Introduction
Due to the severely infiltrative nature of glioma
and the resulting difficulty in performing complete surgical
resection, there is often a high rate of recurrence and metastasis
in glioma patients undergoing surgical treatment alone.
Radiotherapy, chemotherapy and photodynamic therapy exhibit certain
auxiliary functions for the gliomas, however, they are insufficient
according to the published median survival analysis (1). Sonodynamic therapy (SDT) is a novel
therapy for tumor treatment, which focuses the ultrasound energy on
malignant sites located deeper in the tissues, which cooperate with
sonosensitizers, and thus exert significant antitumor effects in
vitro and in vivo. Previous studies have indicated that
ultrasound in combination with porphyrins may induce apoptosis in
glioma cells (2–6). However, a relatively low apoptotic
rate has been identified following SDT (3–6).
Previous studies have indicated that lower frequency and power may
lead to a higher cavitation effect and concomitant biological
effect (7–9). For satisfactory preferential retention
and a long term storage of hematoporphyrin monomethyl ether (HMME)
in glioma tissue, in this study, the C6 glioma cells were treated
with HMME and lower level ultrasound using optimal parameters to
enhance the apoptotic effect in vitro.
Apoptosis is regulated by numerous factors,
including reactive oxygen species (ROS), calcium overload and
mitochondrial damage (10–12). The calcium ion has been proposed to
be an important regulator in apoptosis. A rapid increase in
intracellular Ca2+, [Ca2+]i, has
been identified in various conditions, including
ischemia-reperfusion injury, receptor over-stimulation and
oxidative stress, among others (13–16).
Calcium overload may damage mitochondria, causing the release of
apoptotic promoters and activation of the caspase cascade (17,18).
Honda et al (17) found a
transient increase in [Ca2+]i from outside
the cells in human myelomonocytic lymphoma U937 cells following
ultrasound alone, and Li et al (18) showed an increased
[Ca2+]i, released from internal stores at an
ultrasound frequency of 1 MHz in combination with HMME in C6 glioma
cells (18). This may be attributed
to the ultrasonic cavitation effect, which changes the permeability
of the cytomembrane (19,20). Therefore, overloaded
[Ca2+]i may be obtained from internal and
external sources during the apoptotic process in C6 glioma cells,
as a result of low-level ultrasound and HMME. In the present study,
the source of overloaded [Ca2+]i. was
detected from intracellular and extracellular environments
following SDT.
Previous studies have demonstrated the SDT may
induce apoptosis in C6 glioma cells via the excessive production of
ROS, which was due to the interaction of the ultrasonic cavitation
and sensitizers (7–9,19,20).
The oxidizing effect may damage mitochondria and lead to apoptosis
via the mitochondrial signaling pathway (10–16).
In addition, ROS increases cytosolic calcium in the absence of
extracellular calcium, leading cells into an apoptotic state
(17). Cavitations including
inertial and stable cavitation, are associated with a number of
biological process, including the production of free radicals,
changes in membrane permeability and sonoluminescence, among others
(14–17). Although the mechanism of ROS
production is not clear, the cavitation effect must be involved in
the apoptotic process in SDT and may be relevant to the overloaded
Ca2+ and mitochondrial damage.
Accordingly, in this study we hypothesized that
low-level ultrasound in combination with HMME may increase the
apoptotic rate and the concentration of
[Ca2+]i in C6 glioma cells following SDT-HMME
treatment, which is associated with ROS production, decreased
mitochondrial membrane potential (MMP) and the release of
cytochrome c (cyt-c). The apoptotic rate and
concentration of [Ca2+]i was determined
following SDT-HMME treatment. The L-type Ca2+ channel
antagonist nimodipine was added to cells prior to SDT to detect the
source of overloaded [Ca2+]i.
Materials and methods
Reagents
The C6 glioma cell line was purchased from the
Beijing Institute of Biology, Chinese Academy of Sciences (Beijing,
China) and 2,7-dichlorodihydrofluorescein diacetate (DCFH-DA),
Rhodamine 123 and fluo-3/acetoxymethylester were purchased from
Sigma-Aldrich (St. Louis, MO, USA). Fluorescein isothiocyanate
(FITC)-Annexin-V/propidium iodide (PI) and Hank’s balanced salt
solution (HBSS) were purchased from Beyotime Institute of
Biotechnology (Jiangsu, China) and
3-(4,5-dimethythiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
was purchased from Sigma-Aldrich. Nimodipine was purchased from
Bayer (Leverkusen, Germany). HMME was purchased from Changzhou
Kangmei Chemical Co., Ltd. (Jiangsu, China) and rabbit monoclonal
anti-rat cyt-c antibodies were purchased from Santa Cruz
Biotechnology, Inc. (Santa Cruz, CA, USA).
Cell culture
The C6 glioma cells were cultured in RPMI-1640
medium (Hyclone Laboratories, Inc., Logan, UT, USA) containing 10%
fetal bovine serum (Hyclone Laboratroies, Inc.). The cells were
maintained at 37°C in a humidified atmosphere containing 5%
CO2. One day prior to treatment, the cells were
trypsinized, counted and seeded in six-well plates at a density of
1×106/ml cells per well. Cells were cultured to 70–80%
confluence prior to further experiments.
Ultrasound frequency optimization
To optimize the ultrasound frequency, the cell
viability was investigated by MTT assay as described previously
(3,17). Cells were cultured at 37°C in
six-well plates at a density of 1×106/ml cells per well.
The ultrasound irradiation was carried out at room temperature in a
sponge water bath (depth, 10 mm) using a multi-function ultrasound
device (ultrasound transducer diameter, 20 mm; depth of
penetration, 50 mm; MB-200F, Saifuruide (Beijing) Technology Co.,
Ltd., Beijing, China), the device was customised by the College of
Underwater Acoustic Engineering, Harbin Engineering University
(Harbin, China), the frequency of the device was enabled to alter
between 0.3 and 1.0MHz and the power could be adjusted from 0 to
1.0W. The sponge was placed under the wells, and the probe was
placed under the sponge. The sponge water bath aided the
minimization of acoustic reflections and subsequent standing wave
formations. The pulsed-wave ultrasound parameters were set at 1
W/cm2 for intensity and 60 sec for duration time. The
frequencies varied between 0 and 1.0 MHz. Cells were trypsinized
and transferred to 96-well plates following irradiation. MTT was
added to a final concentration of 0.5 mg/ml. Following 4 h of
culture at 37°C, the supernatant was removed, and 200 μl
dimethylsulfoxide (Sigma-Aldrich) was added. The absorbance was
read at a wavelength of 490 nm using a universal microplate
spectrophotometer (Model 550; Bio-Rad, Hercules, CA, USA). The cell
viability without irradiation was considered as a control for 100%
viability, and thus cell viability was expressed as a percentage of
the control. Cell viability was statistically analyzed to select
the appropriate frequency for further ultrasound experiments.
SDT treatment
The ultrasound and SDT treatments for the C6 glioma
cells were performed as previously described (3). Briefly, cells cultured at 37°C in
six-well plates were randomly divided into control (untreated),
HMME (HMME alone), ultrasound (ultrasound alone) and SDT
(ultrasound + HMME) groups. Each group was placed in six wells.
Cells in the four groups were pretreated with phosphate-buffered
saline (PBS, Ca2+-free), HBSS (containing 1.3 mM
Ca2+, pH 7.4), nimodipine (10 mg/ml in PBS) and
HBSS-nimodipine for the follow-up experiments. HMME was added to
the HMME and SDT group at a final concentration of 10 μg/ml for 2 h
prior to insonation. Then cells in the four different groups were
treated for an instant insonation. The ultrasound treatment was
carried out under the same intensity and time, however, the
frequency was determined by MTT assay. Following treatment, cells
from the sixteen groups were maintained at 37°C in a humidified
atmosphere containing 5% CO2 in the dark for subsequent
experiments.
Measurement of
[Ca2+]i
The concentration of [Ca2+]i
was measured using a confocal laser scanning microscope (Leica TCS
SP5; Leica, Mannheim, Germany) as described previously (18). Cultured C6 glioma cells in 96-well
plates were loaded with 10 μM fluo-3/acetoxymethylester for 30 min
at 37°C prior to SDT. Cells in the different groups were then
washed three times with PBS to remove the extracellular
fluo-3/acetoxymethylester for SDT. Excitation was set at a
wavelength of 488 nm and emission was monitored at a wavelength of
530 nm. Fluorescence images indicating the
[Ca2+]i were captured using a confocal laser
scanning microscope for 30 min (Leica).
Detection of apoptosis
Cell apoptosis was measured by flow cytometry with
double staining of FITC-Annexin-V and PI as previously described in
the groups as determined by the previous confocal laser scanning
assay (11). After 24 h, cells with
and without treatment were harvested, washed twice with PBS and
re-suspended with 0.5 ml PBS at a cell density of 1×106
cells/ml. Next, a total of 10 μl Annexin-V and 5 μl PI were added
to the wells in the dark. Following 30 min of incubation, the cells
were analyzed by flow cytometry (Becton Dickinson, Franklin Lakes,
NJ, USA) to determine the apoptotic rate.
Transmission electron microscopy
After 24 h cells in the groups with or without
treatment were harvested and fixed with 3.0% glutaraldehyde and
1.5% paraldehyde, washed in PBS, and fixed in osmium tetroxide.
Then, cells were dehydrated in an ethanol series, embedded in epoxy
resin and examined under a transmission electron microscope
[JEM-1220EX; Jeol (GmbH), München, Germany].
Production of intracellular ROS
The production of intracellular ROS was assayed by
flow cytometry using DCFH-DA as previously described (11). After 2 h, cells in the groups with
and without treatment were harvested, washed and re-suspended in
500 μl PBS containing DCFH-DA (final concentration, 10 mol/l), and
then incubated at 37°C in the dark for 30 min. The cell analysis
was performed by flow cytometry.
MMP detection
The loss of MMP was detected by flow cytometry using
Rhodamine 123 as previously described (11). After 2 h, Rhodamine 123 was added to
the cells at a final concentration of 200 nmol/l in the dark and
incubated for 30 min. The decrease in MMP was calculated using
CellQuest software (BD Biosciences, Franklin Lakes, NJ, USA).
Detection of cyt-c release from
mitochondria
To quantify cyt-c release, western blot
analysis of cyt-c in the cytosolic fraction was performed as
previously described (11,21). After 24 h, cells were harvested,
washed twice with ice-cold PBS, and incubated in an ice-cold
Tris-sucrose buffer (0.35 M sucrose, 10 mM Tris-HCl, pH 7.5, 1 mM
EDTA, 0.5 mM dithiothreitol and 0.1 mM phenylmethylsulfonyl
fluoride). Following a 40-min incubation, cells were centrifuged at
1,000 × g for 5 min at 4°C, and the supernatant was further
centrifuged at 40,000a × g for 30 min at 4°C. The supernatant was
regarded as the cytosolic fraction, and resolved on SDS-PAGE gel
and analyzed by western blot analysis using a primary rabbit
monoclonal anti-rat antibody against cyt-c and a secondary
goat polyclonal immunoglobulin G anti-rabbit IgG (Santa Cruz
Biotechnology, Inc.). Actin expression was used as the loading
control.
Statistical analysis
Data are expressed as the mean ± standard error of
the mean. Comparisons between the different groups were performed
via factorial design analysis of variance using SPSS, version 11.0
(SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
Optimal parameters for ultrasound
application
Cells were exposed to pulsed-wave ultrasound with an
irradiation time of 60 sec and an average intensity of 1.0
W/cm2. The optimum experimental frequency was determined
by MTT assay according to the cell viability. As shown in Fig. 1, the survival rates were 95.4±1.8,
43.2± 3.2, 57.1± 3.7 and 60.2±2.6% at the frequencies of 0, 0.6,
0.8 and 1 MHz, respectively, following insonation. The survival
rate was decreased significantly in the groups at the frequency of
0.5 MHz. In order to improve the apoptotic rate, 0.5 MHz was
determined as the optimal frequency for the C6 glioma cells.
Measurement of
[Ca2+]i
The concentration of intracellular free calcium was
recorded by a confocal laser scanning microscopy for 30 min in a
single cell with the fluorescent probe fluo-3/acetoxymethylester, a
sensitive Ca2+ probe. The concentration of
[Ca2+]i was increased significantly in the
ultrasound group with PBS, HBSS, nimodipine and HBSS-nimodipine
solution, and further increased in the SDT groups with the same
medium (P<0.05 versus the control-PBS group; 96±7.3 nM; Fig. 2A and B). This revealed that the
overloaded [Ca2+]i was involved in SDT
treatment. Although SDT-PBS treatment significantly increased the
concentration of [Ca2+]i in 1,800s (258±11.8
nM; P<0.05 versus the control-PBS group), a higher elevation of
[Ca2+]i was observed in the SDT-HBSS group
(408±11.6 nM; P<0.05 versus the other groups). In addition, no
significant difference was identified in the elevated concentration
of [Ca2+]i between the SDT-HBSS-nimodipine
(404±12.1 nM) and the SDT-HBSS group (408±11.6 nM) (P>0.05),
which was the same as in the ultrasound-HBSS-nimodipine (181±16.2
nM) and the ultrasound-HBSS group (171±5.5 nM) (P>0.05).
Apoptotic effect
To investigate the association between apoptosis and
overloaded [Ca2+]i, apoptotic rates were
determined in groups with overloaded [Ca2+]i
by flow cytometry. Cells were divided into control, HMME,
ultrasound and SDT groups in PBS and HBSS solutions. The SDT and
ultrasound groups in the two types of solution exhibited a
significant increase in the apoptotic rate (P<0.05, versus the
control-PBS group; 4.2±0.5%; Fig.
3A). Ultrasound-HBSS (26.5±1.1%) and ultrasound-PBS (16.0±0.8%)
treatment exhibited a marginal apoptotic effect (P<0.05, versus
the control-PBS group) (17).
SDT-PBS treatment (49.4±2.6%) exhibited a significant apoptotic
effect (P<0.05, versus the other groups) and SDT-HBSS treatment
(59.9±3.2%) exhibited the highest rate (P<0.05, versus the other
groups).
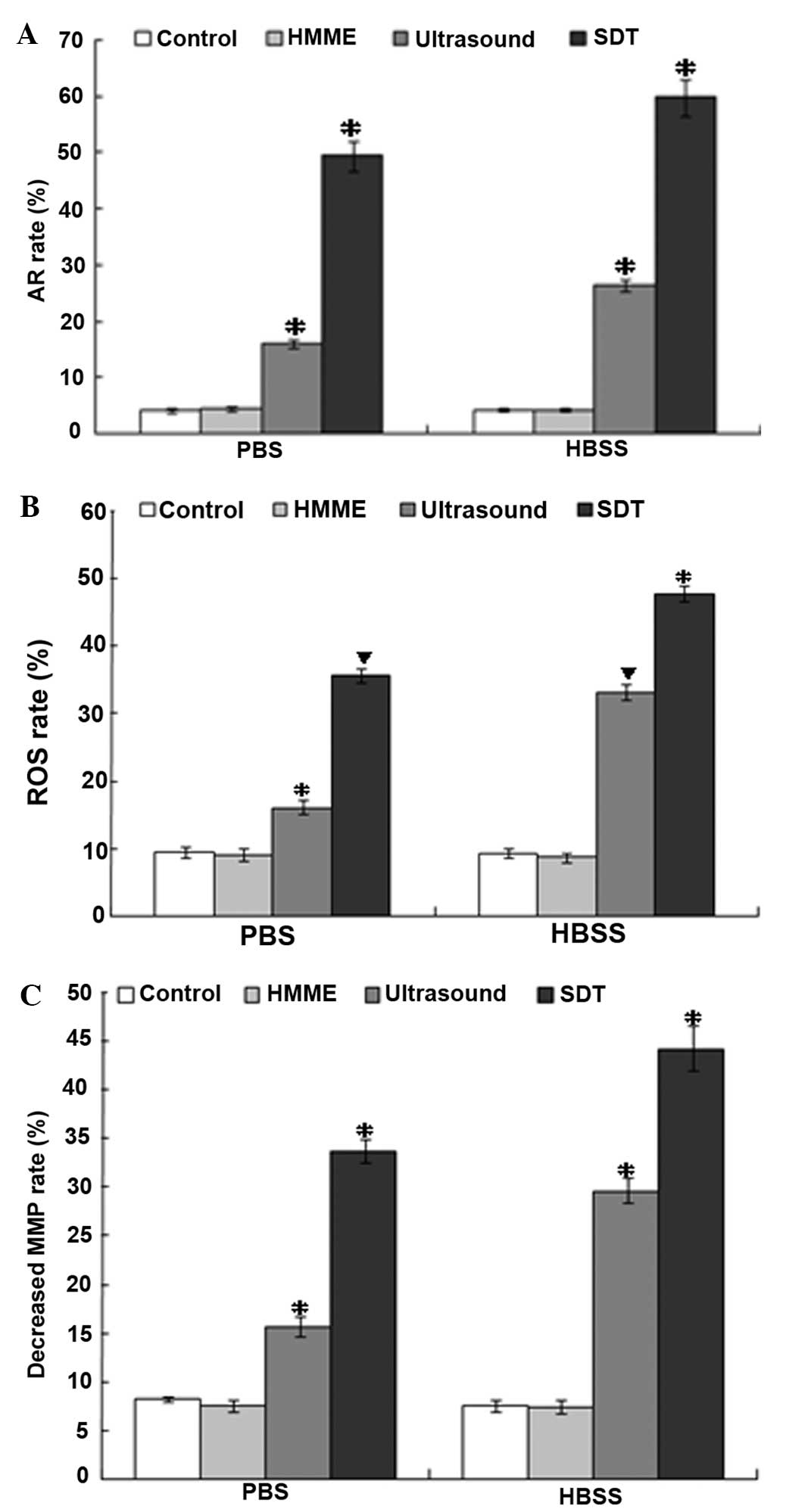 | Figure 3Apoptotic rate, ROS production and
decreased MMP of C6 glioma cells from SDT, ultrasound, HMME and
control groups in PBS and HBSS groups were analyzed by flow
cytometry. (A) The apoptotic rate was increased the most in the
SDT-HBSS group. (B) The production of ROS increased the most in the
SDT-HBSS group. (C) The MMP decreased most in the SDT-HBSS group.
*P<0.05, vs. the other groups. ▼P<0.05,
vs. the other groups except the group with the same symbol
(▼). HMME, hematoporphyrin monomethyl ether; PBS,
phosphate-buffered saline; HBSS, Hank’s balanced salt solution;
MMP, mitochondrial membrane potential; ROS, reactive oxygen
species. |
Intracellular ROS
The production of ROS was determined by flow
cytometry with DCFH-DA. Intracellular ROS significantly increased,
with the exception of the control and HMME groups in PBS and HBSS
(Fig. 3B). The production of
increased ROS in the ultrasound-PBS, ultrasound-HBSS, SDT-PBS and
SDT-HBSS groups were 16.1±1.0, 33.1±1.1, 35.6±1.0 and 47.7±1.2%,
respectively. This increase was concurrent with the increase in
apoptotic rate and overloaded [Ca2+]i in the
same group following SDT treatment (P<0.05, versus the
control-PBS group; 9.4±0.9%; Fig.
3B). However, no significant difference in ROS production was
identified between the ultrasound-HBSS and the SDT-PBS groups
(P>0.05).
Loss of MMP
Cells undergoing apoptosis usually lose their MMP
and appear Rhodamine 123-dim. Thus, MMP was determined by Rhodamine
123 staining and detected by flow cytometry. The MMP decreased
significantly in the ultrasound-PBS (15.7±1.0%) and ultrasound-HBSS
(29.6±1.3%) groups (P<0.05, versus the control-PBS group;
8.2±0.3%); Fig. 3C). Furthermore,
the MMP decreased more clearly in the SDT-PBS (33.7±1.2%) group and
was the largest in the SDT-HBSS group (44.2±2.3%). The decrease in
MMP in the groups was similar to that of the increased ROS and the
overloaded [Ca2+]i in the apoptotic process
by SDT.
Release of cyt-c
Since the MMP was disrupted following SDT treatment,
the modulation of the expression of key signaling molecules of the
mitochondrial signaling pathway under low-level ultrasound and HMME
was investigated. The release of cyt-c was measured by
western blot analysis. The release of cyt-c was upregulated
in the SDT-PBS, SDT-HBSS, ultrasound-PBS and ultrasound-HBSS groups
(P<0.05 versus the control-PBS group; Fig. 4). No significant difference in the
release of cyt-c was identified between the control-HBSS,
HMME-PBS and HMME-HBSS groups (P>0.05 versus the control-PBS
group; Fig. 4).
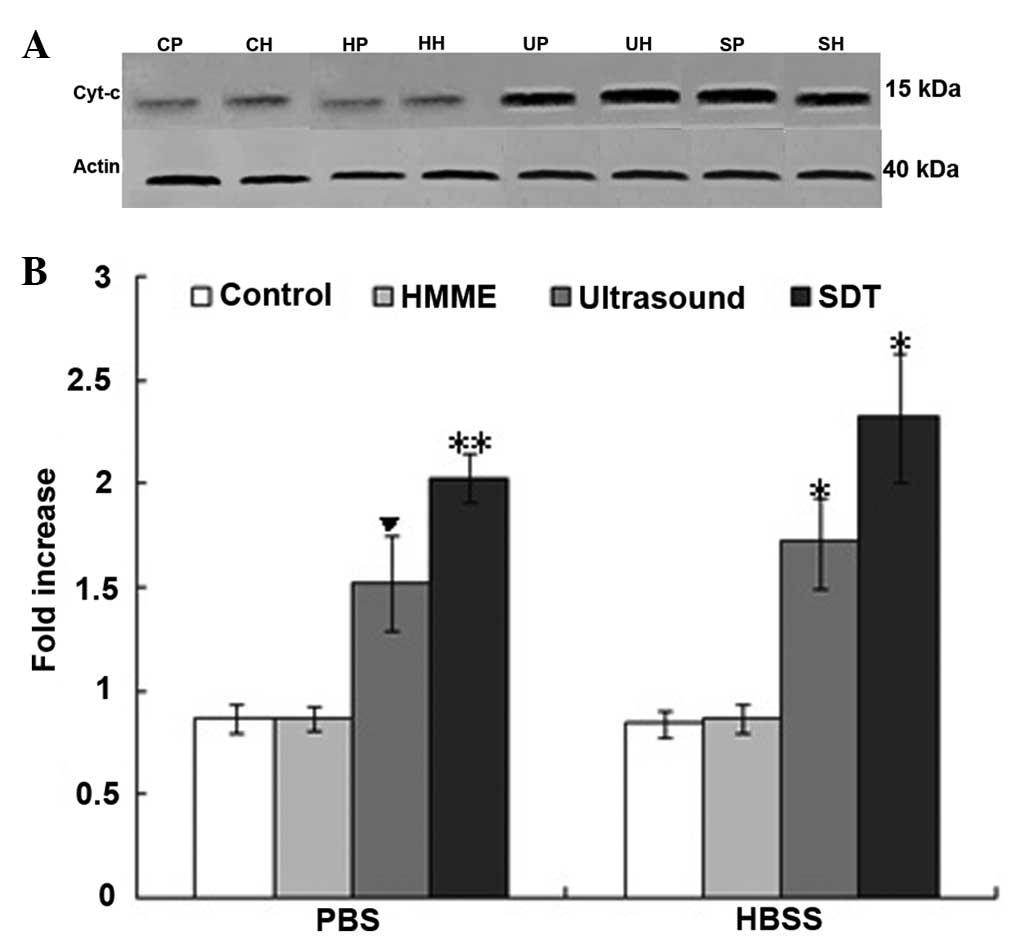 | Figure 4(A) The release of cyt-c from
the control, HMME, ultrasound and SDT groups in PBS and HBSS in C6
glioma cells from the control-PBS (CP), HMME-PBS (HP),
ultrasound-PBS (UP), SDT-PBS (SP), control-HBSS (CH), HMME-HBSS
(HH), ultrasound-HBSS (UH) and the SDT-HBSS (SH) groups were
analyzed using western blot analysis. (B) Upregulation of
cyt-c release was exhibited in the UP, SP, UH and SH groups.
▼P<0.05 vs. the other groups; *P<0.05,
vs. the other groups, with the exception of the SP group; and
**P<0.05, vs. the other groups, with the exception of
the UH and SH groups. Cyt-c, cytochrome c; HMME,
hematoporphyrin monomethyl ether; PBS, phosphate-buffered saline;
HBSS, Hank’s balanced salt solution. |
Morphological changes
In order to further investigate the apoptotic effect
following SDT treatment, transmission electron microscopy was used
to observe the microscopic changes in C6 glioma cells. In the
HMME-PBS/HBSS group and the control-PBS/HBSS group, the cell
membrane and nuclear envelope were intact, the cytoplasm was rich
and mitochondria were integrated. No significant ultra-structural
changes were identified. However, the ultrasound-PBS/HBSS group and
SDT-PBS/HBSS groups exhibited morphological changes, which were
characteristic of apoptosis, including nuclear chromatin
margination, aggregation, condensation, swelling and vacuolization
of mitochondria (Fig. 5).
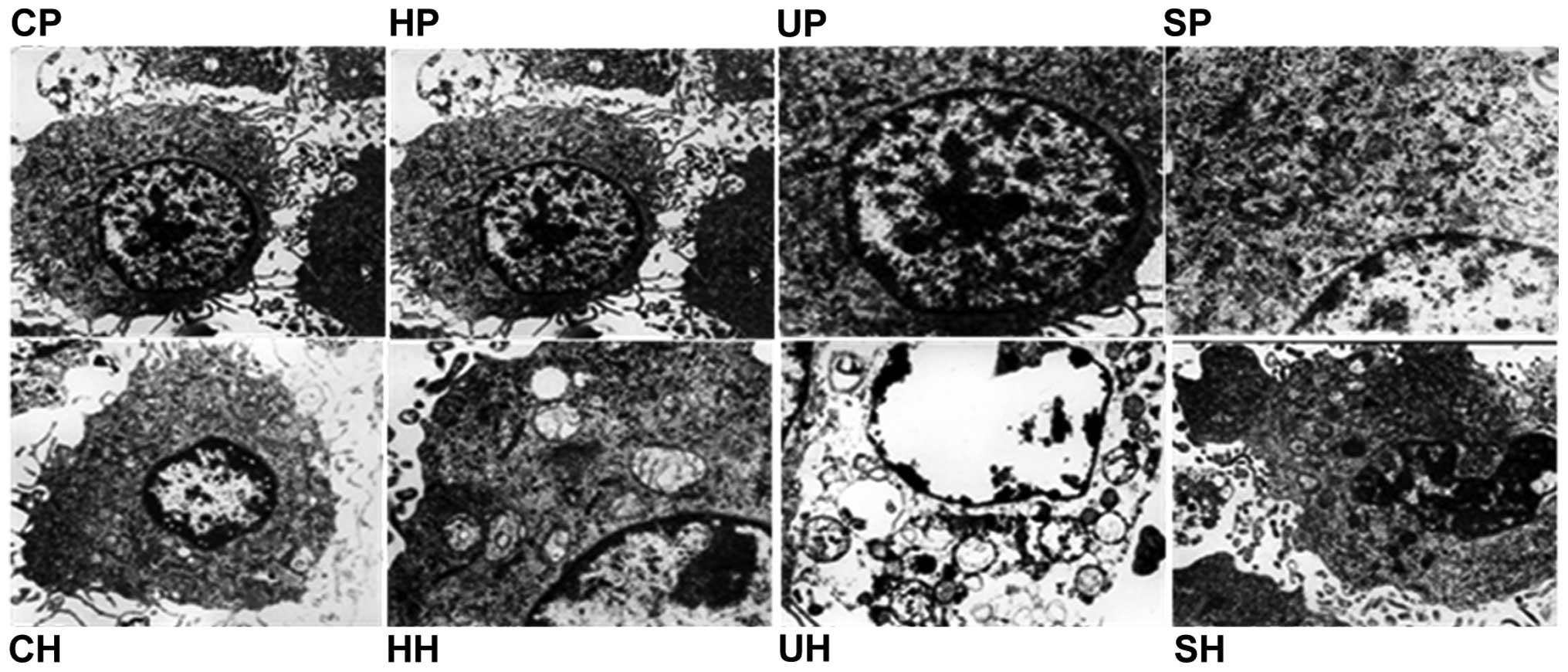 | Figure 5Transmission electron microscopy
images of ultrastructural changes in C6 glioma cells
(magnification, ×10,000). In the UP, UH, SP and SH groups, nuclear
chromatin margination, aggregation, condensation, mitochondrial
swelling and vacuolization were observed. CP, control-PBS; HP,
HMME-PBS; UP, ultrasound-PBS; SP, SDT-PBS; CH, control-HBSS; HH,
HMME-HBSS; UH, ultrasound-HBSS; SH, SDT-HBSS group; HBSS, Hank’s
balanced salt solution; HMME, hematoporphyrin monomethyl ether;
SDT, sonodynamic therapy. |
Discussion
The apoptotic effect induced by SDT was dependent on
ultrasound intensity, frequency, duration time and sonosensitizers,
among others (9,21). Commonly, low-intensity ultrasound is
a term describing intensities <3 W/cm2 (22,23),
and ultrasound frequencies <1 MHz are usually used for drug
delivery, blood-tumor barrier opening and ultrasonic therapy
(22,24). Buldakov et al (24), Bai et al (25) and Jeong et al (26) observed antitumor effects in
vitro and in vivo induced by SDT with ultrasound
intensities of 0.3–2.6 W/cm2 and a frequency of 1 MHz.
In addition, Zhai et al (27) and Ninomiya et al (28) showed an inhibitory effect on growth
of cancer cells induced by SDT when changing the ultrasound
parameters to a frequency of 0.5–1.5 MHz and intensities of 0.4–460
W/cm2 in vitro (27,28).
The lower the frequency and intensity, the higher the cavitation
effect and fewer side effects for the healthy surrounding tissue
(8,19,20).
Therefore, SDT in combination with HMME and the optimized
ultrasound parameters of 1.0 W/cm2, 0.5 MHz and 60 sec
were applied to the C6 glioma cells in the present study. The
results revealed the occurrence of apoptosis by flow cytometry and
transmission electron microscopy following SDT treatment. The
apoptotic rate was significantly increased in the SDT-PBS
(49.4±2.6%) group and further increased in the SDT-HBSS (59.9±3.2%)
group following SDT, which was higher than that in previous studies
whereby apoptotic rate was <40% in C6 glioma cells (3,6,9,18).
Accordingly, the low-level ultrasound in combination with HMME may
increase the apoptotic effect on C6 glioma cells.
Calcium ions are important in living cells and are
the key regulators of cell proliferation and death (15). A crucial requirement for the
regulation of cellular functions by cytosolic Ca2+ is to
maintain a steep concentration gradient between the extracellular
and intracellular environments (16). Within the intracellular space, a
further Ca2+ gradient is established between the
cytoplasm and other organelles, including the endoplasmic reticulum
and mitochondria (17). Any changes
affecting the Ca2+ homeostatic balance ultimately
influences the fate of cells. The overloaded
[Ca2+]i accumulated in the mitochondria may
alter the outer mitochondrial membrane permeability conversely,
leading to the release of cyt-c and other apoptotic factors,
and eventually apoptosis (10–12).
The results of this study revealed that the concentration of
[Ca2+}i was significantly increased in the
SDT group after 30 min when the cells were preincubated in PBS
buffer (Ca2+-free). This indicated that the
[Ca2+]i overload was involved in the SDT
treatment. In order to investigate the source of the overloaded
[Ca2+]i, the cells were preincubated with
HBSS buffer (containing 1.3 mM Ca2+). The results
revealed a further elevation in [Ca2+]i
concentration in the SDT-HBSS group. Therefore, the increased
[Ca2+]i was resultant not only from internal
store release, but also from extracellular medium influx during
early apoptosis (10,21,24).
This result was inconsistent with the previous studies in which the
increased [Ca2+]i was from the intracellular
or extracellular environment (17,18).
This contradiction may be due to the different ultrasound
parameters and the different types of cells used in the experiment.
Liu et al (4) proposed that
the mechanisms of SDT were influenced by multiple factors,
including the nature of the biological model, the sonosensitizer
and the ultrasound parameters, among others. In addition, the
concentration of [Ca2+]i was constant in the
SDT-PBS/HBSS and ultrasound-PBS/HBSS groups when the L-type
voltage-dependent calcium channel antagonist, nimodipine, was
added. This indicated it was ineffective at regulating the
concentration of [Ca2+]i by SDT treatment.
Accordingly, the results indicated that the Ca2+ influx
may be mediated via mechanisms other than voltage-dependent
Ca2+ channels, including promotion of membrane
permeability and membrane perforation, among others (25); however, further confirmation is
required. The results also revealed that the SDT-HBSS group
exhibited the highest [Ca2+]i concentration
and exhibited the highest apoptotic rate. The higher the overload
of [Ca2+]i, the higher the apoptotic rate
following SDT treatment. Hence, the increased
[Ca2+]i from intra- and extracellular sources
contributed to the apoptosis induced by SDT.
Previous studies have revealed that ROS production
and mitochondrial damage were associated with the apoptosis of C6
glioma cells following SDT (3,17,18,24).
In the present study, ROS was increased following SDT using the
lower frequency ultrasound. Oxidative damage of ROS induces
mitochondrial membrane permeabilization in vitro and in
vivo (11,13–16).
When MMP was decreased to a certain extent, cyt-c,
apoptosis-inducing factor and Smac/Diablo were transmitted from the
intermembrane space into the cytosol, which has been defined as an
early stage of apoptosis, and then caspase-9 and-3 were activated,
carrying out the irreversible apoptotic process (12,21,23).
Whether ROS targeted the mitochondria and thereby decreased the MMP
in C6 glioma cells was investigated following SDT using the lower
ultrasound parameter. The results revealed a significant decrease
in MMP and the release of cyt-c. Therefore, mitochondrial
damage was involved in the apoptotic process under the SDT
parameter of the present study. In addition, the mitochondria acted
as calcium buffers by sequestering excess calcium from the cytosol.
Excessive calcium load to the mitochondria may induce an apoptotic
program by stimulating the release of the apoptotic promoting
factors from the mitochondrial intermembrane space to cytosol and
by impairing their function. The results also revealed that the
more ROS production and [Ca2+]i were
increased, the more the MMP was decreased and the apoptotic rate
was increased. Thus, the increased concentration of
[Ca2+]i and the ROS production followed by
the decreased MMP and the release of cyt-c may explain the
higher apoptotic rate following SDT.
In conclusion, SDT under low-level ultrasound and
HMME resulted in an increased [Ca2+]i
concentration from intracellular and extracellular sources,
increased ROS production, a decreased MMP and the release of
cyt-c. This effect may be applied to treat C6 glioma cells
to cause an increased apoptotic effect. Low frequency and low
intensity ultrasound with HMME improved the apoptotic effect in
glioma cells. The overloaded [Ca2+]i was
involved in the mechanism by which apoptosis was stimulated and
enhanced by SDT.
Acknowledgements
This study was supported by the Scientific Reseearch
Foundation of the Affiliated Tumor Hospital of Harbin Medical
University (grant no. 2011-4-006).
References
|
1
|
Li Y, Lupo JM, Parvataneni R, Lamborn KR,
Cha S, Chang SM and Nelson SJ: Survival analysis in patients with
newly diagnosed glioblastoma using pre- and postradiotherapy MR
spectroscopic imaging. Neuro Oncol. 15:607–617. 2013.
|
|
2
|
Rosenthal I, Sostaric JZ and Riesz P:
Sonodynamic therapy - a review of the synergistic effects of drugs
and ultrasound. Ultrason Sonochem. 11:349–363. 2004.
|
|
3
|
Dai S, Hu S and Wu C: Apoptotic effect of
sonodynamic therapy mediated by hematoporphyrin monomethyl ether on
C6 glioma cells in vitro. Acta Neurochir (Wien). 151:1655–1661.
2009.
|
|
4
|
Liu Q, Wang X, Wang P, Xiao L and Hao Q:
Comparison between sonodynamic effect with protoporphyrin IX and
hematoporphyrin on sarcoma 180. Cancer Chemother Pharmacol.
60:671–680. 2007.
|
|
5
|
Liang L, Xie S, Jiang L, Jin H, Li S and
Liu J: The Combined effects of hematoporphyrin monomethyl ether-SDT
and doxorubicin on the proliferation of QBC939 cell lines.
Ultrasound Med Biol. 39:146–160. 2013.
|
|
6
|
Li C, Zhang K, Wang P, Hu J, Liu Q and
Wang X: Sonodynamic antitumor effect of a novel sonosensitizer on
S180 solid tumor. Biopharm Drug Dispos. 35:50–59. 2013.
|
|
7
|
Tsukamoto A, Higashiyama S, Yoshida K,
Watanabe Y, Furukawa KS and Ushida T: Stable cavitation induces
increased cytoplasmic calcium in L929 fibroblasts exposed to 1-MHz
pulsed ultrasound. Ultrasonics. 51:982–990. 2011.
|
|
8
|
El Maalouf J, Béra JC, Alberti L,
Cathignol D and Mestas JL: In vitro sonodynamic cytotoxicity in
regulated cavitation conditions. Ultrasonics. 49:238–243. 2009.
|
|
9
|
Shibaguchi H, Tsuru H and Kuroki M and
Kuroki M: Sonodynamic cancer therapy: a non-invasive and repeatable
approach using low-intensity ultrasound with a sonosensitizer.
Anticancer Res. 31:2425–2429. 2011.
|
|
10
|
Yumita N, Iwase Y, Nishi K, et al:
Sonodynamically-induced antitumor effect of mono-l-aspartyl chlorin
e6 (NPe6). Anticancer Res. 31:501–506. 2011.
|
|
11
|
Bird MJ, Thorburn DR and Frazier AE:
Modelling biochemical features of mitochondrial neuropathology.
Biochim Biophys Acta. 1840:1380–1392. 2014.
|
|
12
|
Reinecke F, Levanets O, Olivier Y, et al:
Metallothionein isoform 2A expression is inducible and protects
against ROS-mediated cell death in rotenone-treated HeLa cells.
Biochem J. 395:405–415. 2006.
|
|
13
|
Tsuru H, Shibaguchi H and Kuroki M,
Yamashita Y and Kuroki M: Tumor growth inhibition by sonodynamic
therapy using a novel sonosensitizer. Free Radic Biol Med.
53:464–472. 2012.
|
|
14
|
Boehmerle W and Endres M: Salinomycin
induces calpain and cytochrome c-mediated neuronal cell death. Cell
Death Dis. 2:e1682011.
|
|
15
|
Green DR and Reed JC: Mitochondria and
apoptosis. Science. 281:1309–1312. 1998.
|
|
16
|
Saelens X, Festjens N, Vande Walle L, van
Gurp M, van Loo G and Vandenabeele P: Toxic proteins released from
mitochondria in cell death. Oncogene. 23:2861–2874. 2004.
|
|
17
|
Honda H, Kondo T, Zhao QL, Feril LB Jr and
Kitagawa H: Role of intracellular calcium ions and reactive oxygen
species in apoptosis induced by ultrasound. Ultrasound Med Biol.
30:683–692. 2004.
|
|
18
|
Li JH, Yue W, Huang Z, Chen ZQ, et al:
Calcium overload induces C6 rat glioma cell apoptosis in
sonodynamic therapy. Int J Radiat Biol. 87:1061–1066. 2011.
|
|
19
|
Wu X, Joyce EM and Mason TJ: Evaluation of
the mechanisms of the effect of ultrasound on Microcystis
aeruginosa at different ultrasonic frequencies. Water Res.
46:2851–2858. 2012.
|
|
20
|
Hasanzadeh H, Mokhtari-Dizaji M, Bathaie
SZ, Hassan ZM, Nilchiani V and Goudarzi H: Enhancement and control
of acoustic cavitation yield by low-level dual frequency
sonication: a subharmonic analysis. Ultrason Sonochem. 18:394–400.
2011.
|
|
21
|
Aggarwal BB and Shishodia S: Molecular
targets of dietary agents for prevention and therapy of cancer.
Biochem Pharmacol. 71:1397–1421. 2006.
|
|
22
|
Xia CY, Liu YH, Wang P and Xue YX:
Low-frequency ultrasound irradiation increases blood-tumor barrier
permeability by transcellular pathway in a rat glioma model. J Mol
Neurosci. 48:281–290. 2012.
|
|
23
|
Fulda S and Debatin KM: Extrinsic versus
intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene.
25:4798–4811. 2006.
|
|
24
|
Buldakov MA, Hassan MA, Zhao QL, et al:
Influence of changing pulse repetition frequency on chemical and
biological effects induced by low-intensity ultrasound in vitro.
Ultrason Sonochem. 16:392–397. 2009.
|
|
25
|
Bai WK, Shen E and Hu B: The induction of
the apoptosis of cancer cell by sonodynamic therapy: a review. Chin
J Cancer Res. 24:368–373. 2012.
|
|
26
|
Jeong EJ, Seo SJ, Ahn YJ, Choi KH, Kim KH
and Kim JK: Sonodynamically induced antitumor effects of
5-aminolevulinic acid and fractionated ultrasound irradiation in an
orthotopic rat glioma model. Ultrasound Med Biol. 38:2143–2150.
2012.
|
|
27
|
Zhai BJ, Shao ZY, Wu F and Wang ZB:
Reversal of multidrug resistance of human hepatocarcinoma HepG2/Adm
cells by high intensity focused ultrasound. Ai Zheng. 22:1284–1288.
2003.(In Chinese).
|
|
28
|
Ninomiya K, Noda K, Ogino C, Kuroda S and
Shimizu N: Enhanced OH radical generation by dual-frequency
ultrasound with TiO2 nanoparticles: its application to targeted
sonodynamic therapy. Ultrason Sonochem. 21:289–294. 2014.
|



















