Introduction
The presence of the CpG island (CGI) methylator
phenotype (CIMP) in colorectal cancers (CRCs) has been supported by
the fact that one group of CRCs has few methylated promoter CGIs
and another group harbors simultaneous aberrant methylation of
multiple promoter CGIs (1,2). CIMP-positive CRCs have distinct
clinical and histological features, including a female predominance
and proximal location, and show genetic characteristics, including
frequent KRAS/BRAF mutations and microsatellite
instability (MSI) (3,4). CIMP is initially defined using
cancer-specific CIMP markers (CDKN2A, MINT1, MINT2, MINT31
and MLH1) in CRCs (2), but
in 2006, Weisenberger et al (5) challenged the application of these
classic CIMP markers and insisted upon the efficacy of novel marker
panels to endorse the CIMP as a distinctive molecular feature of
CRCs. Although based on a systematic analysis of a large number of
CRCs with aberrant methylation of numerous promoter CGIs, later
studies failed to emulate the original results using the same
markers selected by Weisenberger et al (4,6). No
matter how the markers are selected, CIMP is certain to be involved
in CRC development as the third molecular pathway, following
chromosomal instability and MSI. Ogino et al showed that
CIMP-positive CRC was a predictor of a low cancer-specific
mortality rate in a large cohort study (4). By contrast, using different CIMP
marker panels, this characteristic of CIMP-positivity was not
observed in patients with stage III CRCs (7) or stage II/III CRCs treated by surgery
alone (8). The response to
5-fluorouracil (5-FU)-based adjuvant chemotherapy in CIMP-positive
CRC is also contradictory (9–11),
although this therapy is essential to reduce the tumor recurrence
of stage II or III CRC patients following curative resection.
Hypermethylation of promoter CGIs can prevent
transcription of tumor suppressor or mismatch repair genes, such as
MutL homolog 1 (MLH1), and occurs at an early stage of
colorectal carcinogenesis. Methylation of promoter CGIs followed by
transcriptional silencing of MLH1 is present in ~70% of
sporadic MSI CRCs (8,12,13).
However, MLH1 is usually included in CIMP marker sets of
promoter CGIs, and up to 60% of CIMP-positive CRCs have aberrant
methylation of MLH1 (14).
This may be one of the reasons for the clinical and pathological
resemblance between CIMP-positive and MSI CRCs. The high frequency
of serrated polyps with MLH1 gene promoter methylation in
individuals with MSI CRC suggests the presence of a serrated
pathway in colorectal carcinogenesis (15). More recently, genetic and epigenetic
profiles of a variety of colorectal polyps have demonstrated that
sessile serrated adenomas/polyps may be precursor lesions for MSI
CRCs and follow the CIMP pathway (16). Since a considerable fraction of
advanced CRCs in the adenoma-adenocarcinoma sequence had a remnant
adenomatous element within the tumors and coexisting extralesional
adenomas (17), it is important to
examine whether CIMP-positive CRCs have similar morphological
characteristics regarding serrated polyps.
In the present study, a series of CRCs were
retrospectively examined for aberrant methylation using an
alternative panel of promoter CGIs of cancer-related genes. The
panel consisted of promoter CGIs of tumor suppressor genes (p16,
GATA5, TSLC1, HLTF and ID4), DNA repair genes
(MGMT), metastases suppressor genes (TIMP3, CDH4 and
CDH13), angiogenesis inhibitors (TSP1) and genes with
apoptosis-related properties (HRK, CACNA1G and
RSASF1A). The majority of these genes are not involved as
classical or novel CIMP markers of CRC (2,5,14). The
panel also contained two novel genes that were originally cloned in
pancreatic cancer, which were methylated in the cancer cells, but
not in the normal pancreas or colonic mucosa (18). The CIMP was defined by comparing the
observed distribution of CRCs by the number of aberrant
methylations of these genes with the calculated distribution. The
CIMP status was correlated with the methylation status of
MLH1 and with clinicopathological parameters, with
particular reference to neighboring lesions, such as conventional
adenoma and serrated lesions, in and around tumors.
Materials and methods
Patient population and DNA
preparation
Neoplastic specimens were collected from consecutive
patients who underwent CRC resection at Kyushu University Hospital
(Fukuoka, Japan) between 1996 and 2004. From these tumor specimens,
104 CRC frozen tissues were used and the frozen tissue of 15
corresponding normal mucosae were also collected. Clinical data and
the patient status at the last follow-up were obtained from medical
records. Informed consent to harvest the tissue for the studies was
obtained from all patients, and the Kyushu University Hospital
Human Research Ethics Committee approved the study. Genomic DNA was
prepared from cryostat sections of the frozen cancer tissue and
corresponding normal mucosa specimens and was extracted by QIAamp
DNA Mini kit (Qiagen, Hilden, Germany). Hematoxylin and
eosin-stained sections of formalin-fixed and paraffin-embedded
surgical specimens were evaluated to determine tumor
differentiation and stage. All polyps present in the specimen were
also sectioned and prepared for histological examination.
Bisulfite modification and
methylation-specific polymerase chain reaction (MSP) assay
The methylation status of each gene was verified by
MSP, as described by Herman et al (19). Genomic DNA from the cancer tissue
and the normal mucosa was treated with bisulfite for 16 h at 50°C
to convert unmethylated cytosine to thymine. Polymerase chain
reaction (PCR) primers for each gene were designed to be specific
for the methylated sequence and the promoter region of each gene.
Three to six CpG sites were included in each primer pair to achieve
optimal specificity for the determination of methylation. MSP was
carried out on 1 μl bisulfite-treated DNA with the following
amplification conditions: 95°C for 5 min, followed by 40 cycles of
94°C for 30 sec, annealing for 30 sec and 72°C for 30 sec, with a
final extension at 72°C for 5 min. All PCRs were performed with
CpGenome Universal Methylated DNA (Chemicon International,
Temecula, CA, USA) as a positive control for methylated alleles and
with no DNA as a negative control. The primer sequences and the
specific annealing temperatures for the 15 genes and MLH1
are shown in Table I. PCR products
(5 μl) were separated by 3% agarose gel electrophoresis and
visualized under ultraviolet illumination, following ethidium
bromide staining. The presence of PCR products indicated the
presence of methylated template sequences in the original genomic
DNA.
 | Table IMethylated primer sequences and
annealing temperatures used in methylation-specific polymerase
chain reaction. |
Table I
Methylated primer sequences and
annealing temperatures used in methylation-specific polymerase
chain reaction.
| Gene | F/R | Sequences | Temperature, °C | Size, bp |
|---|
| p16 | F |
5′-TAATAGTATTTTTTTCGAGTATTC-3′ | 54 | 123 |
| R |
5′-TTCTTCCTCCGATACTAACG-3′ | | |
| hMLH1 | F |
5′-TAATAGGAAGAGCGGATAGC-3′ | 54 | 106 |
| R |
5′-TCTATAAATTACAAATCTCTTCG-3′ | | |
| TIMP3 | F |
5′-GGGTCGATGAGGTAATGC-3′ | 64 | 116 |
| R |
5′-TACGCCGCTACCTAAACG-3′ | | |
| MGMT | F |
5′-GTTTTAGATTTCGTTTTACGTC-3′ | 54 | 145 |
| R |
5′-CCAAATCGCAAACGATACG-3′ | | |
| TSP1 | F |
5′-GAAAGTTTTTGCGTTATTTCGC-3′ | 64 | 130 |
| R |
5′-CTTAACGCACACGAACTCG-3′ | | |
| CACNA1G | F |
5′-TTTTAGATTCGGTTTTAGTTGC-3′ | 54 | 140 |
| R |
5′-AACTCCGATTACCGAATTCG-3′ | | |
| CDH4 | F |
5′-GTTTTCGGTGTCGGGTATC-3′ | 66 | 105 |
| R |
5′-CGACAACTTACCCGAAACG-3′ | | |
| H-CAD | F |
5′-TTCGCGGGGTTCGTTTTTC-3′ | 67 | 147 |
| R |
5′-AATAAATCAACAACAACATCACG-3′ | | |
| GATA5 | F |
5′-TTCGGGTCGTTGAGGTTTC-3′ | 64 | 140 |
| R |
5′-CAAAATCACGTAACTCTACG-3′ | | |
| RASSF1A | F |
5′-CGAGAGCGCGTTTAGTTTC-3′ | 58 | 103 |
| R |
5′-CAAAATCCAAACTAAACGACG-3′ | | |
| HLTF | F |
5′-CGTTTCGTTGTTATTTAAAGAC-3′ | 60 | 132 |
| R |
5′-CCGCAAACACCGCAATCG-3′ | | |
| HRK | F |
5′-AATTTCGCGTTTTTTAGTTGTC-3′ | 54 | 115 |
| R |
5′-GAAAAAAAAAATTACATCATCCG-3′ | | |
| KIRREL2 | F |
5′-TTGGGGGCGTTTATTCGTC-3′ | 62 | 105 |
| R |
5′-GCCCCCCGAAAACTCCG-3′ | | |
| SLC13A5 | F |
5′-GTTTAGCGTCGAGGTTATC-3′ | 67 | 137 |
| R |
5′-TACGAAACGAAATTATCACCG-3′ | | |
| ID4 | F |
5′-ATTTTTCGTTTTTTAGTATCGTTC-3′ | 62 | 104 |
| R |
5′-ACGCGCGAACCGAATCG-3′ | | |
| TSLC1 | F |
5′-TAATCGTTGTATTAGATCGAC-3′ | 60 | 103 |
| R |
5′-TAAATTTACAACGTCTAATTCG-3′ | | |
Statistical analysis
The primary variable in this study was the
distribution of carcinomas falling into each classification of the
number of aberrantly methylated genes. The observed distribution of
the 104 CRCs was compared with the expected distribution by
χ2 test (goodness-of-fit test) under the assumptions
that promoter methylation of each gene occurred randomly and was
distributed equally in the carcinomas. The association between CIMP
status and clinicopathological parameters was assessed by Fisher’s
exact test, analysis of variance or Mann-Whitney U test. Event time
distributions for overall survival (OS) and relapse-free survival
(RFS) of our 104 CRC patients were estimated with the Kaplan-Meier
method. Hazard ratios (HRs) of tumor-relapse, according to the
clinicopathological features and the CIMP status in tumors, were
analyzed by Cox proportional hazard models. All P-values were
two-sided, and P<0.05 was considered to indicate a statistically
significant difference.
Results
Classification of CIMP and methylation of
each gene in CRCs
The number of aberrantly methylated genes in each
CRC ranged between zero and 14. The expected distribution of
carcinomas having each number of aberrantly methylated genes among
104 CRCs was calculated, assuming that hypermethylation of the 15
genes occurred independently and was spread randomly across the 104
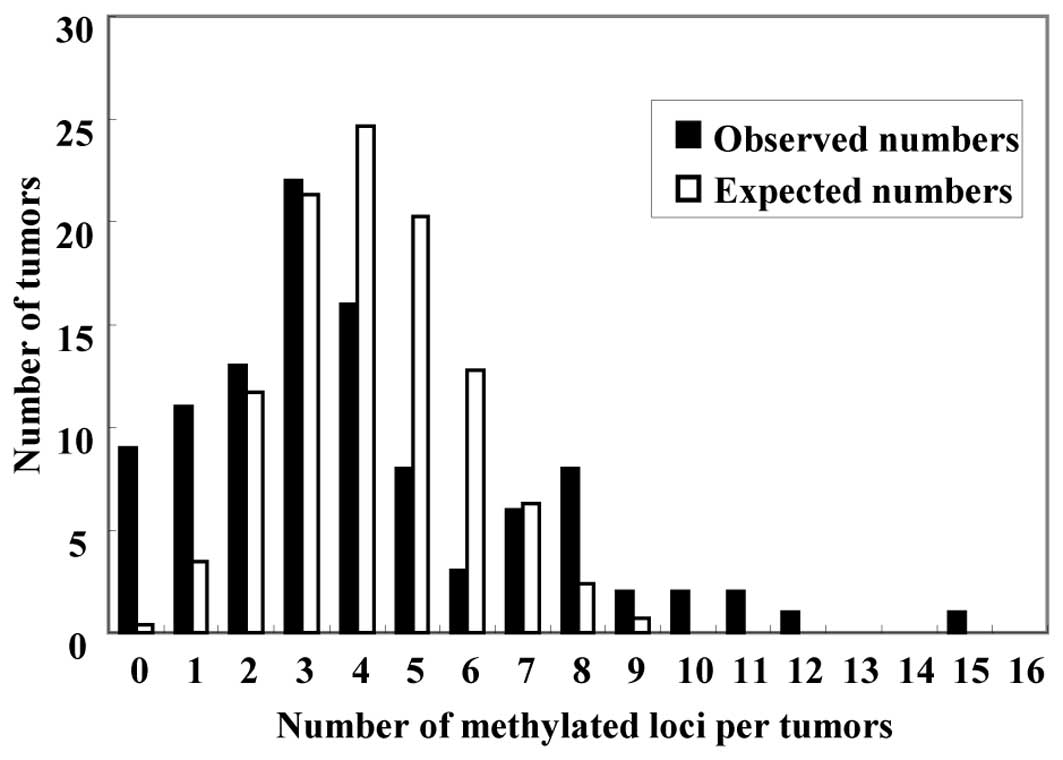
CRCs. The expected distribution followed a unimodal pattern, with
the largest number of carcinomas having four methylated genes
(Fig. 1, white bars). In the
expected distribution, ten CRCs (9.6% of the 104 CRCs) were
predicted to have seven or more methylated genes, and the number of
CRCs without any methylated genes was zero (0% of the 104 CRCs).
The observed distribution of carcinomas having each number of
aberrantly methylated genes did not follow the expected
distribution (Fig. 1, black bars).
Carcinomas with methylation of seven or more of the 15 genes were
classified as CIMP-high (CIMP-H), carcinomas with methylation of
one to six genes were classified as CIMP-low (CIMP-L) and
carcinomas without methylation were classed as CIMP-negative
(CIMP-N). There were 19 (18.3%) CIMP-H CRCs, 76 (73.1%) CIMP-L CRCs
and 9 (8.7%) CIMP-N CRCs. The observed distribution of methylated
genes in each group differed significantly from the expected
distribution (P<0.001), thus, methylation of these genes did not
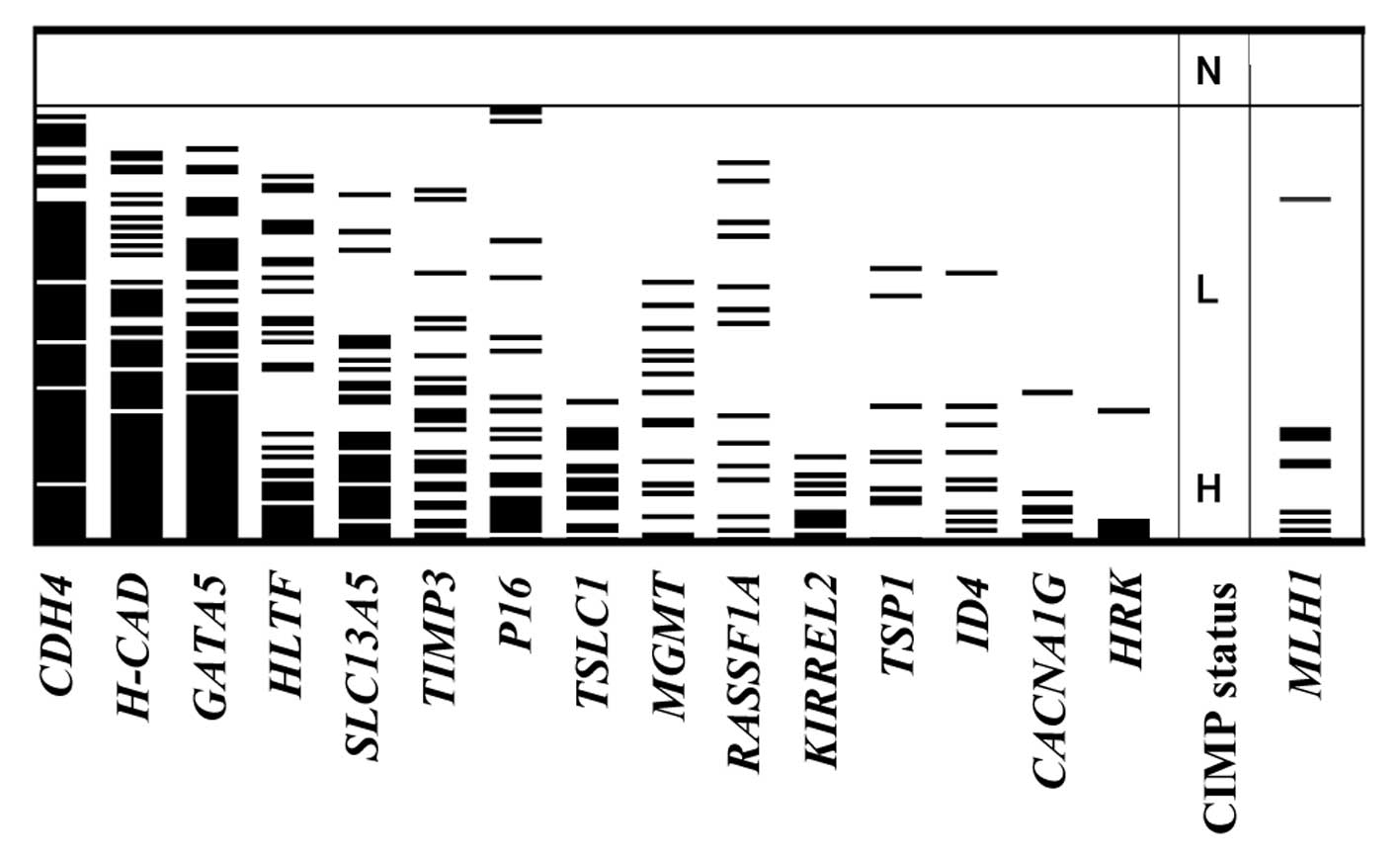
happen at random in the 104 CRCs. Methylation of each gene and the
CIMP status are summarized in Fig.
2. The frequency of hypermethylation of the 15 genes in the 104
CRCs ranged from 5.8% (HRK) to 77.9% (CDH4), whereas
methylated templates were not detected in the 15 normal colonic
mucosae.
CIMP status, clinicopathological
parameters and MLH1 methylation
The association between CIMP status, the
clinicopathological features and MLH1 methylation are shown
in Table II. There was no
significant correlation between the CIMP status and the parameters
among the 104 patients with respect to age, gender, tumor size,
histological tumor grade or tumor stage. In total, 12 patients
exhibited distant metastases, the majority of which were liver
metastases, and three patients presented with peritoneal metastases
at diagnosis. MLH1 methylation was detected in 10 (9.6%) of
104 CRCs. Six of these were classified as CIMP-H (31.6% of 19
CIMP-H tumors) and four were CIMP-L (5.3% of 76 CIMP-L tumors), but
none of the nine CIMP-N tumors exhibited MLH1 methylation
(P=0.005). The patients with CIMP-N CRCs had more frequent distant
metastases compared with those with CIMP-H/L tumors (44.4, 15.8 and
10.5%, respectively, P=0.023). 5-FU-based chemotherapy was
post-operatively performed in 63.2% of patients with CIMP-H CRCs,
64.5% of those with CIMP-L and 77.8% of those with CIMP-N. Within
the median follow-up time of 60 months, two (40.0%) out of five
patients with stage 0-III CIMP-N and 22 (32.4%) out of 68 patients
with stage 0-III CIMP-L developed tumor recurrence following
curative resection, while only one (6.3%) out of 16 patients with
stage 0-III CIMP-H tumors developed recurrence (P=0.093).
 | Table IIAssociation between the CIMP and the
clinicopathological features of 104 colorectal cancers. |
Table II
Association between the CIMP and the
clinicopathological features of 104 colorectal cancers.
| Features | Total | CIMP-N | CIMP-L | CIMP-H | P-valuea |
|---|
| No. of
patients | 104 (100.0) | 9 (8.7) | 76 (73.1) | 19 (18.3) | |
| Mean age,
years | 63.4 | 60.7 | 62.9 | 66.6 | 0.317 |
| Gender, n (%) |
| Male | 51 (49.0) | 5 (55.6) | 35 (46.1) | 10 (52.6) | |
| Female | 53 (51.0) | 4 (44.4) | 41 (53.8) | 9 (47.4) | 0.471 |
| Tumor location, n
(%) |
| Proximal | 42 (40.4) | 1 (11.1) | 32 (42.1) | 9 (47.4) | |
| Distal | 62 (59.6) | 8 (88.9) | 44 (57.9) | 10 (52.6) | 0.118 |
| Mean tumour size,
mm | 48.2 | 47.7 | 49.7 | 43.7 | 0.466 |
| Histology, n
(%) |
|
Differentiated | 90 (86.5) | 7 (77.8) | 67 (91.3) | 16 (84.2) | |
|
Undifferentiated | 14 (13.5) | 2 (22.2) | 9 (8.7) | 3 (15.8) | 0.680 |
| Lymphatic invasion,
n (%) |
| Negative | 51 (49.0) | 5 (55.6) | 38 (50.0) | 8 (42.1) | |
| Positive | 53 (51.0) | 4 (44.4) | 38 (50.0) | 11 (58.9) | 0.760 |
| Venous invasion, n
(%) |
| Negative | 66 (57.4) | 5 (55.6) | 47 (60.3) | 14 (73.7) | |
| Positive | 38 (42.6) | 4 (44.4) | 29 (39.7) | 5 (26.3) | 0.543 |
| Tumor stage, n
(%) |
| 0 | 2 (1.9) | 0 (0.0) | 2 (2.6) | 0 (0.0) | |
| I | 16 (15.4) | 1 (11.1) | 12 (15.8) | 3 (15.8) | |
| II | 32 (30.8) | 3 (33.3) | 21 (20.2) | 8 (42.1) | |
| III | 39 (37.5) | 1 (11.1) | 33 (43.4) | 5 (26.3) | |
| IV | 15 (14.4) | 4 (44.4) | 8 (10.5) | 3 (15.8) | 0.227 |
| Distant metastases
at diagnosis, n (%) |
| Negative | 89 (85.6) | 5 (55.6) | 68 (89.5) | 16 (84.2) | |
| Positive | 15 (14.4) | 4 (44.4) | 8 (10.5) | 3 (15.8) | 0.023 |
| Postoperative
chemotherapy, n (%) |
| No | 35 (33.7) | 2 (22.2) | 26 (34.2) | 7 (36.8) | |
| Yes | 69 (66.3) | 7 (77.8) | 50 (65.8) | 12 (63.2) | 0.719 |
| Tumor
recurrenceb, n (%) |
| Negative | 64 (71.9) | 3 (60.0) | 46 (61.6) | 15 (93.7) | |
| Positive | 25 (28.1) | 2 (40.0) | 22 (32.4) | 1 (6.3) | 0.093 |
| MLH1
methylation, n (%) |
| − | 94 (90.4) | 9 (100.0) | 72 (94.7) | 13(68.4) | |
| + | 10 (9.6) | 0 (0.0) | 4 (5.3) | 6 (31.6) | 0.005 |
The rate of tumor recurrence in the CIMP-N and
CIMP-L tumors was similar; therefore, 104 CRCs were divided into
two groups, CIMP-L/N and CIMP-H, for further survival time
analysis. Kaplan-Meier survival curves representing the OS rates of
all patients and the RFS rate of 89 patients with stage 0-III
tumors, according to CIMP status, are shown in Fig. 3A and B. Patients with CIMP-H CRCs
exhibited a significantly improved RFS rate compared with those
with CIMP-L/N CRCs (Fig. 3B;
five-year RFS rate, 93.8 vs. 67.1%; log-rank test, P=0.044),
although there was no significant difference in OS rate (Fig. 3A; five-year OS rate, 79.0 vs. 68.2%;
P=0.383). Cox regression univariate analysis revealed that CIMP-H
was a better prognostic indicator for tumor recurrence following
curative resection [HR, 0.167; 95% confidence interval (CI),
0.001–0.789], with stage 0-II tumors, an absence of lymphatic and
venous invasion and MLH1 methylation as better prognostic
factors (Table III). Although the
multivariate analysis revealed that tumor stage 0-II was a
significantly better prognostic factor (Table III), the HR of the multivariate
analysis for tumor recurrence in CIMP-H tumors was consistently low
in stage 0-II (HR, <0.001; 95% CI, 0.000–2.281) and in stage III
(HR, 0.455; 95% CI, 0.003–2.228) tumors.
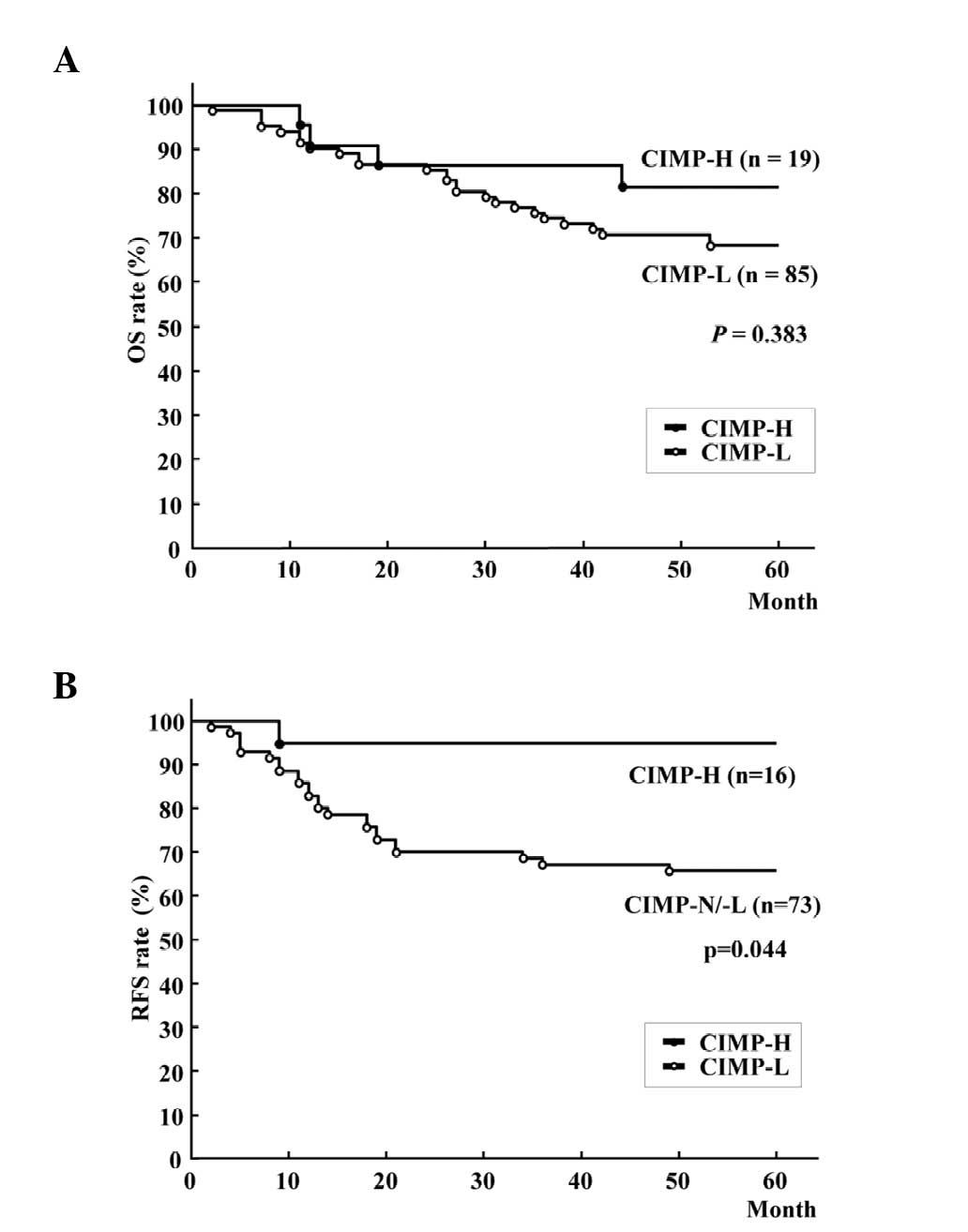 | Figure 3Kaplan-Meier survival curves for (A)
OS rate of all patients and (B) RFS of patients following curative
resection of colorectal cancer. OS rate was not significantly
different between patients with CIMP-H and CIMP-L/-N (five-year OS
rate, 79.0% vs. 68.2%; log-rank test, P=0.383), while patients with
CIMP-H tumors had better RFS rates than those with CIMP-N/L tumors
(five-year RFS rate, 93.8 vs. 67.1%; log-rank test, P=0.044).
CIMP-H, CpG island methylator phenotype-high; CIMP-L, CIMP-low;
CIMP-N, CIMP-negative; RFS, recurrence-free survival; OS, overall
survival. |
 | Table IIIUnivariate and multivariate analysis
of risk factors for recurrence-free survival in stage 0-III
colorectal cancer patients. |
Table III
Univariate and multivariate analysis
of risk factors for recurrence-free survival in stage 0-III
colorectal cancer patients.
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|---|
| Factor | HR | 95% CI | P-value | HR | 95% | CI P-value |
|---|
| Age, years
(>60/≤60) | 0.639 | 0.287–1.409 | 0.264 | | | |
| Gender
(male/female) | 0.541 | 0.229–1.120 | 0.132 | | | |
| Tumor location
(proximal/distal) | 0.683 | 0.278–1.536 | 0.364 | | | |
| Tumor size, mm
(<44/≥44) | 0.961 | 0.435–2.134 | 0.919 | | | |
| Histology
(diff/undiff) | 0.872 | 0.302–3.686 | 0.827 | | | |
| Depth of tumor
(T0–2/T3, T4) | 0.416 | 0.098–1.201 | 0.112 | | | |
| Tumor stage (stage
0-II/III) | 0.247 | 0.096–0.568 | 0.001 | 0.395 | 0.146–0.969 | 0.042 |
| Pathological
lymphatic invasion (negative/positive) | 0.448 | 0.189–0.994 | 0.048 | 0.527 | 0.220–1.187 | 0.123 |
| Pathological venous
invasion (negative/positive) | 0.305 | 0.135–0.674 | 0.004 | 0.503 | 0.212–1.156 | 0.106 |
| Post-operative
chemotherapy (yes/no) | 0.722 | 0.295–1.624 | 0.440 | | | |
| CIMP status
(CIMP-H/L/N) | 0.167 | 0.001–0.789 | 0.019 | 0.292 | 0.016–1.421 | 0.149 |
| MLH1
methylation (positive/negative) | <0.001 | 0.601–0.601 | 0.013 | <0.001 |
<0.001–1.888 | 0.141 |
Coexistent lesions within tumors and in
the normal mucosae surrounding CRC
In total, 11 (10.6%) of 104 CRCs presented with
neighboring conventional adenoma, but no CRC had adjacent serrated
lesions. Among the CRCs with adenoma, two were T0, three were T1,
one was T2 and five were T3. Four (21.1%) out of 19 CIMP-H CRCs
presented with coexistent adenomas, together with six (7.9%) out of
76 CIMP-L and one (11.1%) out of nine CIMP-N CRCs. Two out of four
CIMP-H CRCs with adenoma were located in the right colon and the
others were in the left colon/rectum; two were T1 and two were T3
tumors. One had MLH1 promoter hypermethylation and the
remaining three had no MLH1 hypermethylation. Among the 104
CRC resections, serrated lesions, including five hyperplastic
polyps and one serrated adenoma, were present in the normal mucosae
around the tumors of four specimens, while conventional adenomas
were detected in 25 specimens. In the tumor specimens containing
serrated lesions, two CRCs were located in the right colon and two
in the left colon/rectum. The serrated lesions were present in one
specimen with CIMP-H, in two with CIMP-L and in one with CIMP-N
CRC, while adenomatous lesions were distributed in five specimens
with CIMP-H, 17 with CIMP-L and three with CIMP-N CRC.
Discussion
In the present study, according to the number of
hypermethylations of 15 promoter CGIs, an almost bimodal
distribution of CRCs indicated the presence of the distinct
subclass of CRC, termed CIMP-H, which is recognized by an
accumulation of hypermethylation of promoter CGIs. In other words,
tumor-specific aberrant methylation in promoter CGIs may assemble
itself in CIMP-H and randomly occur in remaining CRCs. Although
this bimodal distribution in tumors has been demonstrated using
several gene marker panels (5,14),
Yamashita et al (20)
doubted the presence of CIMP, claiming that tumor-specific somatic
hypermethylation of six genes (MLH1, p16, p14, MGMT,
APC and CDH1) was an age-dependent feature and that the
distribution of the number of tumors harboring their markers was
normal (20). This inconsistency
could have been due to the different marker panels used in each
study. For instance, APC gene methylation has been inversely
linked to classical CIMP-H CRCs (21). Weisenberger et al
demonstrated the bimodal distribution of tumors using 14 novel CIMP
markers, but the histogram of the methylation frequency of the five
classic CIMP markers showed only one peak (5). In the present study, all 15 markers,
with the exception of one (CACNA1G), differed from
Weisenberger’s 14 markers, while the histograms resembled each
other. Furthermore, Ogino et al also identified that the
CIMP classification error decreased along with an increasing number
of markers from one to seven (14).
Thus, in addition to the selection of markers, the number of
promoter CGIs examined is crucial for the detection of CIMP. The
mechanism of this epigenetic instability is unresolved, thus, the
definition of CIMP cannot be faultless and depends on the
distribution of the methylation frequency of selected markers.
Following the identification of concurrent
methylation of several classic CIMP markers in hyperplastic
polyposis, large hyperplastic polyps and serrated adenomas
(22,23), the serrated lesions, particularly
sessile serrated adenomas/polyps, have been described as
conceivable precursors of the serrated pathway to CIMP-H CRCs
(16,24). In the present study, serrated
lesions contiguous with CRC were not found in any of the CIMP-H
tumors. Although this may account for the notion that the ancestor
lesion would be replaced by an aggressive successor, >20% of
CIMP-H CRCs had concomitant adenomatous lesions that indicated the
adenoma-carcinoma pathway of their tumorigenesis. The definition of
CIMP-H in the present study was different from the classic or novel
CIMP classification, thus the present CIMP-H CRCs may differ from
those on the advocated serrated pathway. Jass (25) proposed that one of the molecular
subtypes of CRCs that is characterized by CIMP-L, KRAS
mutation and microsatellite stable/MSI-L, originates from adenoma
or serrated polyps. Although the present study did not examine this
molecular discrimination, certain CIMP-H CRCs that have coexistent
adenomas can be classified in this subtype. These CIMP-H CRCs may
arise from serrated components of mixed hyperplastic and
adenomatous polyps, but these polyps are rare. Only one CIMP-H CRC
specimen exhibited serrated lesions around the tumor, and each CIMP
subtype did not vary in the frequency of serrated lesions in the
colonic mucosa around the tumors. Thus, the results failed to
support the serrated pathway in CIMP-H CRC tumorigenesis, but
suggested that a certain fraction of CRCs showing promoter
hypermethylation of multiple cancer-related genes is derived from
conventional adenoma.
In univariate analysis, the patients with CIMP-H
tumors exhibited a significantly improved disease-free survival
rate compared with those with CIMP-L/N tumors following curative
resection. Similar results have been obtained from a large cohort
(4), but this prognostic advantage
of CIMP-H is often challenged (7,8,26). Any
attempt to involve MLH1 in the CIMP marker panel (14) and to correlate CIMP-H with
BRAF mutation (5) would
confuse the aforementioned argument further. MLH1
methylation followed by MSI-high (MSI-H) is known to be associated
with a good prognosis in patients with sporadic CRC (27–29),
while BRAF mutation is one of the genetic markers of a
shorter survival time (4,30). Similarly, this controversy over the
prognosis of patients with CIMP-H tumors is occasionally explained
by the presence of the CIMP subtypes harboring various prognostic
features, CIMP-H/MSI-H with frequent BRAF mutation and
CIMP-H/MSS with occasional BRAF, but a dominant KRAS
mutation (31). Patients with MSI-H
CRCs do not benefit from 5-FU-based adjuvant chemotherapy (32,33).
Although CIMP-H was associated with MLH1 methylation in the
present study, MLH1 methylation was not an independent
factor for an improved RFS rate. Additionally, 68.4% of CIMP-H
tumors had no MLH1 methylation and 63.2% of patients with
CIMP-H tumors received post-operative 5-FU-based adjuvant
chemotherapy in the present study. Thus, the improved RFS rates of
patients with CIMP-H tumors was not solely a result of MSI-H
following MLH1 methylation. Iacopetta et al
demonstrated that CIMP-H was a predictor of the survival benefit
from 5-FU-based chemotherapy in CRC patients (9). Therefore, this prognostic advantage of
patients with CIMP-H CRCs could be augmented by 5-FU-based adjuvant
chemotherapy. However, 5-FU-based adjuvant chemotherapy was
recently shown not to improve, but to worsen the disease-free
survival of patients with stage II or III CIMP-positive CRC
(34). The criteria of CIMP, the
proportion of MLH methylation in CIMP-H tumors and the number of
patients receiving adjuvant chemotherapy largely differed between
these studies. Thus, a large cohort study based on universal CIMP
consent using coherent CIMP markers is required to resolve this
critical issue.
Little is known about the CRCs that are without
methylation of any promoter CGIs; named CIMP-N in the present
study. The absence of aberrant methylation of any promoter CGIs in
these patients confers possible global hypomethylation, which has
been often associated with chromosomal instability in CRC (35,36).
Instead, an inverse association between CIMP-H and chromosomal
instability has been shown (37,38).
Similar to the association between the shorter survival time and
LINE-1 hypomethylation among CRC patients (39), frequent metastases at the time of
diagnosis in patients with CIMP-N CRC suggest that CIMP-N tumors
are more aggressive than CIMP-L/H tumors. In the present study, the
histogram of the CIMP-L/N CRCs was almost a Gaussian distribution
and the number of tumors in this subset was small, therefore, the
meaning of this phenotype in CRCs remains uncertain.
There were certain limitations to this study.
Firstly, BRAF and KRAS were not sequenced. Although
the BRAF mutation is one of the genetic traits of CIMP-H
CRCs, the BRAF mutation in CIMP has varied in each study,
ranging from 21.6% using a classic CIMP marker set (26), to 73% using novel markers (5). The mechanism connecting the
BRAF mutation and aberrant methylation of promoter CGIs
remains unclear, and the importance of this mutation to predict
prognosis was not proven in recent larger studies involving
>1,000 CRC patients (27,40).
Additionally, MSI status was not examined in the present study.
Finally, this was a single-institution retrospective study, and the
numbers of patients and genes examined were not sufficient to allow
definitive conclusions.
The panel of promoter CGIs in this study included
KIRREL2 and SLC13A5, which have previously been
identified as the differentially-methylated CGIs in pancreatic
cancer and cloned using methylated CpG island
amplification-representational difference analysis from pancreatic
cancer cell lines (18).
Cancer-specific methylation of these CGIs, loss of expression of
these genes in CRC cell lines that had hypermethylation of these
promoter CGIs and restoration of their expression by
5-aza-2-deoxycytidine treatment (data not shown) suggest possible
involvement of promoter methylation of these genes in colorectal
carcinogenesis. For example, SLC13A5, a member of the solute
carrier (SLC) families and a
Na+/sulfate/selenate/thiosulfate/carboxylate symporter
(41), is one of the hallmarks of
CIMP in renal cell carcinoma (42).
SLC13A5 is differentially methylated between glioblastoma
and normal brain tissue, as shown by whole-genome integrative
analysis (43). Certain SLC family
members increase chemosensitivity against anticancer drugs by
mediating the cellular uptake of hydrophilic drugs (44). One of the sodium transporter
families also has tumor suppressor activity, and aberrant
methylation of promoter CGI is detected in aberrant crypt foci,
which is considered to be the initial lesion of the serrated
adenoma-carcinoma pathway (45).
Thus, future studies on the novel target gene for aberrant promoter
methylation would shed light on our understanding of cancer
epigenetics and the carcinogenesis of CRC.
References
|
1
|
Shen L and Issa JP: Epigenetics in
colorectal cancer. Curr Opin Gastroenterol. 18:68–73. 2002.
|
|
2
|
Toyota M, Ahuja N, Ohe-Toyota M, et al:
CpG island methylator phenotype in colorectal cancer. Proc Natl
Acad Sci USA. 96:8681–8686. 1999.
|
|
3
|
Hawkins N, Norrie M, Cheong K, et al: CpG
island methylation in sporadic colorectal cancers and its
relationship to microsatellite instability. Gastroenterology.
122:1376–1387. 2002.
|
|
4
|
Ogino S, Nosho K, Kirkner GJ, et al: CpG
island methylator phenotype, microsatellite instability, BRAF
mutation and clinical outcome in colon cancer. Gut. 58:90–96.
2009.
|
|
5
|
Weisenberger DJ, Siegmund KD, Campan M, et
al: CpG island methylator phenotype underlies sporadic
microsatellite instability and is tightly associated with BRAF
mutation in colorectal cancer. Nat Genet. 38:787–793. 2006.
|
|
6
|
Shen L, Toyota M, Kondo Y, et al:
Integrated genetic and epigenetic analysis identifies three
different subclasses of colon cancer. Proc Natl Acad Sci USA.
104:18654–18659. 2007.
|
|
7
|
Ahn JB, Chung WB, Maeda O, et al: DNA
methylation predicts recurrence from resected stage III proximal
colon cancer. Cancer. 117:1847–1854. 2011.
|
|
8
|
Van Rijnsoever M, Grieu F, Elsaleh H, et
al: Characterisation of colorectal cancers showing hypermethylation
at multiple CpG islands. Gut. 51:797–802. 2002.
|
|
9
|
Iacopetta B, Kawakami K and Watanabe T:
Predicting clinical outcome of 5-fluorouracil-based chemotherapy
for colon cancer patients: is the CpG island methylator phenotype
the 5-fluorouracil-responsive subgroup? Int J Clin Oncol.
13:498–503. 2008.
|
|
10
|
Shen L, Catalano PJ, Benson AB III, et al:
Association between DNA methylation and shortened survival in
patients with advanced colorectal cancer treated with
5-fluorouracil based chemotherapy. Clin Cancer Res. 13:6093–6098.
2007.
|
|
11
|
Van Rijnsoever M, Elsaleh H, Joseph D, et
al: CpG island methylator phenotype is an independent predictor of
survival benefit from 5-fluorouracil in stage III colorectal
cancer. Clin Cancer Res. 9:2898–2903. 2003.
|
|
12
|
Arnold CN, Goel A, Compton C, et al:
Evaluation of microsatellite instability, hMLH1 expression and
hMLH1 promoter hypermethylation in defining the MSI phenotype of
colorectal cancer. Cancer Biol Ther. 3:73–78. 2004.
|
|
13
|
Samowitz WS, Albertsen H, Herrick J, et
al: Evaluation of a large, population-based sample supports a CpG
island methylator phenotype in colon cancer. Gastroenterology.
129:837–845. 2005.
|
|
14
|
Ogino S, Kawasaki T, Kirkner GJ, et al:
Evaluation of markers for CpG island methylator phenotype (CIMP) in
colorectal cancer by a large population-based sample. J Mol Diagn.
9:305–314. 2007.
|
|
15
|
Hawkins NJ and Ward RL: Sporadic
colorectal cancers with microsatellite instability and their
possible origin in hyperplastic polyps and serrated adenomas. J
Natl Cancer Inst. 93:1307–1313. 2001.
|
|
16
|
Gaiser T, Meinhardt S, Hirsch D, et al:
Molecular patterns in the evolution of serrated lesion of the
colorectum. Int J Cancer. 132:1800–1810. 2013.
|
|
17
|
George SM, Mäkinen MJ, Jernvall P, et al:
Classification of advanced colorectal carcinomas by tumor edge
morphology: evidence for different pathogenesis and significance of
polypoid and nonpolypoid tumors. Cancer. 89:1901–1909. 2000.
|
|
18
|
Ueki T, Toyota M, Skinner H, et al:
Identification and characterization of differentially methylated
CpG islands in pancreatic carcinoma. Cancer Res. 61:8540–8546.
2001.
|
|
19
|
Herman JG, Graff JR, Myöhänen S, et al:
Methylation-specific PCR: a novel PCR assay for methylation status
of CpG islands. Proc Natl Acad Sci USA. 93:9821–9826. 1996.
|
|
20
|
Yamashita K, Dai T, Dai Y, et al: Genetics
supersedes epigenetics in colon cancer phenotype. Cancer Cell.
4:121–131. 2003.
|
|
21
|
Iacopetta B, Grieu F, Li W, et al: APC
gene methylation is inversely correlated with features of the CpG
island methylator phenotype in colorectal cancer. Int J Cancer.
119:2272–2278. 2006.
|
|
22
|
Chan AO, Issa JP, Morris JS, et al:
Concordant CpG island methylation in hyperplastic polyposis. Am J
Pathol. 160:529–536. 2002.
|
|
23
|
Park SJ, Rashid A, Lee JH, et al: Frequent
CpG island methylation in serrated adenomas of the colorectum. Am J
Pathol. 162:815–822. 2003.
|
|
24
|
Young J and Jass JR: The case for a
genetic predisposition to serrated neoplasia in the colorectum:
hypothesis and review of the literature. Cancer Epidemiol
Biomarkers Prev. 15:1778–1784. 2006.
|
|
25
|
Jass JR: Classification of colorectal
cancer based on correlation of clinical, morphological and
molecular features. Histopathology. 50:113–130. 2007.
|
|
26
|
Barault L, Charon-Barra C, Jooste V, et
al: Hypermethylator phenotype in sporadic colon cancer: study on a
population-based series of 582 cases. Cancer Res. 68:8541–8546.
2008.
|
|
27
|
Hutchins G, Southward K, Handley K, et al:
Value of mismatch repair, KRAS, and BRAF mutations in predicting
recurrence and benefits from chemotherapy in colorectal cancer. J
Clin Oncol. 29:1261–1270. 2011.
|
|
28
|
Popat S, Hubner R and Houlston RS:
Systematic review of microsatellite instability and colorectal
cancer prognosis. J Clin Oncol. 23:609–618. 2005.
|
|
29
|
Sinicrope FA, Foster NR, Thibodeau SN, et
al: DNA mismatch repair status and colon cancer recurrence and
survival in clinical trials of 5-fluorouracil-based adjuvant
therapy. J Natl Cancer Inst. 103:863–875. 2011.
|
|
30
|
Samowitz WS, Sweeney C, Herrick J, et al:
Poor survival associated with the BRAF V600E mutation in
microsatellite-stable colon cancers. Cancer Res. 65:6063–6069.
2005.
|
|
31
|
Ang PW, Loh M, Liem N, et al:
Comprehensive profiling of DNA methylation in colorectal cancer
reveals subgroups with distinct clinicopathological and molecular
features. BMC Cancer. 10:2272010.
|
|
32
|
Carethers JM, Smith EJ, Behling CA, et al:
Use of 5-fluorouracil and survival in patients with
microsatellite-unstable colorectal cancer. Gastroenterology.
126:394–401. 2004.
|
|
33
|
Ribic CM, Sargent DJ, Moore MJ, et al:
Tumor microsatellite-instability status as a predictor of benefit
from fluorouracil-based adjuvant chemotherapy for colon cancer. N
Engl J Med. 349:247–57. 2003.
|
|
34
|
Jover R, Nguyen TP, Perez-Carbonell L, et
al: 5-Fluorouracil adjuvant chemotherapy does not increase survival
in patients with CpG island methylator phenotype colorectal cancer.
Gastroenterology. 140:1174–1181. 2011.
|
|
35
|
Matsuzaki K, Deng G, Tanaka H, et al: The
relationship between global methylation level, loss of
heterozygosity, and microsatellite instability in sporadic
colorectal cancer. Clin Cancer Res. 11:8564–8569. 2005.
|
|
36
|
Rodriguez J, Frigola J, Vendrell E, et al:
Chromosomal instability correlates with genome-wide DNA
demethylation in human primary colorectal cancers. Cancer Res.
66:8462–8468. 2006.
|
|
37
|
Derks S, Postma C, Carvalho B, et al:
Integrated analysis of chromosomal, microsatellite and epigenetic
instability in colorectal cancer identifies specific associations
between promoter methylation of pivotal tumour suppressor and DNA
repair genes and specific chromosomal alterations. Carcinogenesis.
29:434–439. 2008.
|
|
38
|
Goel A, Nagasaka T, Arnold CN, et al: The
CpG island methylator phenotype and chromosomal instability are
inversely correlated in sporadic colorectal cancer.
Gastroenterology. 132:127–138. 2007.
|
|
39
|
Ogino S, Nosho K, Kirkner GJ, et al: A
cohort study of tumoral LINE-1 hypomethylation and prognosis in
colon cancer. J Natl Cancer Inst. 100:1734–1738. 2008.
|
|
40
|
Roth AD, Tejpar S, Delorenzi M, et al:
Prognostic role of KRAS and BRAF in stage II and III resected colon
cancer: results of the translational study on the PETACC-3, EORTC
40993, SAKK 60–00 trial. J Clin Oncol. 28:466–474. 2010.
|
|
41
|
He L, Vasiliou K and Nebert DW: Analysis
and update of the human solute carrier (SLC) gene superfamily. Hum
Genomics. 3:195–206. 2009.
|
|
42
|
Arai E, Chiku S, Mori T, et al:
Single-CpG-resolution methylome analysis identifies
clinicopathologically aggressive CpG island methylator phenotype
clear cell renal cell carcinomas. Carcinogenesis. 33:1487–1493.
2012.
|
|
43
|
Etcheverry A, Aubry M, de Tayrac M, et al:
DNA methylation in glioblastoma: impact on gene expression and
clinical outcome. BMC genomics. 11:7012010.
|
|
44
|
Huang Y and Sadée W: Membrane transporters
and channels in chemoresistance and -sensitivity of tumor cells.
Cancer Lett. 239:168–182. 2006.
|
|
45
|
Li H, Myeroff L, Smiraglia D, et al:
SLC5A8, a sodium transporter, is a tumor suppressor gene silenced
by methylation in human colon aberrant crypt foci and cancers. Proc
Natl Acad Sci USA. 100:8412–8417. 2003.
|