications in the
manual group was 50%, and 57.1% in the staple group (P=0.782). The
complications involving the anastomosis port were 0 and 28.6%, with
no significant difference (P=0.200) (Fig. 2A and B and Table IV).
 | Table IVComplication of the two groups. |
Table IV
Complication of the two groups.
| Variables | Manual, n (%) | Staple, n (%) | P-value |
|---|
| n | 8 (100.0) | 7 (100.0) | |
| Bleeding shock | 1 (12.5) | 1 (14.3) | 0.919 |
| Leakage | 0 (0.0) | 1 (14.3) | 0.467 |
| Stricture | 0 (0.0) | 1 (14.3) | 0.467 |
| Infection of
abdominal | 2 (25.0) | 2 (28.6) | 0.662 |
| Infection of
wound | 1 (12.5) | 1 (14.3) | 0.919 |
Morphological changes in the anastomotic
port
The diameter of the anastomotic port in the manual
group was 3.04±0.07 cm, while this was 2.24±0.25 in the staple
group (P=0.004). The thickness of the anastomotic port in the
posterior wall of the manual group was 2.94±0.06 cm, which was
thinner than that of the staple group (5.07±0.85 cm) (P=0.002), and
also thinner than in its anterior wall (4.22±0.16 cm) (P=0.036).
However, no significant difference was identified between the
staple group and the anterior wall of the manual group (P=0.179;
Figs. 2CD and 3A–C). Anastomotic stricture was also
identified in the staple group, with a diameter of <1 cm, while
the thickness of the wall was 1 cm (Fig. 3A).
Pathological formation of the anastomotic
port
Compared with the staple group, inflammation of the
anastomotic port was marginal in the manual group, with scores of
2.10±0.97 versus 1.58±0.83 (P=0.063), and the stricture was neater
than that in the controls (2.40±0.75 vs. 1.79±0.88; P=0.020). The
total score of the posterior wall was lower than that of the
anterior walls, which were sutured by double-layer interrupted
suture with silk thread (9.50±2.51 vs. 11.00±2.00; P=0.030)
(Fig. 4A–F and Table V).
 | Table VPathological formation of the
anastomotic port between the two groups. |
Table V
Pathological formation of the
anastomotic port between the two groups.
| Group | Score (mean ±
SD) | P-value |
|---|
| Fibrosis |
| Manual | 1.92±0.78 | 0.354 |
| Staple | 2.15±0.88 | |
| Inflammation |
| Manual | 1.58±0.83 | 0.063 |
| Staple | 2.10±0.97 | |
| Stricture |
| Manual | 1.79±0.88 | 0.020 |
| Staple | 2.40±0.75 | |
| Total of slice |
| Manual | 5.29±0.70 | 0.018 |
| Staple | 6.65±1.95 | |
| Total of
samples |
| Manual | 21.17±4.75 | 0.171 |
| Staple | 26.60±7.33 | |
|
Anterior-posterior |
| Anterior wall of
manual | 11.00±2.00 | 0.030 |
| Posterior | 9.50±2.51 | |
Discussion
In this study, single-layer suture was combined with
continuous suture in back wall anastomosis at challenging surgical
sites. The study showed that this combined suture is technically
possible to perform, and that, under experimental conditions, the
novel anastomosis appears to be as safe as stapled sutures. The
postoperative complications were reduced or the same as those in
stapled sutures; however, the expenditure was evidently
reduced.
The predominant complications of anastomosis are
leakage, stricture and infection. To date, the superiority of
staple suture has remained controversial. It has been reported that
in previous hand-sewn and staple groups, the incidence of leakage
following esophagogastric anastomosis is 0–21.9 and 0–25.8%,
respectively, and 0–19.5% and 0–32.8%, respectively, for stricture
(16–24). In rectal cancer, leakage following
low anterior resection with the double stapling technique is
2.6–17% (25–28), and no significant difference has
been identified between the morbidity or mortality rates in
hand-sewn and stapled techniques (14,29).
The results of the current study demonstrated that the incidence
rate of total complications was 50%, and anastomosis complication
was 13.3%. The results were higher than those identified in the
previously described studies, but lower than a study which reported
postoperative minor complications in 70.9% of patients and serious
complications in 22.6% of patients following esophagogastric
anastomosis (30). The reasons of
complication in the present study may have also involved the
following: i) Preoperative preparation, due to the challenges of
bowel preparation and implantation of the gastric pipe; and ii)
insufficient nutrition supply for animals following surgery.
However, in the same conditions, the manual group demonstrated a
lower rate of complications and higher safety than the staple
group, providing evidence for its advantage.
The predominant challenges of anastomosis are due to
exposure, particularly for anastomosis of the back wall in
difficult locations. The anastomosis becomes invisible due to
blockade by surrounding tissues, and even in the course of staple
suture, the proceeding is not visible. However, continuous suture
does not require a butt joint, which increases exposure to the back
wall and contributes to convenience and safety.
Mechanical integrity and tissue viability are
emphasized in gastrointestinal anastomosis. It has been shown in an
experimental and comparative study, that single-layer anastomosis
is as strong as two-layer suturing in the small intestine and
colon, and may guarantee mechanical integrity (19). Tissue viability has also been found
to closely correlate with a healthy blood supply and good
nutritional status of suture line (7,10). The
blood flow is always reduced in the suture line compared with the
normal mucosa, and of the three anastomotic methods (stapled
suture, two-layered manual and single-layered manual suture), the
blood flow of suture line increases in turn (9,10) and,
therefore, the mechanism requires further investigation.
In the present study, no significant difference was
identified between surgical time in the manual and staple suture
groups. Continuous suture contributes to reduced surgical time, and
does not increase complication in episiotomy (31). Staple anastomosis reduces surgical
time by ≤30 min due to cutting and suturing tissues only once
(32); however, to acquire a safe
anastomosis, a sharp blade and a sufficient amount of tissue
cutting is required, which may lead to surgical issues and prolong
surgery time.
The complication of stricture has been found to
closely correlate with the diameter of the anastomosis and the
thickness of the wall. Few studies regarding anastomotic ports have
included morphological observations. In the current study, a
smaller diameter and increased thickness of the anastomotic port
were identified in the staple group, with the thickness of the
anastomotic wall in one sample reaching 1 cm, and a narrow diameter
of <0.8 cm. Staple suture is a double-layer suture and, in
certain cases, an additional strengthening suture is required to
reinforce the suture with a third layer; however, this may cause
too much tissue to turn inwards and induce stricture formation
(10). Staplers with several
external diameters allow the resection of various diameters and the
dissection of various surface areas (33). However, selecting a stapler is
almost impossible due to fixed types. By contrast, single-layer
suture causes little inward tissue movement, which may reduce the
anastomotic stricture, and continuous suture has been confirmed to
contribute to the adjustment of the anastomotic diameter (34).
Fibrosis is an important factor for anastomosis
stricture, and, in this study, a decreasing number of inflammation
and fibroblast cells were identified by microscope, as well as a
slightly irregular structure in the novel manual suture group
compared with the staple suture and conventional manual suture
groups. These results provided evidence that manual sutures may
efficiently reduce anastomotic complication, and support the view
that staple sutures increase the rate of postoperative anastomotic
stricture (21). The results are
consistent with a meta-analysis of randomized and controlled
trials, comparing hand-sewn with stapled esophagogastric
anastomosis in other studies (7).
In conclusion, the results of the current study
suggest that single-layer continuous suture in anastomosis of the
posterior wall of the digestive tract is a novel method with
feasibility and safety. This novel method simplifies the surgical
approach and can be easily applied clinically, in particular, it
can be used in challenging anastomosis of special anatomic sites.
In addition, this method reduces expenditure and deserves
generalization in the future.
Acknowledgements
The authors would like to thank for Dr Ma Feng, Dr
Wang Haohua and Dr Yang Hui from the Dream Factory Surgical
Experiment Laboratory of Xi’an Jiaotong University for aiding in
the animal experiments, and Professor Liu Ningna and Hu Yuwen from
the Pathology Department of Xi’an Gaoxin Hospital for assistance
with the pathological experiment.
References
|
1
|
Briel JW, Tamhankar AP, Hagen JA, et al:
Prevalence and risk factors for ischemia, leak, and stricture of
esophageal anastomosis: gastric pull-up versus colon interposition.
J Am Coll Surg. 198:536–542. 2004.
|
|
2
|
Kirat HT, Kiran RP, Lian L, Remzi FH and
Fazio VW: Influence of stapler size used at ileal pouch-anal
anastomosis on anastomotic leak, stricture, long-term functional
outcomes, and quality of life. Am J Surg. 200:68–72. 2010.
|
|
3
|
Polk HC, Cheadle WG and Frankllin GA:
Principles of operative surgery. Sabiston Textbook of Surgery: The
Biological Basis of Modern Surgical Practice. Towsend CM: 16th
edition. WB Saunders Company; St. Louis, MO: pp. 163–170. 2001
|
|
4
|
Graffner H, Andersson L, Löwenhielm P and
Walther B: The healing process of anastomoses of the colon. A
comparative study using single, double-layer or stapled
anastomosis. Dis Colon Rectum. 27:767–771. 1984.
|
|
5
|
Orringer MB, Marshall B and Iannettoni MD:
Eliminating the cervical esophagogastric anastomotic leak with a
side-to-side stapled anastomosis. J Thorac Cardiovasc Surg.
119:277–288. 2000.
|
|
6
|
Docherty JG, McGregor JR, Akyol AM, Murray
GD and Galloway DJ: Comparison of manually constructed and stapled
anastomoses in colorectal surgery. West of Scotland and Highland
Anastomosis Study Group. Ann Surg. 221:176–184. 1995.
|
|
7
|
Markar SR, Karthikesalingam A, Vyas S,
Hashemi M and Winslet M: Hand-sewn versus stapled oesophago-gastric
anastomosis: systematic review and meta-analysis. J Gastrointest
Surg. 15:876–884. 2011.
|
|
8
|
Ziv Y, Fazio VW, Church JM, et al: Stapled
ileal pouch anal anastomoses are safer than handsewn anastomoses in
patients with ulcerative colitis. Am J Surg. 171:320–323. 1996.
|
|
9
|
Orsay CP, Bass EM, Firfer B, Ramakrishnan
V and Abcarian H: Blood flow in colon anastomotic stricture
formation. Dis Colon Rectum. 38:202–206. 1995.
|
|
10
|
Chung RS: Blood flow in colonic
anastomoses. Effect of stapling and suturing. Ann Surg.
206:335–339. 1987.
|
|
11
|
Wong J, Cheung H, Lui R, et al:
Esophagogastric anastomosis performed with a stapler: the
occurrence of leakage and stricture. Surgery. 101:408–415.
1987.
|
|
12
|
Berrisford RG, Page RD and Donnelly RJ:
Stapler design and strictures at the esophagogastric anastomosis. J
Thorac Cardiovasc Surg. 111:142–146. 1996.
|
|
13
|
Ozkan O and Ozgentaş HE: Open guide suture
technique for safe microvascular anastomosis. Ann Plast Surg.
55:289–291. 2005.
|
|
14
|
Valdivieso A, Sarabia S, Pocino R, et al:
Is it worth using mechanical sutures in gastric surgery? Acta Chir
Belg. 95(Suppl 4): S179–S181. 1995.
|
|
15
|
Fok M, Ah-Chong AK, Cheng SW and Wong J:
Comparison of a single layer continuous hand-sewn method and
circular stapling in 580 oesophageal anastomoses. Br J Surg.
78:342–345. 1991.
|
|
16
|
Law S, Fok M, Chu KM and Wong J:
Comparison of hand-sewn and stapled esophagogastric anastomosis
after esophageal resection for cancer: a prospective randomized
controlled trial. Ann Surg. 226:169–173. 1997.
|
|
17
|
Hsu HH, Chen Js, Huang PM, Lee JM and Lee
YC: Comparison of manual and mechanical cervical esophagogastric
anastomosis after esophageal resection for squamous cell carcinoma:
a prospective randomized controlled trial. Eur J Cardiothorac Surg.
25:1097–1101. 2004.
|
|
18
|
Laterza E, de’Manzoni G, Veraldi GF, et
al: Manual compared with mechanical cervical oesophagogastric
anastomosis: a randomised trial. Eur J Surg. 165:1051–1054.
1999.
|
|
19
|
Valverde A, Hay JM, Fingerhut A and
Elhadad A: Manual versus mechanical esophagogastric anastomosis
after resection for carcinoma: a controlled trial. French
Associations for Surgical Research. Surgery. 120:476–483. 1996.
|
|
20
|
Walther B, Johansson J, Johnsson F, Von
Holstein CS and Zilling T: Cervical or thoracic anastomosis after
esophageal resection and gastric tube reconstruction: a prospective
randomized trial comparing sutured neck anastomosis with stapled
intrathoracic anastomosis. Ann Surg. 238:803–814. 2003.
|
|
21
|
Luechakiettisak P and Kasetsunthom S:
Comparison of hand-sewn and stapled in esophagogastric anastomosis
after esophageal cancer resection: a prospective randomized study.
J Med Assoc Thai. 91:681–685. 2008.
|
|
22
|
Okuyama M, Motoyama S, Suzuki H, et al:
Hand-sewn cervical anastomosis versus stapled intrathoracic
anastomosis after esophagectomy for middle or lower thoracic
esophageal cancer: a prospective randomized controlled study. Surg
Today. 37:947–952. 2007.
|
|
23
|
Craig SR, Walker WS, Cameron EW and
Wightman AJ: A prospective randomized study comparing stapled with
handsewn oesophagogastric anastomoses. J R Coll Surg Edinb.
41:17–19. 1996.
|
|
24
|
George WD: Suturing or stapling in
gastrointestinal surgery: a prospective randomize study. West of
Scotland and Highland Anastomosis Study Group. Br J Surg.
78:337–341. 1991.
|
|
25
|
Kuroyanagi H, Oya M, Ueno M, et al:
Standardized technique of laparoscopic intracorporeal rectal
transaction and anastomosis for low anterior resection. Surg
Endosc. 22:557–561. 2008.
|
|
26
|
Bärlehner E, Benhidjeb T, Anders S and
Schicke B: Laparoscopic resction for rectal cancer Outcomes in 194
patients and review of the literature. Surg Endosc. 19:757–766.
2005.
|
|
27
|
Scheidbach H, Schneider C, Konradt J, et
al: Laparoscopic abdominoperineal resection and anterior resection
with curative intent for carcinoma of the rectum. Surg Endosc.
16:7–13. 2002.
|
|
28
|
Karanjia ND, Corder AP, Holdsworth PJ and
Heald RJ: Risk of peritonitis and fatal septicaemia and the need to
defunction the low anastomosis. Br J Surg. 78:196–198. 1991.
|
|
29
|
Dziki AJ, Duncan MD, Harmon JW, et al:
Advantages of handsewn over stapled bowel anastomosis. Dis Colon
Rectum. 34:442–448. 1991.
|
|
30
|
Henriques AC, Godinho CA, Saad R Jr, et
al: Esophagogastric anastomosis with invagination into stomach: New
technique to reduce fistula formation. World J Gastroenterol.
16:5722–5726. 2010.
|
|
31
|
Valenzuela P, Saiz Puente MS, Valero JL,
et al: Continuous versus interrupted sutures for repair of
episiotomy or second-degree perineal tears: a randomised controlled
trial. BJOG. 116:436–441. 2009.
|
|
32
|
Shoji Y, Nihei Z, Hirayama R and Mishima
Y: Experiences with the linear cutter technique for performing
Roux-en-Y anastomosis following total gastrectomy. Surg Today.
25:27–31. 1995.
|
|
33
|
Hirahara N, Monma H, Shimojo Y, et al:
Reconstruction of the esophagojejunostomy by double stapling method
using EEA™ OrVil™ in laparoscopic total gastrectomy and proximal
gastrectomy. World J Surg Oncol. 9:552011.
|
|
34
|
Park KJ, Woo JS, Jeong SS and Yi JH:
Continuous ‘over and over’ suture for tricuspid ring annuloplasty.
Korean J Thorac Cardiovasc Surg. 45:19–23. 2012.
|