Introduction
Invasive ductal carcinoma (IDC) of the breast is a
malignant disease, which affects numerous females worldwide
(1). Chemotherapy in the
neoadjuvant and adjuvant settings is widely administered for the
treatment of breast cancer (2).
However, despite its success, resistance to chemotherapeutic agents
is a common occurrence that is often attributable to mechanisms of
multidrug resistance (MDR) (3,4).
Although proteins that mediate this resistance mechanism have been
identified and have the potential to serve as biomarkers or
prognostic indicators of outcome, the function of these MDR-related
proteins (MRPs) in IDC of the breast has not been extensively
investigated.
The critical proteins that mediate MDR in tumors
include MRP, p-glycoprotein (P-gp), topoisomerase 2α (Topo2α),
thymidylate synthase (TS) and glutathione-S-transferase π
(GST-π). A number of these proteins have been investigated in other
tumor types, including esophageal, colorectal and endometrial
cancer. In numerous instances, resistance is achieved by an
increased efflux of chemotherapeutic agents out of the tumor.
Occasionally, this is an acquired problem, as while certain tumors
are initially responsive, they become resistant following prolonged
treatment. In other cases, tumors fail to respond to therapy at
all, a mechanism known as de novo resistance (5).
The function of these MRPs in IDC of the breast has
not been extensively investigated. Notably, the expression of MRP,
P-gp, Topo2α, TS and GST-π exhibit the potential to serve as
biomarkers for the disease and have prognostic significance. The
aim of this study was to examine the expression of MRP, P-gp,
Topo2α, TS and GST-π in breast IDC, and assess their association
with clinicopathological variables, as well as their prognostic
significance. The results may aid clinicians in the design of
unique treatment regimens for each individual patient.
Materials and methods
Patients and specimens
Samples were obtained from patients with IDC of the
breast who underwent primary surgery at Beijing Tiantan Hospital,
Capital University of Medical Sciences (Beijing, China) between
2005 and 2007.
Prior to patient enrolment, the expression of MRP,
P-gp, Topo2α, TS, GST-π, ER, PR, HER2 and pP53 was analyzed by
staining the excised tumor tissue. In total, samples from 156
female patients were analyzed. The patient age ranged between 32
and 75 years (median age, 52 years). No distant metastases were
detected in any patients during pre-operative examination.
Lumpectomy and axillary dissection was performed in 20 cases,
radical mastectomy in 24 cases and modified radical mastectomy in
112 cases. Immunohistochemical staining was performed at Beijing
Tiantan Hospital. The clinicopathological data are shown in
Table I.
 | Table ICorrelation between MRP, P-gp,
Topo-2α, TS and GST-π and the clinicopathological variables and
status of ER, PR, HER2 and p53. |
Table I
Correlation between MRP, P-gp,
Topo-2α, TS and GST-π and the clinicopathological variables and
status of ER, PR, HER2 and p53.
| | MRP | P-gp | Topo-2α | TS | GST-π |
|---|
| |
|
|
|
|
|
|---|
| Variable | n | +/++/+++, n (%) | χ2 | P-value | +/++/+++, n (%) | χ2 | P-value | +/++/+++, n (%) | χ2 | P-value | +/++/+++, n (%) | χ2 | P-value | +/++/+++, n (%) | χ2 | P-value |
|---|
| Age, years | | | 0.177 | 0.674 | | 0.556 | 0.456 | | 1.626 | 0.202 | | 1.442 | 0.230 | | 0.032 | 0.859 |
| <50 | 68 | 15 (22.1) | | | 19 (27.9) | | | 60 (88.2) | | | 32 (47.1) | | | 27 (39.7) | | |
| ≥50 | 88 | 17 (19.3) | | | 20 (22.7) | | | 71 (80.7) | | | 33 (37.5) | | | 37 (42.0) | | |
| Tumor size, cm | | | 1.546 | 0.214 | | 1.074 | 0.300 | | 1.897 | 0.168 | | 1.979 | 0.160 | | 0.891 | 0.345 |
| ≤2.0 | 63 | 16 (25.4) | | | 13 (20.6) | | | 56 (88.9) | | | 22 (34.9) | | | 23 (36.5) | | |
| >2.0 | 93 | 16 (17.2) | | | 26 (28.0) | | | 75 (80.7) | | | 43 (46.2) | | | 41 (44.1) | | |
| Lymph nodes | | | 1.403 | 0.236 | | 3.280 | 0.070 | | 0.060 | 0.806 | | 42.281 | 0.000a | | 0.071 | 0.790 |
| Negative | 97 | 17 (17.5) | | | 29 (29.9) | | | 82 (84.5) | | | 21 (21.6) | | | 39 (40.2) | | |
| Positive | 59 | 15 (25.4) | | | 9 (15.3) | | | 49 (83.1) | | | 44 (74.6) | | | 25 (42.4) | | |
| Clinical Stages | | | 0.693 | 0.707 | | 0.789 | 0.674 | | 2.548 | 0.280 | | 1.450 | 0.484 | | 0.164 | 0.921 |
| I | 23 | 5 (21.7) | | | 7 (30.4) | | | 18 (78.3) | | | 8 (34.8) | | | 9 (39.1) | | |
| II | 97 | 18 (18.6) | | | 22 (22.7) | | | 85 (87.6) | | | 44 (45.4) | | | 41 (42.3) | | |
| III | 36 | 9 (25.0) | | | 10 (27.8) | | | 28 (77.8) | | | 13 (36.1) | | | 14 (38.9) | | |
| Histological
grade | | | 0.080 | 0.961 | | 20.226 | 0.000a | | 4.460 | 0.108 | | 3.855 | 0.146 | | 35.032 | <0.001a |
| I | 37 | 7 (18.9) | | | 4 (10.8) | | | 27 (73.0) | | | 11 (29.7) | | | 15 (40.5) | | |
| II | 91 | 19 (20.9) | | | 19 (20.9) | | | 79 (86.8) | | | 39 (42.9) | | | 24 (26.4) | | |
| III | 28 | 6 (21.4) | | | 16 (57.1) | | | 25 (89.3) | | | 15 (53.6) | | | 25 (89.3) | | |
| ER status | | | 0.685 | 0.164 | | 0.010 | 0.921 | | 1.799 | 0.180 | | 2.572 | 0.109 | | 17.407 | <0.001a |
| +/++/+++ | 107 | 21 (19.6) | | | 27 (25.2) | | | 87 (81.3) | | | 40 (37.4) | | | 32 (29.9) | | |
| − | 49 | 11 (22.4) | | | 12 (24.5) | | | 44 (89.8) | | | 25 (51.0) | | | 32 (65.3) | | |
| PR status | | | 0.288 | 0.592 | | 3.174 | 0.075 | | 2.288 | 0.130 | | 2.623 | 0.105 | | 3.101 | 0.078 |
| +/++/+++ | 91 | 20 (22.0) | | | 18 (19.8) | | | 73 (80.2) | | | 33 (36.3) | | | 32 (35.2) | | |
| − | 65 | 12 (18.5) | | | 21 (32.3) | | | 58 (89.2) | | | 32 (49.2) | | | 32 (49.2) | | |
| HER-2 status | | | 0.003 | 0.959 | | 2.261 | 0.133 | | 0.109 | 0.741 | | 0.012 | 0.912 | | 3.026 | 0.082 |
| 2+/3+ | 64 | 13 (20.3) | | | 20 (31.3) | | | 53 (82.8) | | | 27 (42.2) | | | 21 (32.8) | | |
| 0/1+ | 92 | 19 (20.7) | | | 19 (20.7) | | | 78 (84.8) | | | 38 (41.3) | | | 43 (46.7) | | |
| p53 status | | | 1.887 | 0.170 | | 0.103 | 0.749 | | 0.143 | 0.705 | | 0.712 | 0.399 | | 1.272 | 0.259 |
| +/++/+++ | 117 | 27 (23.1) | | | 30 (25.6) | | | 99 (84.6) | | | 51 (43.6) | | | 45 (38.5) | | |
| − | 39 | 5 (12.8) | | | 9 (23.1) | | | 32 (82.1) | | | 14 (35.9) | | | 19 (48.7) | | |
The follow-up period consisted of the time from the
first day following surgery until December 2012. Survival time was
calculated from the first day following surgery until mortality or
the last follow-up. The study was approved by the ethics committee
of Beijing Tiantan Hospital Affiliated to Capital Medical
University (Beijing, China). All patients provided written informed
consent.
Immunohistological analysis
All tumor tissues were fixed in neutral buffered 4%
formaldehyde and embedded in paraffin. Immunohistochemical staining
was performed using an avidin-biotin peroxidase system (SP-9000
kit; Zhongshan Goldenbridge Biotechnology Co., Ltd., Beijing,
China). All primary antibodies and reagents were purchased from
Beijing Zhongshan Goldenbridge Biotechnology Co., Ltd. The
following monoclonal antibodies were used: MRP (OCRL-1), P-gp
(C494), Topo2α (3F6), TS (TS106), GST-π (LW29), ER (1D5), PR (1A6),
HER2 (CB11) and p53 (DO7). Antigen retrieval for all proteins, with
the exception of GST-π, was performed in citrate buffer (pH 6.0) by
autoclaving for 180 sec at 100°C. Staining was performed using the
LabVision Autostainer360 System (Maixin. Bio. Co. Ltd., Fuzhou,
China).
Positive staining of the tumor cells was determined
by the appearance of a brown-yellow color. Protein staining scores
were defined as follows: 0, negative or <10% of tumor cells
stained positive; +1, 10–25% of cells stained positive; +2, 26–75%
of cells stained positive; and +3, >75% of cells stained
positive. HER-2 overexpression was scored based on the degree of
membrane staining according to the manufacturer’s instructions for
the HercepTest (6). The following
parameters were applied for the assessment of HER-2 expression: 0,
no membrane staining or membrane staining in <10% of tumor
cells; 1+, faint/barely perceptible partial membrane staining in
>10% of tumor cells; 2+, weak to moderate staining of the entire
membrane in >10% of tumor cells; and 3+, marked staining of the
entire membrane in >10% of tumor cells. A score of either 0 or
1+ was considered negative and scores of 2+ and 3+ were considered
positive for HER2 overexpression. For each sample, ≥10 fields
(magnification, ×200) were randomly selected for analysis, whereby
>500 positive cells were counted, and the average was
calculated. Scores were assigned independently by two different
pathologists. In the case of a discordant result, additional fields
were counted and analyzed.
Statistical analysis
Statistical analyses were carried out using SPSS
19.0 software (SPSS Inc., Chicago, IL, USA). Pearson’s
χ2 test and Spearman’s correlation coefficient were used
to analyze the association between MDR protein expression and
clinicopathological variables, as well as ER, PR, HER2 and p53
status. Similar calculations were performed to assess the
association between the five MDR proteins analyzed in the study.
Survival analysis was used to determine prognostic significance
using Kaplan-Meier analysis and the Cox regression model. P<0.05
was considered to indicate a statistically significant
difference.
Results
Patient characteristics
A total of 23 (14.7%) cases exhibited stage I
disease, 97 cases (62.2%) exhibited stage II disease and 36 cases
(23.1%) presented with stage III. The median follow-up time was 61
months (range, 7–94 months). No patients were lost to follow-up. A
total of 132 patients (84.6%) received adjuvant chemotherapy,
including anthracycline-based compounds (111 cases in total; 98
cases received anthracycline drugs + cyclophosphamide +
fluorouracil, and 13 cases received anthracyclines + paclitaxel) or
a cyclophosphamide + methotrexate + fluorouracil-based regimen for
4–8 cycles (21 cases). Additionally, 33 cases (21.2%) received
adjuvant radiotherapy (60Co or linear accelerator) at a dose 50 Gy,
or 60 Gy for breast-conserving surgery. A total of 46 cases (29.5%)
exhibited recurrent metastasis. Of these, 19 cases exhibited
metastasis to the lung, 16 cases exhibited liver metastasis, 9
cases exhibited bone metastasis and two cases exhibited brain
metastasis. A total of 43 mortalities occurred, including five
mortalities due to non-cancer-associated causes, such as
cardiovascular disease (Table
I).
Association between MDR protein
expression and clinicopathological variables, including HER-2, ER,
PR and p53 status
The expression of MRP, P-gp, Topo2α, TS and GST-π
was detected in 20.5% (32/156), 25.0% (39/156), 84.0% (131/156),
41.7% (65/156) and 41.0% (64/156) of cases examined, respectively.
Representative staining for each of the aforementioned proteins are
shown in Fig. 1. Pearson
χ2 analysis revealed that MRP and Topo2α protein
expression did not correlate with patient age, tumor size, axillary
lymph node metastasis, histological grade, HER-2 overexpression or
expression status of ER, PR and p53. P-gp expression was
significantly higher in grade III tumors compared with grade I
(χ2=16.060; P<0.001) and grade II
(χ2=13.563; P<0.001) tumors. No significant
difference in GST-π staining was identified between grade I and II
tumors (χ2=2.492; P=0.114), however, there was a
significant difference between tumors of grades I and III
(χ2=16.001; P<0.001) and II and III
(χ2=34.998; P<0.001). GST-π expression was highest in
grade III IDC breast tissue. GST-π expression was also increased in
ER-negative tumors (65.3%; χ2=17.407; P<0.001) with a
Spearman’s correlation coefficient of −0.437 (P<0.001). TS
expression (74.6%; 44/59 cases) was increased in breast cancer
cases with axillary lymph node metastasis (χ2=42.281;
P<0.001) (Table I).
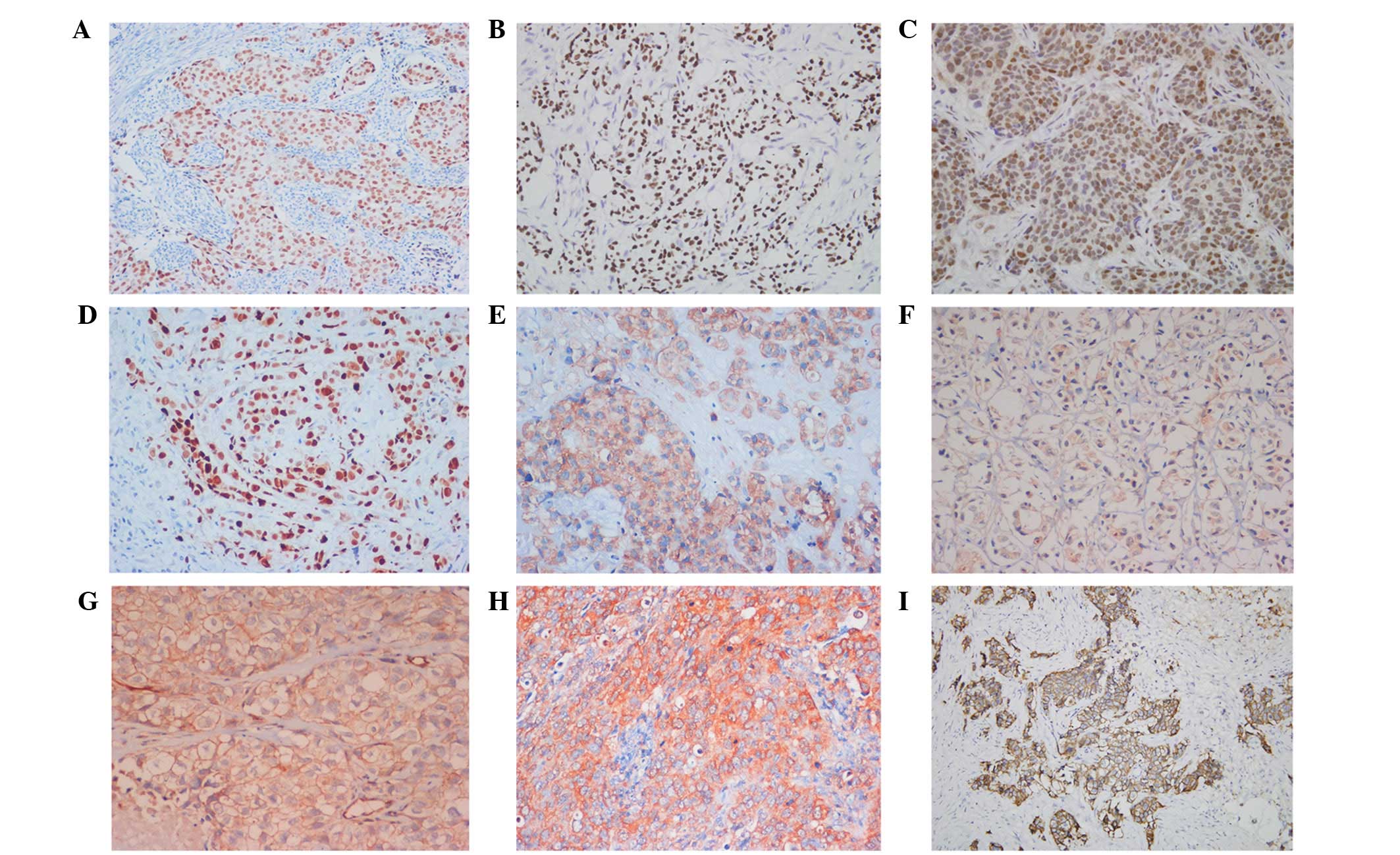 | Figure 1Positive immunohistochemical staining
of (A) ER, (B) PR, (C) p53, (D) Topo2α, (E) GST-π, (F) TS, (G) P-gp
and (H) MRP, and (I) HER-2 overexpression in invasive ductal
carcinoma of breast (streptomycin avidin-peroxidase, magnification,
×200). ER, estrogen receptor; PR, progesterone receptor; Topo2α,
topoisomerase 2α; GST-π, glutathione-S-transferase; TS,
thymidylate synthase; P-gp, p-glycoprotein; MRP, multidrug
resistance-related protein; HER-2, human epidermal growth factor
receptor 2. |
Association between the expression of
MRP, TS, Topo2α, P-gp and GST-π proteins
Pearson’s χ2 test was performed to
examine the associations between the five MRPs. No significant
correlation was identified between MRP protein expression and the
expression of TS (χ2=3.523; P=0.061), Topo2α
(χ2=2.409; P=0.121), P-gp (χ2=3.355; P=0.067)
or GST-π (χ2=2.769; P=0.096). Furthermore, no
significant association was identified between TS expression and
the expression of Topo2α (χ2=1.308; P=0.253), P-gp
(χ2=1.064; P=0.302) or GST-π (χ2=2.047;
P=0.153). Similarly, no significant correlation was identified
between Topo2α and GST-π (χ2=0.599; P=0.439) or P-gp
(χ2=0.397; P=0.529). However, a significant positive
correlation was identified between P-gp and GST-π
(χ2=20.348; P<0.001) with a Spearman’s correlation
coefficient of 0.319 (P<0.001).
TS and GST-π expression are associated
with poor overall survival
Positive staining of MRP, P-gp and Topo2α were not
found to significantly correlate with changes in overall survival
(Fig. 2). Kaplan-Meier
survival analyses revealed that patients with tumor specimens that
stained positive for TS expression exhibited significantly poorer
overall survival rates than patients with TS-negative tumors
(P=0.001; Fig. 2B). Similarly,
patients with GST-π-positive tumors had a poorer overall survival
rate compared with patients with GST-π-negative breast tumors
(P=0.001; Fig. 2C). TS and GST-π
were then examined to determine whether they represent independent
prognostic factors in the disease. As shown in Table II, Cox univariate analysis revealed
that positive staining for TS or GST-π was associated with a
significantly increased risk of mortality in breast carcinoma
patients (TS, P=0.002; GST-π, P=0.001). These factors were also
positively associated with histological grade (P<0.001). Cox
multivariate analysis indicated that TS and GST-π were independent
prognostic factors (P=0.018 and P=0.001, respectively) and that
tumor grade was a predictor of a poor survival outcome
(P<0.001).
 | Table IICox univariate and multivariate
analysis of five-year overall survival on MDR proteins and
clinicopathological variables. |
Table II
Cox univariate and multivariate
analysis of five-year overall survival on MDR proteins and
clinicopathological variables.
| MDR proteins and
clinicopathological variables | Univariate | Multivariate |
|---|
|
|
|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| MRP | 1.023
(0.739–1.865) | 0.905 | 1.281
(0.806–2.036) | 0.295 |
| TS | 1.634
(0.988–2.161) | 0.002a | 1.481
(1.070–2.048) | 0.018a |
| Topo2α | 1.291
(0.809–2.059) | 0.284 | 1.557
(0.949–2.555) | 0.080 |
| P-gp | 1.032
(0.845–1.096) | 0.855 | 0.753
(0.506–1.120) | 0.161 |
| GST-π | 1.683
(0.917–2.325) | 0.001a | 1.853
(1.284–2.674) | 0.001a |
| Age | 0.976
(0.872–1.085) | 0.115 | 1.016
(0.966–1.069) | 0.538 |
| Menstrual | 1.789
(0.645–7.016) | 0.060 | 1.862
(0.551–6.295) | 0.317 |
| Tumor size | 1.002
(0.502–1.592) | 0.995 | 0.959
(0.482–1.908) | 0.905 |
| Lymph node | 1.660
(0.635–3.194) | 0.098 | 1.528
(0.719–3.243) | 0.271 |
| Histological
grade | 3.471
(1.122–4.125) | <0.001a | 3.089
(1.819–5.243) | <0.001a |
Discussion
MDR is a common mechanism by which tumor cells
become resistant to numerous chemotherapeutic agents. While this
MDR response is multi-faceted, it typically involves the
upregulation of several key proteins that promote the efflux of
drugs out of tumor cells, decreasing their biological efficacy. In
the present study, the expression of several key MDR-associated
proteins, including MRP, P-gp, Topo2α, TS and GST-π, was
investigated in IDC of the breast. The study analyzed the
expression of these factors together with several
clinicopathological variables and overall survival. We hypothesized
that the expression of these factors may be useful as biomarkers of
the disease and may explain the occurrence of chemotherapy
resistance.
The expression of one of the proteins examined,
P-gp, was significantly higher in grade III tumors compared with
grade I and II tumors. Previous studies have demonstrated that this
protein promotes the efflux of a number of anticancer drugs,
including anthracyclines, vinca alkaloids, taxanes,
epipodophyllotoxins and doxorubicin, out of tumor cells (7,8). P-gp
is also involved in the secretion of anticancer agents into bile,
urine and the intestinal lumen, which markedly affects the
pharmacokinetic properties and bioavailability of therapeutically
administered compounds (9). Linn
et al (10) evaluated P-gp
expression in 92 primary and 12 metastatic breast cancers and found
that P-gp expression was associated with a poor prognosis. In
another study, metastatic breast cancer patients negative for P-gp
expression (P=0.06) exhibited a longer progression-free survival
time following docetaxel treatment compared with patients
exhibiting P-gp-positive tumors (11). In the present study, P-gp and GST-π
expression were found to be positively correlated (r=0.319;
P<0.0001). Additionally, the expression of the two proteins was
elevated in grade III tumors. Consistent with the findings of the
present study, Cui et al (12) identified a positive correlation
(r=0.429; P<0.01) between P-gp and GST-π in 76 breast cancer
patients prior to treatment. Furthermore, another study
investigated the correlation between the expression of P-gp, GST
and metallothioneins (MTs) and the response to various chemotherapy
regimens in triple--negative (ER-, PR- and HER2-negative) breast
cancer patients (13). The
chemotherapy-treated groups demonstrated improved three-year
relapse-free survival rates (P<0.05), which were associated with
the expression of P-gp, GST and MT. These results, in addition to
the results of the present study, indicate a function for these
MRPs in the outcome of breast cancer and its response to
chemotherapy.
As aforementioned, GST-π expression was highest in
grade III IDC breast tumors and also increased in ER-negative
disease. Notably, patients with tumors staining positive for GST-π
were also found to exhibit poorer overall survival rates than
patients with GST-π-negative tumors. Thus, the results of this
study indicated that GST-π is associated with a worse prognosis and
may potentially drive disease progression. These findings are
supported by previous studies. For example, resistance against
drugs and environmental insults is conferred by the glutathione
metabolic pathway (4). The GSTs are
a family of enzymes involved in the metabolism of a broad range of
xenobiotics, which have been shown to inactivate platinum drugs,
doxorubicin, cyclophosphamide and etoposide (14,15).
In a previous study, the expression of GST-π was investigated in 21
primary untreated human breast tumors (16) and in agreement with the findings of
the present study, the mean expression of GST-π in ER-negative
tumors was found to be 5-fold greater than the mean expression in
ER-positive tumors. These findings were also consistent with
another study examining 189 breast cancer cases (17). Overall, patients with ER-negative
tumors may exhibit increased resistance to chemotherapeutic
regimens due to increased GST-π levels. The results of the present
study indicate an association between ER and GST-π, which requires
additional investigation in the future.
Topo2 is an essential nuclear DNA-binding enzyme
that controls and modifies the topological states of DNA by
combining nuclease, helicase and ligase activities (18). Topo2α is a specific isoform that is
located on chromosome 17q21 in close proximity to HER-2. The exact
association between Topo2α and HER-2 remains unclear. While certain
studies have found no association between Topo2α and HER2
overexpression (19–21), other studies have reported that the
increased expression of Topo2α is associated with HER-2
amplification or overexpression (22,23).
To better define the clinical relevance of Topo2α expression,
larger prospective studies are required. In this study, positive
staining for Topo2α was identified in 84.0% (131/156) of the cases
examined, indicating that Topo2α expression may be significant in
breast cancer. Notably, Mukherjee et al (24) revealed that the expression of Topo2α
prior to the administration of chemotherapy significantly
correlated with the pathological complete response to neoadjuvant
anthracycline treatment. Another study reported that ER is an
independent predictive factor for pathological response to three
different pre-operative chemotherapy regimens in primary breast
tumors (25), however, the
expression of PR, Topo2, P-gp, MRP and GST-π were not predictive of
the pathological response to the three treatment regimens.
TS is a folate-dependent enzyme involved in
pyrimidine synthesis that is crucial for cellular proliferation and
growth (26). TS also catalyzes the
methylation of deoxyuridine monophosphate to deoxythymidine
monophosphate, an essential precursor of DNA biosynthesis (27). In the present study, TS expression
was found to be elevated in cases of invasive breast carcinoma with
lymph node metastasis. Thus, these breast cancers are more
aggressive and exhibit a poorer overall prognosis. Furthermore, a
similar association between TS levels and prognosis has been
reported in other tumor types, including colorectal, rectal and
gastric cancers (28–30). TS overexpression is a biomarker of
5-fluorouracil (5-FU) resistance in human cancer cells (31). However, 5-FU combined with low-dose
trichostatin A (50 nmol/l) has been shown to restore 5-FU-mediated
cytotoxicity in 5-FU-resistant cancer cells in combination with the
downregulation of TS protein expression (31). In another study of advanced-stage
breast cancer patients, lower TS levels were associated with an
improved response to the chemotherapy drug pemetrexed.
Additionally, in a number of patients, continuous administration of
pemetrexed has been found to decrease TS levels (32). Brandi et al (33) revealed that patients with low levels
of TS and high levels of p53 responded better to docetaxel.
Overall, these results indicate that low levels of TS may be
associated with an improved breast cancer prognosis and response to
chemotherapy administration.
In conclusion, the assessment of MDR protein
expression in breast cancer may be a useful predictor of prognosis
and the response to chemotherapy. Ultimately, this information may
aid clinicians in the design of unique treatment regimens for each
individual patient.
References
|
1
|
Liu H, Liu Y and Zhang JT: A new mechanism
of drug resistance in breast cancer cells: fatty acid synthase
overexpressionme-mediated palmitate overproduction. Mol Cancer
Ther. 7:263–270
|
|
2
|
Cance WG, Carey LA, Calvo BF, et al:
Long-term outcome of neoadjuvant therapy for locally advanced
breast carcinoma: effective clinical downstaging allows breast
preservating and predicits outstanding local control and survival.
Ann Surg. 236:295–303. 2002.
|
|
3
|
Sachelarie I, Grossbard ML, Chadha M, et
al: Primary systemic therapy of breast cancer. Oncologist.
11:574–589. 2006.
|
|
4
|
LaPensee EW and Ben-Jonathan N: Novel
roles of prolactin and estrogens in breast cancer: resistance to
chemotherapy. Endocr Relat Cancer. 17:R91–R107. 2010.
|
|
5
|
Coley HM: Mechanisms and strategies to
overcome chemotherapy resistance in metastatic breast cancer.
Cancer Treat Rev. 34:378–390. 2008.
|
|
6
|
Jacobs TW, Gown AM, Yaziji H, Barnes MJ
and Schnitt SJ: Specificity of HercepTest in determining HER-2/neu
status of breast cancers using the United States Food and Drug
Administration-approved scoring system. J Clin Oncol. 17:1983–1987.
1999.
|
|
7
|
Gottesman MM, Fojo T and Bates SE:
Multidrug resistance in cancer: role of ATP-dependent transporters.
Nat Rev Cancer. 2:48–58. 2002.
|
|
8
|
Sekine I and Saijo N: Polymorphisms of
metabolizing enzymes and transporter proteins involved in the
clearance of anticancer agents. Ann Oncol. 12:1515–1525. 2001.
|
|
9
|
Orlowski S and Garrigos M: Multiple
recognition of various amphiphilic molecules by the multidrug
resistance P-glycoprotein: molecular mechanisms and pharmacological
consequences coming from functional interactions between various
drugs. Anticancer Res. 19:3109–3123. 1999.
|
|
10
|
Linn SC, Giaccone G, van Diest PJ, et al:
Prognostic relevance of P-glycoprotein expression in breast cancer.
Ann Oncol. 6:679–685. 1995.
|
|
11
|
Savas B, Bozcuk H, Özdo M, et al:
Molecular and clinical parameters which determine the docetaxel
response in metastatic breast cancer. J Clin Oncol.
22:97322004.
|
|
12
|
Cui SD, Liu ZZ, Liu H, Li LF, Yang H and
Li WL: The relationship between 99mTc-MIBI scintimammography of
breast cancer and multidrug-resistant proteins. Zhonghua Zhong Liu
Za Zhi. 27:606–608. 2005.(In Chinese).
|
|
13
|
Chekhun VF, Zhylchuk VE, Lukyanova NY,
Vorontsova AL and Kudryavets YI: Expression of drug resistance
proteins in triple-receptor-negative tumors as the basis of
individualized therapy of the breast cancer patients. Exp Oncol.
31:123–124. 2009.
|
|
14
|
Jakoby WB: The glutathione S-transferases:
a group of multifunctional detoxification proteins. Adv Enzymol
Relat Areas Mol Biol. 46:383–414. 1978.
|
|
15
|
Tew KD: Glutathione-associated enzymes in
anticancer drug resistance. Cancer Res. 54:4313–4320. 1994.
|
|
16
|
Moscow JA, Townsend AJ, Goldsmith ME, et
al: Isolation of the human anionic glutathione S-transferase cDNA
and the relation of its gene expression to estrogen-receptor
content in primary breast cancer. Proc Natl Acad Sci USA.
85:6518–6522. 1988.
|
|
17
|
Gilbert L, Elwood LJ, Merino M, et al: A
pilot study of pi-class glutathione S-transferase expression in
breast cancer: correlation with estrogen receptor expression and
prognosis in node-negative breast cancer. J Clin Oncol. 11:49–58.
1993.
|
|
18
|
Colozza M, Azambuja E, Cardoso F, Sotiriou
C, Larsimont D and Piccart MJ: Proliferative markers as prognostic
and predictive tools in early breast cancer: where are we now? Ann
Oncol. 16:1723–1739. 2005.
|
|
19
|
Coon JS, Marcus E, Gupta-Burt S, et al:
Amplification and overexpression of topoisomerase IIalpha predict
response to anthracycline-based therapy in locally advanced breast
cancer. Clin Cancer Res. 8:1061–1067. 2002.
|
|
20
|
MacGrogan G, Rudolph P, Mascarel Id, et
al: DNA topoisomerase IIalpha expression and the response to
primary chemotherapy in breast cancer. Br J Cancer. 89:666–671.
2003.
|
|
21
|
Campiglio M, Somenzi G, Olgiati C, et al:
Role of proliferation in HER2 status predicted response to
doxorubicin. Int J Cancer. 105:568–573. 2003.
|
|
22
|
Depowski PL, Rosenthal SI, Brien TP,
Stylos S, Johnson RL and Ross JS: Topoisomerase IIalpha expression
in breast cancer: correlation with outcome variables. Mod Pathol.
13:542–547. 2000.
|
|
23
|
Rudolph P, Olsson H, Bonatz G, et al:
Correlation between p53, c-erbB-2, and topoisomerase II alpha
expression, DNA ploidy, hormonal receptor status and proliferation
in 356 node-negative breast carcinomas: prognostic implications. J
Pathol. 187:207–216. 1999.
|
|
24
|
Mukherjee A, Shehata M, Moseley P, Rakha
E, Ellis I and Chan S: Topo2alpha protein expression predicts
response to anthracycline combination neo-adjuvant chemotherapy in
locally advanced primary breast cancer. Br J Cancer. 103:1794–1800.
2010.
|
|
25
|
Wang L, Jiang Z, Sui M, Shen J, Xu C and
Fan W: The potential biomarkers in predicting pathologic response
of breast cancer to three different chemotherapy regimens: a case
control study. BMC Cancer. 9:2262009.
|
|
26
|
Navalgund LG, Rossana C, Muench AJ and
Johnson LF: Cell cycle regulation of thymidylate synthetase gene
expression in cultured mouse fibroblasts. J Biol Chem.
255:7386–7390. 1980.
|
|
27
|
Carreras CW and Santi DV: The catalytic
mechanism and structure of thymidylate synthase. Annu Rev Biochem.
64:721–762. 1995.
|
|
28
|
Aschele C, Debernardis D, Casazza S, et
al: Immunohistochemical quantitation of thymidylate synthase
expression in colorectal cancer metastases predicts for clinical
outcome to fluorouracil-based chemotherapy. J Clin Oncol.
17:1760–1770. 1999.
|
|
29
|
Johnston PG, Fisher ER, Rockette HE, et
al: The role of thymidylate synthase expression in prognosis and
outcome of adjuvant chemotherapy in patients with rectal cancer. J
Clin Oncol. 12:2640–2647. 1994.
|
|
30
|
Suda Y, Kuwashima Y, Tanaka Y, Uchida K
and Akazawa S: Immunohistochemical detection of thymidylate
synthase in advanced gastric cancer: a prognostic indicator in
patients undergoing gastrectomy followed by adjuvant chemotherapy
with 5-fluoropyrimidines. Anticancer Res. 19:805–810. 1999.
|
|
31
|
Lee JH, Park JH, Jung Y, et al: Histone
deacetylase inhibitor enhances 5-fluorouracil cytotoxicity by
down-regulating thymidylate synthase in human cancer cells. Mol
Cancer Ther. 5:3085–3095. 2006.
|
|
32
|
Gomez HL, Santillana SL, Vallejos CS, et
al: A phase II trial of pemetrexed in advanced breast cancer:
clinical response and association with molecular target expression.
Clin Cancer Res. 12:832–838. 2006.
|
|
33
|
Brandi M, Calascibetta A, Cabibi D, et al:
Relationship between thymidylate synthase expression and p53 levels
with the treatment of cyclophosphamide, methotrexate,
5-fluorouracil chemotherapy (CMF) versus docetaxel (TXT) in locally
advanced carcinoma of the breast. J Clin Oncol. 24:105462006.
|
















