Introduction
Gastrointestinal stromal tumors (GISTs) are unusual
mesenchymal tumors that are commonly found in the stomach (60–70%),
and are also found in the small intestine (20–25%); only 5% of all
GISTs originate in the rectum. Complete surgical resection is the
main therapy for patients with resectable GISTs (1). Patients with rectal GISTs usually
undergo extensive procedures, such as abdominoperineal resections
(APRs) or low anterior resections (LARs), which may have little
benefit in a number of cases, particularly when considering the
fact that there is no evidence that extensive surgery prolongs
survival or delays recurrence (2,3).
Local excision of anorectal tumors includes the use
of transrectal, transsacral and transvaginal approaches (4–6).
However, less invasive approaches for the local resection of rectal
GISTs are often inadequate due to the size of the mass and its
exophytic growth.
In total, 80–95% of GISTs typically express cluster
of differentiation (CD)117, a tyrosine kinase growth factor
receptor (c-KIT), which can be detected immunohistochemically in
order to discriminate GISTs from other mesenchymal gastrointestinal
neoplasms (7,8). c-KIT also serves as the target for
drug therapy with imatinib mesylate (IM; Glivec®), a
c-KIT and platelet-derived growth factor receptor (PDGFR)-α
inhibitor. IM is now the standard treatment for patients with
locally unresectable or metastatic GISTs, and is approved for use
in the adjuvant therapy of resectable GISTs. A previous study
concluded that pre-operative IM for rectal GISTs is associated with
improved surgical margins, and disease-free and overall survival
(9).
There are a few studies that have focused on IM
adjuvant therapy; these studies have found that following local
resection for rectal GISTs, IM is better than, or at least not
inferior to, radical surgical LAR or APR (9–11). The
present study reports two cases of rectal GISTs that were treated
by IM adjuvant therapy and subsequent transsacral local resection.
The study was approved by the Ethics Committee of The Second
Affiliated Hospital, Zhejiang University School of Medicine
(Hangzhou, China), and written informed patient consent was
obtained.
Case report
Case 1
A 38-year-old male was referred to The Second
Affiliated Hospital with the chief complaint of a change in stool
shape that had been apparent for two months. The patient’s past
medical history was unremarkable. A clinical examination did not
detect any palpable abdominal masses. A digital examination of the
rectum revealed a mass of ~5 cm in diameter on the anterior rectal
wall, ~5 cm above the anal verge. The mass was hard, elastic and
immobile, with a smooth, high tension surface. Routine laboratory
tests of the serum and urine showed no abnormalities, while the
analysis of tumor markers also returned normal results.
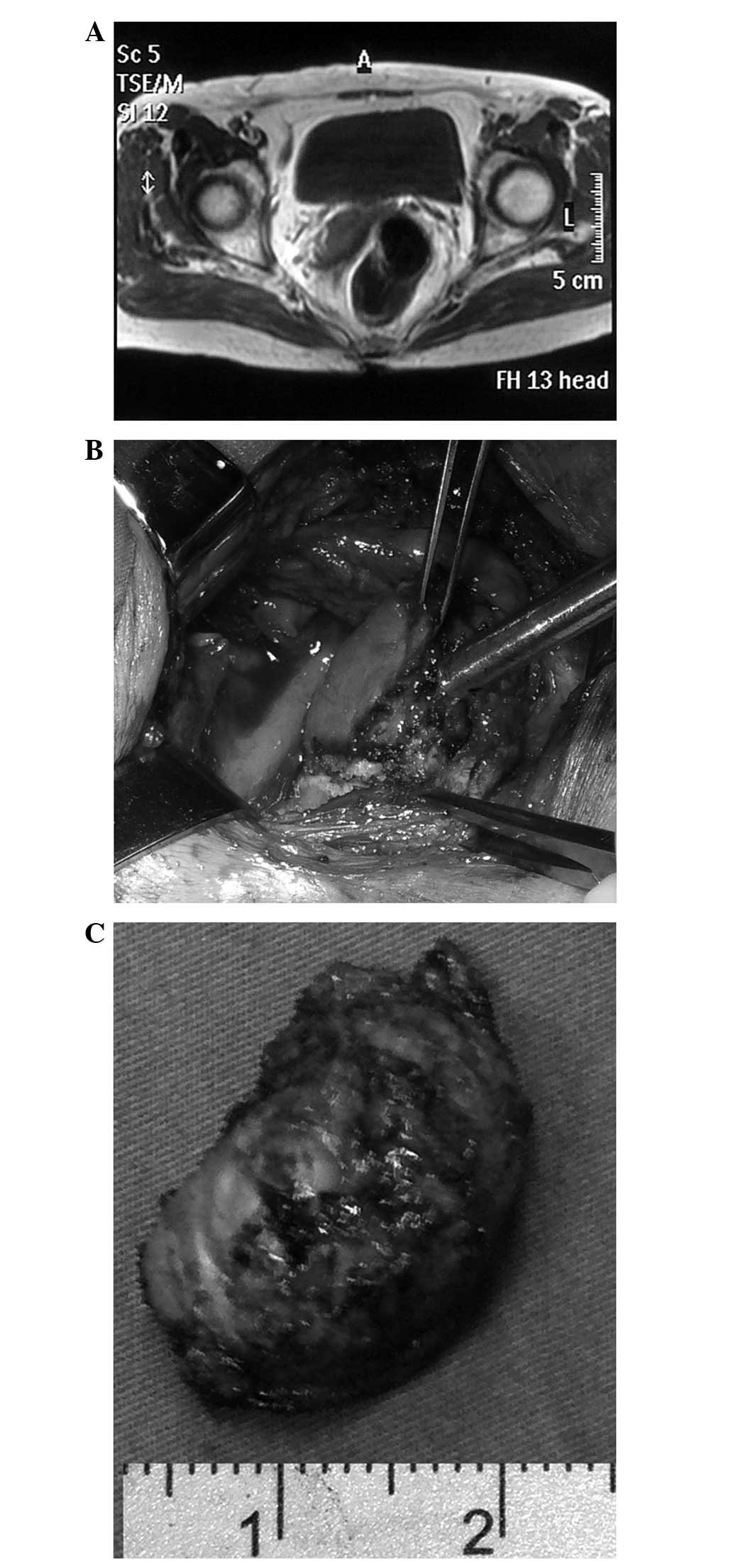
Magnetic resonance imaging (MRI) revealed a solitary
tumor measuring 4.9×3.6 cm, with a clear boundary. The tumor
exhibited extramural growth on the right anterior wall of the lower
rectum, with compression displacement of the prostatic gland, but
there was no evidence of either pelvic lymphadenopathy or distant
metastasis (Fig. 1A). Transrectal
ultrasound-guided biopsy samples showed the presence of a spindle
cell tumor and strong immunohistochemical positivity for CD117
(Fig. 1C), CD34 and discovered on
GIST-1. However, the samples were negative for α-smooth muscle
actin (SMA) and desmin. From the results of these examinations, a
rectal GIST was diagnosed.
Due to the size and localization of the lesion, IM
neoadjuvant therapy was recommended. Therefore, the patient
received a single daily dose of 400 mg Glivec for 7 months and was
followed up within 3 months by CT or MRI scans to assess the
effects. During IM therapy, the tumor continued to shrink (from
4.9×3.6 to 3.3×2.3 cm) (Fig. 1B),
with the only side-effect being mild fatigue, and no evidence of
progression. Subsequent to 7 months of IM therapy, CT and MRI scans
showed no further significant change in tumor size. Therefore, the
patient underwent a transsacral local resection (Fig. 1E).
The tumor, which measured 3.5×3 cm, was solid with a
clear boundary on the cut sections (Fig. 1F). Histopathological examination
revealed that in local areas, the tumor existed with hyaline
degeneration of the tumor cells, with <3 mitoses per 50
high-power fields (HPFs). The resection margins were uninvolved on
all sides, and there was no lymph node metastasis. Following the
surgery, the patient suffered the complication of rectal leakage,
which was successfully managed by drainage. To date, no recurrence
has been observed in the 24-month follow-up period, subsequent to 5
months of additional post-operative IM treatment.
Case 2
A 76-year-old female presented with a 3-month
history of a change in stool shape. A pelvic MRI scan showed a
solid tumor measuring 4.5×4.0 cm, with clear boundary. The tumor
exhibited extramural growth on the right anterior wall of the lower
rectum, with compression of the wall of the vagina (Fig. 2A).
A transrectal ultrasound-guided biopsy showed a
spindle cell stromal tumor with >5 mitoses per 50 HPFs, and
immunohistochemical positivity for CD117 and CD34. The neoplastic
cells were negative for α-SMA and desmin. The pathologic findings
were consistent with a high-risk GIST
The patient began therapy with 400 mg IM once daily,
and was followed-up within 3 months with transrectal ultrasound and
MRI scans. Subsequent to IM therapy for 3 months, the tumor had
shrunk to 3.0×2.0 cm in size and there were no side-effects or
evidence of progression. The patient requested surgery to remove
the tumor, and therefore underwent a transsacral local resection
(Fig. 2B).
The tumor, which measured 2.3×1.9 cm, was soft and
had a light-yellow parenchyma, with focal cysts on the cut sections
(Fig. 2C). Histopathological
examination revealed that there was local necrosis of the tumor
cells. The tumor cells were strongly positive for CD117 and CD34,
and negative for SMA and S-100 protein. There were <3 mitoses
per 50 HPFs. To date, the post-operative course has been
satisfactory, and there has been no recurrence for 28-months
without IM treatment.
Discussion
GISTs are the most common mesenchymal tumor of the
gastrointestinal tract, and are likely to arise from the precursor
interstitial cells of Cajal. GISTs are common in the stomach
(60–70% of cases) and small intestine (30%), and occur rarely in
the rectum (5%), esophagus, colon, pancreas, appendix, omentum,
mesentery and retroperitoneum (1,2).
The symptoms of a rectal GIST do not generally
differ from those of other rectal tumors. Occasionally, no symptoms
are present. As for diagnosis of rectal GIST, digital examination
of the rectum, transanal ultrasound and colonoscopy are essential
and part of the same workup that is used for other rectal masses.
Pre-operative biopsies are a vital part of the diagnosis of a GIST,
as they provide immunohistochemical data, such as the positivity
for CD117 and CD34, and the mitotic count. In total, ~95% of GISTs
express CD117, and ~70% are CD34-positive (12). Furthermore, MRI or CT scans are
required to determine the extent of local invasion and to detect
the possible metastases. The size, site and mitotic index of the
GISTs are the most used prognostic factors and aid risk
stratification of recurrence. The National Institute of Health has
defined those lesions with a diameter of >10 cm, a mitotic rate
of >10/50 HPF or a diameter of 5 cm, and a mitotic rate of
>5/50 HPF as the tumors that are at a high-risk of metastasis
(13).
Surgery is the primary treatment of choice for
patients with localized or potentially resectable GISTs. Various
surgical procedures may be considered, including local excision,
LAR and APR with total mesorectal excision (TME). The only
potentially curative treatment for GISTs is complete surgical
resection with negative tumor margins (1,12).
Compared with rectal adenocarcinoma, rectal GISTs exhibit two
specific features that may significantly affect surgical
management. Firstly, metastases are extremely rare in the
locoregional lymph nodes, and secondly, GISTs typically show a
tendency to grow away from the intestinal lumen (2). So for the surgical management of a
large GIST arising in the lower rectum, radical surgery, including
LAR and APR with TME, may have little benefit (15). A previous study found that GISTs
>5 cm in diameter that were removed by APR, LAR or local
excision demonstrated no significant differences with regard to
survival. The study suggested that the natural history of these
GISTs partly cancels out the benefit of radical surgery (2).
The surgery most frequently proposed for the local
excision of anorectal tumors is a transrectal approach with
application of various dilators. This approach is most suitable for
tumors whose distal margin from the dentate line is ~3 cm (6,16).
Other possible approaches for local excision include the
transvaginal route, and the transcoccygeal or trans-sphincteric
approach. Transvaginal local excision for rectal carcinoma has also
been performed in patients with T1 and T2 rectal cancers. The
average distance from the dentate line that best fits this approach
is ~4 cm. The possible complication of a rectovaginal fistula
occurs at a low rate and is treated conservatively (16).
Transcoccygeal (transsacral) excision, is suitable
for higher lesions (average distance from the dentate line, 5 cm)
located in the posterior wall of the rectum (5,17,18).
This location requires a paracoccygeal incision between the anus
and coccyx, an S5 or coccygeal transection, and an incision of
Waldeyer’s fascia, with exposure of the perirectal fat. The tumor
may be excised through a wedge resection or even a segmental
resection with an end-to-end anastomosis. However, certain
post-operative complications have also been described, including
wound infections, urinary retention, fecal fistulae, fecal
incontinence and hemorrhage (5,19).
In addition, the trans-sphincteric approach is well
suited for exophytic GISTs located anteriorly and in the lower
third of the rectum. This approach requires the sphincter to be
divided; the exposure of the lower rectum is similar to a
transcoccygeal approach, but there is rising concern regarding
long-term continence problems (4,16).
However, less invasive approaches for local
resection of rectal GISTs are often inadequate due to the size of
the mass and its exophytic growth. The larger the tumor, the more
difficult it is to obtain tumor-free margins. An alternative
approach would be the use of pre-operative IM therapy for large
rectal GISTs, which may result in tumor shrinkage (20). In a previous study, neoadjuvant
therapy with IM was used prior to local excision via the Kraske
approach (17), which showed that
preoperative IM therapy resulted in the shrinkage of GISTs and
exhibited a clear benefit with regard to local excision.
In total, 80–95% of GISTs typically express CD117, a
c-kit proto-oncogene, which can be detected immunohistochemically
in order to discriminate GISTs from other mesenchymal
gastrointestinal neoplasms (7,8). c-kit
also serves as the target for drug therapy with IM, a c-kit and
PDGFR-α inhibitor. IM is now standard treatment for patients with
locally unresectable or metastatic GISTs, and is approved for use
in the adjuvant therapy of resectable GISTs.
The 10 European Organization for Research and
Treatment of Cancer Soft Tissue and Bone Sarcoma Group sarcoma
centers (19) and the Radiation
Therapy Oncology Group (RTOG) phase II study (RTOG0132) (22) evaluated and analyzed the safety and
efficacy of neoadjuvant IM for patients with locally advanced
primary GISTs. The results showed indicated excellent long-term
results in locally advanced GISTs treated with neoadjuvant IM in
routine practice and found that the complications of surgery and IM
toxicity were minimal. The approach is therefore feasible
The recommended duration of pre-operative IM therapy
in the adjuvant setting is not known. The median time to the best
response in all responding patients was ~4 months (107 days), and
the majority of responses occurred within 9 months of treatment
(23). Verweij et al
(23) recommend that studies on
neoadjuvant IM therapy should be designed with the duration of
treatment ranging between 4 and 6 months. In the present cases,
surgery was performed following 3 to 7 months of treatment, in
order for the tumor shrinkage to have stabilized.
Several case studies have demonstrated that the use
of pre-operative IM enables organ-sparing surgery and improves
surgical outcomes in patients with rectal GISTs (17,24,29).
There are a few studies that have shown that the use
of IM adjuvant therapy and subsequent local resection is better
than, or at least not inferior to, LAR or APR for anorectal GISTs
(9,10,11).
The present study reports the cases of two rectal GISTs that were
treated by IM adjuvant therapy and subsequent transsacral local
resections. There were no severe complications, except a slight
fistula, and no recurrence and metastasis occurred after >2
years of follow-up. Neoadjuvant IM therapy following the local
resection of anorectal GISTs in the literature is also summarized
in the present study, and the results are shown in Table I.
 | Table ISummary of the anorectal
gastrointestinal stromal tumors cases from the literature that
underwent neoadjuvant IM therapy following local resection. |
Table I
Summary of the anorectal
gastrointestinal stromal tumors cases from the literature that
underwent neoadjuvant IM therapy following local resection.
| First author, year
(ref.) | Cases, n | Local excision | Pre-operative IM,
n | Post-operative IM,
n | Risk of
recurrence | Outcome |
|---|
| Fujimoto et
al, 2013 (25) | 5 | Laprascopic ISR | 5 | 3 | High for 3 | ANED |
| Agaimy et al,
2013 (10) | 16 | 6 cases | 3 | 7 | High for 13 | Incomplete resection
associated with high local recurrence rates |
| Centonze et
al, 2013 (4) | 2 | 2 cases | 2 | 2 | High | ANED |
| Tielen et al,
2013 (11) | 32 | 8 cases | 22 | Yes | N/A | Pre-operative IM did
not lead to less extensive surgery |
| Jacob et al,
2012 (9) | 39 | 21 cases for local
excision | 16 | N/A | N/A | 5 recurrence, 5
metastasis cases |
| Lagos et al,
2012 (26) | 1 | Transanal | No | Yes | High | ANED |
| Wang et al,
2011 (17) | 3 | Transsacral | Yes | N/A | N/A | ANED |
| Hara et al,
2011 (27) | 1 | Transvaginal | No | No | High | ANED |
| Matsushima and Kayo,
2007 (18) | 2 | Transsacral | N/A | N/A | Medium | ANED |
| Gervaz et al,
2008 (28) | 1 | Transsacral | No | No | High | N/A |
| Shelly et al,
2005 (29) | 1 | Transanal | Yes | N/A | High | ANED |
| Miettinen et
al, 2001 (2) | 144 | 24 cases | No | No | N/A | No difference in
survival between radical and local resection |
| Present study | 2 | Transsacral | 2 | 2 | High | ANED |
The literature review found that radical surgery did
not always generate a better outcome than local excision for
anorectal GISTs. In a study of 144 cases of anorectal GISTs,
Miettinen et al (2) found
that there was no significant difference in the survival rates
between patients who underwent radical surgery and local excision.
Radical surgery, including LAR or APR, possibly affected or
sacrificed anal sphincter function and was associated with high
mortality and morbidity. The natural history of these tumors may
partly cancel out the benefit of radical surgery.
Jakob et al (9) concluded that if pre-operative IM was
used, it was associated with improved surgical margins and local
disease-free, total disease-free and overall survival. Local
excision did not incur elevated local recurrence rates. The study
found that 5 out of 21 local excisions for anorectal GISTs incurred
local recurrence, as these patients underwent local excision with
positive margins. Complete resection is recommended to achieve
local disease control. The study also found that 5 out of 39
patients without IM therapy incurred metastasis (9).
Laparoscopic surgery has been a breakthrough in the
field of rectal cancer surgery. Fujimoto et al (25) reported the cases of five patients
who were treated by a combination of neoadjuvant IM therapy and
laparoscopic sphincter-preserving surgery [intersphincteric
resection (ISR) or modified ISR] for a large rectal GIST. All
patients underwent complete surgical resection macroscopically and
microscopically, including one case with a complete response,
thereby avoiding a radical excision and preserving the anus
(25).
From the present study and the literature, it can be
observed that IM therapy plus local excision does not incur severe
complications and colorectal dysfunction. Therefore, the
pre-operative use of IM combined with subsequent local resection is
a viable therapeutic option for anorectal GISTs and allows less
extensive resections. In the present study, there were no severe
complications, except a slight fistula, and no recurrence and
metastasis occurred after more than two years of follow-up. The key
point of this therapy strategy is to obtain a tumor-free margin and
to preserve the function of the anal sphincter.
References
|
1
|
Tran T, Davila JA and El-Serag HB: The
epidemiology of malignant gastrointestinal stromal tumors: an
analysis of 1,458 cases from 1992 to 2000. Am J Gastroenterol.
100:162–168. 2005.
|
|
2
|
Miettinen M, Furlong M, Sarlomo-Rikala M,
Burke A, Sobin LH and Lasota J: Gastrointestinal stromal tumors,
intramural leiomyomas, and leiomyosarcomas in the rectum and anus:
a clinicopathologic, immunohistochemical, and molecular genetic
study of 144 cases. Am J Surg Pathol. 25:1121–1133. 2001.
|
|
3
|
Miettinen M and Lasota J: Histopathology
of gastrointestinal stromal tumor. J Surg Oncol. 104:865–873.
2011.
|
|
4
|
Centonze D, Pulvirenti E, Pulvirenti
D’Urso A, Franco S, Cinardi N and Giannone G: Local excision with
adjuvant imatinib therapy for anorectal gastrointestinal stromal
tumors. Tech Coloproctol. 17:571–574. 2013.
|
|
5
|
Christiansen J: Excision of mid-rectal
lesions by the Kraske sacral approach. Br J Surg. 67:651–652.
1980.
|
|
6
|
Koscinski T, Malinger S and Drews M: Local
excision of rectal carcinoma not-exceeding the muscularis layer.
Colorectal Dis. 5:159–163. 2003.
|
|
7
|
Corless CL, Fletcher JA and Heinrich MC:
Biology of gastrointestinal stromal tumors. J Clin Oncol.
22:3813–3825. 2004.
|
|
8
|
Hirota S, Isozaki K, Moriyama Y, et al:
Gain-of-function mutations of c-kit in human gastrointestinal
stromal tumors. Science. 279:577–580. 1998.
|
|
9
|
Jakob J, Mussi C, Ronellenfitsch U, et al:
Gastrointestinal stromal tumor of the rectum: results of surgical
and multimodality therapy in the era of imatinib. Ann Surg Oncol.
20:586–592. 2013.
|
|
10
|
Agaimy A, Vassos N, Märkl B, et al:
Anorectal gastrointestinal stromal tumors: a retrospective
multicenter analysis of 15 cases emphasizing their high local
recurrence rate and the need for standardized therapeutic approach.
Int J Colorectal Dis. 28:1057–1064. 2013.
|
|
11
|
Tielen R, Verhoef C, van Coevorden F, et
al: Surgical management of rectal gastrointestinal stromal tumors.
J Surg Oncol. 107:320–323. 2013.
|
|
12
|
Miettinen M, Sobin LH and Sarlomo-Rikala
M: Immunohistochemical spectrum of GISTs at different sites and
their differential diagnosis with a reference to CD117 (KIT). Mod
Pathol. 13:1134–1142. 2000.
|
|
13
|
Dematteo RP, Gold JS, Saran L, et al:
Tumor mitotic rate, size, and location independently predict
recurrence after resection of primary gastrointestinal stromal
tumor (GIST). Cancer. 112:608–615. 2008.
|
|
14
|
Connolly EM, Gaffney E and Reynolds JV:
Gastrointestinal stromal tumours. Br J Surg. 90:1178–1186.
2003.
|
|
15
|
Hassan I, You YN, Dozois EJ, et al:
Clinical, pathologic, and immunohistochemical characteristics of
gastrointestinal stromal tumors of the colon and rectum:
implications for surgical management and adjuvant therapies. Dis
Colon Rectum. 49:609–615. 2006.
|
|
16
|
Fu T, Liu B, Zhang S, Wang D and Zhang L:
Transvaginal local excision of rectal carcinoma. Curr Surg.
60:538–542. 2003.
|
|
17
|
Wang JP, Wang T, Huang MJ, Wang L, Kang L
and Wu XJ: The role of neoadjuvant imatinib mesylate therapy in
sphincter-preserving procedures for anorectal gastrointestinal
stromal tumor. Am J Clin Oncol. 34:314–316. 2011.
|
|
18
|
Matsushima K and Kayo M: Transsacral
approach to resect a gastrointestinal stromal tumor in the rectum:
report of two cases. Surg Today. 37:698–701. 2007.
|
|
19
|
Terkivatan T, den Hoed PT, Lange JF Jr,
Koot VC, van Goch JJ and Veen HF: The place of the posterior
surgical approach for lesions of the rectum. Dig Surg. 22:86–90.
2005.
|
|
20
|
Machlenkin S, Pinsk I, Tulchinsky H, et
al: The effect of neoadjuvant imatinib therapy on outcome and
survival after rectal gastrointestinal stromal tumour. Colorectal
Dis. 13:1110–1115. 2011.
|
|
21
|
Rutkowski P, Gronchi A, Hohenberger P, et
al: Neoadjuvant imatinib in locally advanced gastrointestinal
stromal tumors (GIST): the EORTC STBSG experience. Ann Surg Oncol.
20:2937–2943. 2013.
|
|
22
|
Eisenberg BL, Harris J, Blanke CD, et al:
Phase II trial of neoadjuvant/adjuvant imatinib mesylate (IM) for
advanced primary and metastatic/recurrent operable gastrointestinal
stromal tumor (GIST): early results of RTOG 0132/ACRIN 6665. J Surg
Oncol. 99:42–47. 2009.
|
|
23
|
Verweij J, Casali PG, Zalcberg J, et al:
Progression-free survival in gastrointestinal stromal tumours with
high-dose imatinib: randomised trial. Lancet. 364:1127–1134.
2004.
|
|
24
|
Demetri GD, von Mehren M, Antonescu CR, et
al: NCCN Task Force report: update on the management of patients
with gastrointestinal stromal tumors. J Natl Compr Canc Netw.
8(Suppl 2): S1–S44. 2010.
|
|
25
|
Fujimoto Y, Akiyoshi T, Konishi T,
Nagayama S, Fukunaga Y and Ueno M: Laparoscopic
sphincter-preserving surgery (intersphincteric resection) after
neoadjuvant imatinib treatment for gastrointestinal stromal tumor
(GIST) of the rectum. Int J Colorectal Dis. 29:111–116. 2014.
|
|
26
|
Lagos AC, Marques I, Reis J, Martins I and
Neves B: Malignant rectal gastrointestinal stromal tumour: case
report and review of literature. J Gastrointest Cancer. Mar
8–2012.(Epub ahead of print).
|
|
27
|
Hara M, Takayama S, Arakawa A, Sato M,
Nagasaki T and Takeyama H: Transvaginal resection of a rectal
gastrointestinal stromal tumor. Surg Today. 42:909–912. 2012.
|
|
28
|
Gervaz P, Huber O, Bucher P, Sappino P and
Morel P: Trans-sacral (Kraske) approach for gastrointestinal
stromal tumour of the lower rectum: old procedure for a new
disease. Colorectal Dis. 10:951–952. 2008.
|
|
29
|
Lo SS, Papachristou GI, Finkelstein SD,
Conroy WP, Schraut WH and Ramanathan RK: Neoadjuvant imatinib in
gastrointestinal stromal tumor of the rectum: report of a case. Dis
Colon Rectum. 48:1316–1319. 2005.
|