Introduction
Hepatocellular carcinoma (HCC) is the most common
form of liver cancer and the third leading cause of cancer-related
mortality worldwide (1–3). Its incidence has increased in recent
years. The etiology of HCC includes alcohol abuse, chronic viral
hepatitis, environmental carcinogens or genetic disorders. Although
several risk factors for HCC development are known, the therapeutic
options for this disease are very limited. Hepatic resection
remains the most effective treatment (4), but the prognosis of HCC is generally
poor, with high postoperative recurrence and invasiveness of
primary tumor responses. Therefore, it is important to explore
novel molecular targets for treatment strategies that have the
potential to significantly improve the prognosis of HCC.
The role of Hedgehog (Hh) signaling in human cancer
has been established through the studies of basal cell nevus
syndrome (5), a rare hereditary
disorder with a high risk of basal cell carcinomas. The activation
of Hh signaling has been observed in numerous types of cancer, such
as prostate cancer, gastrointestinal cancer and HCC (6–8). The
Hh signaling pathway is a highly conserved system, which plays a
crucial role in cell differentiation, proliferation and tissue
patterning (9). In vertebrate
organisms, the signaling pathway is initiated by the ligands
(Desert, Indian and Sonic hedgehog) that bind to the membranous
receptor patched (Ptch). Ptch alleviates the suppression on
smoothened (Smo) that triggers a series of intracellular events by
activating glioma-associated oncogenes (Gli1, Gli2 and Gli3) that
induce the expression of numerous target genes and regulate
differentiation, proliferation and extracellular matrix
interactions (9–11). Gli1 is one of downstream effectors
of Hh signaling and acts as a transcription factor to promote cell
growth and inhibition of apoptosis (12,13).
Gli1 is overexpressed in several cancer tissues including
glioblastoma (14), breast cancer
(15) and pancreatic adenocarcinoma
(16). Moreover, the transcription
of Hh signaling-associated molecules (such as Shh, Smo and Gli1) is
also overexpressed in some cases of HCC (17,18).
Numerous types of cancer cells require increased
glucose uptake with a concomitant decrease in oxidative
phosphorylation, even in the presence of oxygen. This phenomenon of
aerobic glycolysis with increased lactate production has been named
as the Warburg effect (19). A
previous study demonstrated that expression of pyruvate kinase M2
(PKM2 or M2-PK) is a key event in determining this metabolic
phenotype, and tumor expression of M2 provides a proliferative
advantage in vitro and in vivo (20). Pyruvate Kinase (PK) is a key
regulatory enzyme in glycolysis and it has four known isoforms,
including L, R, M1 and M2. PKM2 plays a central role in the
metabolism of cancer cells and is expressed in a broad range of
human cancers. PKM2 can directly regulate gene transcription, which
may occur in both active tetrameric and inactive dimeric forms
(21–23). In addition, certain tyrosine kinases
may also be responsible for the Warburg effect in cancer, as they
can phosphorylate glycolytic enzymes, including PKM2, and then
promote tumor growth (24).
However, the exact role of PKM2 in tumor growth and
maintenance is not clear. Moreover, there is currently no study
showing a correlation between PKM2 and Hh signaling. The present
study identified a novel regulatory mechanism for PKM2, as a
regulator for Gli1 expression in HCC.
Materials and methods
Cell lines and reagents
HepG2 cells were purchased from the ATCC (Manassas,
VA, USA) and L-O2, Huh-7 and Hep3B cells were purchased from the
National platfom of Experimental Cell Resources for Sci-Tech
(Beijing, China) and maintained in Dulbecco’s modified Eagle’s
medium (DMEM; Hyclone, Logan, UT, USA) with 10% (v/v) fetal bovine
serum (FBS; Gibco-BRL, Carlsbad, CA, USA). 293T cells were
purchased from the cell bank and maintained in RPMI-1640 (Hyclone)
with 10% (v/v) FBS. The cells were incubated at 37°C in a 5%
CO2 humidified atmosphere for 24 or 48 h The pS-FLAG-SBP
(SBP) vector was provided by Dr Xin Zheng (The First Affiliated
Hospital of Xi’an Jiaotong University, Xi’an, China), and the
pcDNA3-Gli1 human Gli1 expression vector and pIRES2-S-SBP-FLAG
plasmid were provided by Dr Xin Zheng. Vector PLKO was purchased
from Addgene (Cambridge, MA, USA), it is a replication-incompetent
lentiviral vector for the expression of shRNAs. Rabbit polyclonal
anti-human PKM2 and mouse monoclonal anti-human ACTB antibodies
were purchased from Cell signaling Technology, Inc. (Beverly, MA,
USA), while rabbit polyclonal anti-human Gli1 and mouse monoclonal
anti-human HA antibodies were purchased from Santa Cruz
Biotechnology, Inc. (Santa Cruz, CA, USA). GFP-PKM2 was obtained
from OriGene Technologies, Inc., tagge with green fluorescent
protein. Patients provided written informed consent.
Patients and tissue samples
A total of 63 patients at the The First Affiliated
Hospital of Xi’an Jiaotong University with HCC were enrolled in the
study between January 2009 and October 2009, including 49 males and
14 females (mean age, 52 years; range, 35–71 years) who had not
received pre-operative chemotherapy or embolization. Following
routine X-ray, abdominal ultrasonography and computed tomography,
all patients underwent liver resection, including curative
resection for early HCC and palliative resection for advanced HCC.
Tumor tissue and matched adjacent normal tissue specimens (>2 cm
distant from the resection margin) were collected and immediately
stored in liquid nitrogen for quantitative polymerase chain
reaction (qPCR) and paraformaldehyde for immunohistochemistry,
respectively. Clinical data were obtained from the patients’
medical records. Subsequently, histopathological Edmonson
classification, clinical tumor-node-metastasis (TNM) grading,
maximum tumor diameter and the normal tumor-adjacent tissues were
all confirmed by an experienced pathologist who was blinded to the
clinical information.
Written informed consent was obtained from all
patients. The ethics committee of Xi’an Jiaotong University (Xi’an,
China) approved all protocols according to the 1975 Declaration of
Helsinki.
Immunohistochemistry
Immunohistochemistry was performed on
paraformaldehyde-fixed paraffin sections. The sections were dewaxed
and dehydrated. Following rehydration, endogenous peroxidase
activity was blocked for 30 min using a methanol solution
containing 0.3% hydrogen peroxide. After antigen retrieval in
citrate buffer, the sections were blocked overnight at 4°C, and
then separately incubated with the primary antibodies directed
against Gli1 and PKM2, at 4°C overnight. The primary antibody was
detected using biotinylated polyclonal goat anti-mouse IgG (H+L)
and polyclonal goat anti-rabbit IgG (H+L) secondary antibodies
(Zhongshan Golden Bridge Biotechnology Co., Ltd., Beijing, China)
according to the manufacturer’s recommendations. The staining of
the sections was performed using the avidin-biotin-peroxidase
complex (Zhongshan Golden Bridge Biotechnology Co., Ltd.) for Gli1
and PKM2. The sections were visualized with diaminobenzidine and
counterstained with hematoxylin, then dehydrated in alcohol and
xylene and mounted onto glass slides.
All sections were assessed independently by two
experienced pathologists. The staining results for the two proteins
(Gli1 and PKM2) were semi-quantitatively expressed by an
immunohistochemical score combined with the percentage of tumor
cells showing specific immunoreactivity. Staining intensity was
scored as follows: 0, none; 1, weak; 2, moderate; and 3, strong.
The percentage of positive carcinoma cells was scored as follows:
0, <5%; 1, 6–25%; 2, 26–50%; 3, 51–75%; and 4, >75%. The
staining intensity and average percentage of positive tumor cells
were assayed for 10 independent high-magnification (x400) fields
(Olympus CX21; Olympus Corporation, Tokyo, Japan). The total score
was calculated by multiplying the staining intensity score by the
percentage of positive tumor cells score. Sections with a total
score of >1 were defined as exhibiting positive staining for the
above two proteins.
Cell lysis, immunoprecipitation and
western blotting
293T cell transfections, protein extract
preparations, immunoprecipitation and western blot analysis were
performed as previously described (25,26).
Briefly, for immunoprecipitation, cells were lysed with ice-cold
NETN100 buffer [20 mM Tris-HCl, pH 8.0 (Sangon Biotech Co., Ltd.,
Shanghai, China), 100 mM NaCl (Sangon Biotech Co., Ltd.), 1 mM EDTA
(Sangon Biotech Co., Ltd.), 0.5% Nonidet P-40 (Amresco, Solon, OH,
USA)] containing 10m M NaF and 50 mM b-glycerophosphate, and then
subjected to sonication for 12 sec. Supernatants were incubated
with indicated antibodies (anti-PKM2, -Gli1, -ACTB and -HA) and
G-protein-conjugated sepharose beads (Amersham Pharmacia Biotech,
Inc., Piscataway, NJ, USA). Precipitates were washed three times
with NETN100, and then subjected to SDS-PAGE and western blotting
with the indicated antibodies. To examine the PKM2 expression, cell
pellets were lysed with 400 ml NETN100 buffer. Following
centrifugation at 13,000 × g for 20 min, the supernatants were
termed 100 mM NaCl samples. The insoluble pellets were collected,
washed with ice-cold phosphate-buffered saline (PBS) and incubated
with 400 ml NETN300 buffer (20 mM Tris-HCl, pH 8.0, 300 mM NaCl, 1
mM EDTA, 0.5% Nonidet P-40) on ice. After centrifugation, the
supernatants were termed 300 mM NaCl samples. The remaining pellets
were washed twice with ice-cold PBS and then treated with 200 ml
0.2 N HCl. The supernatants were neutralized with 40 ml 1 N NaOH,
and termed 0.2 N HCl fractions. Each fraction sample was loaded
onto 7.5% SDS-PAGE gels for western blotting with the indicated
antibodies. Western blotting was quantified using Quantity One
version 4.6.2 (Bio-Rad Laboratories, Philadelphia, PA, USA).
RNA isolation and qPCR
The expression of Gli1 in HepG2 cells was determined
by reverse transcription of total RNA, followed by qPCR analysis.
Total RNA (1 μg) was reverse-transcribed with random hexamers using
Superscript II reverse transcriptase (Invitrogen Life Technologies,
Carlsbad, CA, USA) according to the manufacturer’s instructions.
qPCR was performed on a Bio-Rad iCycler using iQ™ SYBR Green
(Bio-Rad Laboratories, Inc., Hercules, CA, USA) with the following
primers: Forward, 5′-GAAGGTGAAGGTCGGAGT-3′ and reverse,
5′-GTCCAGGCTGGCATCCGACA-3′ for Gli1; forward,
5′-GAAGGTGAAGGTCGGAGT-3′ and reverse, 5′-GAAGATGGTGATGGGATTTC-3′
for GAPDH; forward,
5′-GGCAGAGGCTGCCATtTAtCAtTTaCAgTTgTTcGAGGAACTCCGCCGCCT-3′ and
reverse 5′-AGGCGGCGAGTTCCTCGAACAACTGTAAATGAT AAATGGCAGCCTCTGCC-3′
for PKM2 shRNA-resistant 1408; and forward,
5′-AGAGGCTGCCATCTAtCAtTTaCAgTTgTTcGAaGAACTCCGCCGCCTGGC-3′ and
reverse, 5′-GCCAGGCGGCGGAGTTCTTCGAACAACTGTAAAT GATAGATGGCAGCCTCT-3′
for PKM2 shRNA-resistant 1411. PKM2 shRNA resistant 1408 and PKM2
shRNA resistant 1411 were used to generate the PKM2 shRNA resistant
1408 and PKM2 shRNA resistant 1411 plasmids (QuikChange Site
Directed Mutagenesis kit, Stratagene, Agilent Technologies, Inc.,
Santa Clara, CA, USA).
Glutathione S-transferase (GST) pull-down
assay
293T cells were used for the GST pull-down assay as
they exhibit a higher transfection efficiency than the other cell
lines. First, to produce 293T cells overexpressing HA-tagged Glil,
the 293T cells were grown in DMEM containing 10% FBS. Next,
2×106 cells were seeded in 10-cm dishes 24 h prior to
transfection with 5 mg of the HA-Gli1 plasmid using Lipofectamine
2000 reagent (Invitrogen Life Technologies), according to
manufacturer’s instructions. Subsequently, 1 mg of GST-PKM2 or GST
(OriGene Technologies, Inc., Rockville, MD, USA) as a control was
incubated with the cell lysates from the 293T cells overexpressing
HA-tagged Gli1. Glutathione beads (Sigma-Aldrich, St. Louis, MO,
USA) were then added and incubated for 2 h. The bound proteins were
eluted with sample loading buffer and analyzed by immunoblotting
with HA antibodies. For endogenous immunoprecipitation, 293T cell
lysates were immunoprecipitated with normal mouse immunoglobulin G
as a control, followed by incubation with protein A beads
(Sigma-Aldrich). The bound proteins were subjected to immunoblot
analysis with PKM2 antibody.
Transfection
293T cells were grown in DMEM containing 10% FBS. A
total of 2×106 cells were seeded in 10-cm dishes 24 h
prior to transfection. Cells were subsequently transfected with 5
mg GFP-PKM2, HA-Gli1, PKM2 shRNA 1408 or shRNA 1411, and vector
PLKO plasmids using Lipofectamine 2000 reagent (Invitrogen Life
Technologies), according to the manufacturer’s instructions.
Statistical analysis
Data are presented as the mean ± standard error of
the mean. Mann-Whitney U tests were used for statistical analysis
unless otherwise indicated.
Results
Gli1 and PKM2 expression in HCC
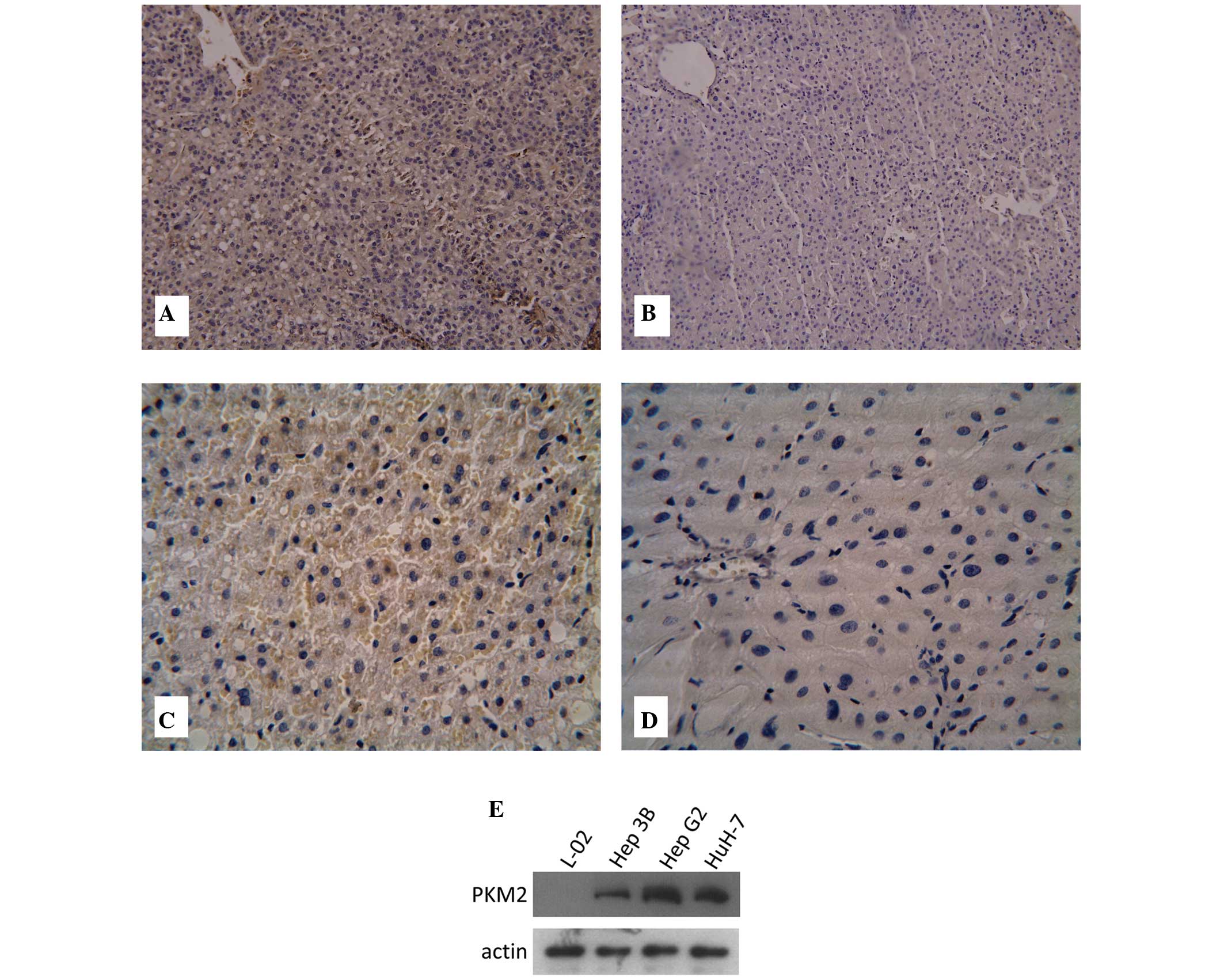
To examine Gli1 expression in HCC, a total of 63
pairs of HCC and adjacent normal tissues from HCC patients were
examined by immunohistochemistry. Gli1 protein levels in HCC and
adjacent normal tissues were assessed using a Gli1-specific
antibody. It was found that Gli1 protein expression was positive in
57 out of 63 tumor tissues (90.48%), including 17 (26.98%) highly
positive (+++) cases. By contrast, Gli1 protein expression was
observed in 21 of 63 normal tissues (33.33%) and none of these
tissues were highly positive (Fig. 1A
and B and Table I). Cox
regression analysis showed that there was a significant correlation
between Gli1 expression and tumor invasiveness, including
histological differentiation, portal vein tumorous thrombogenesis,
lymph node invasion and TNM stage (Table II).
 | Table IExpression of Gli1 protein in the HCC
tumor tissues and adjacent normal tissues. |
Table I
Expression of Gli1 protein in the HCC
tumor tissues and adjacent normal tissues.
| Pathological
type | n | Gli1 expression
level, n | Positive n (%) | χ2 | P-value |
|---|
|
|---|
| − | + | ++ | +++ |
|---|
| HCC tissues | 63 | 6 | 8 | 32 | 17 | 57 (90.48) | 43.6 | <0.05 |
| Adjacent normal
tissues | 63 | 42 | 18 | 3 | 0 | 21 (33.33) | | |
 | Table IIPrognostic factors in Cox
proportional-hazards model. |
Table II
Prognostic factors in Cox
proportional-hazards model.
| Parameter | RR | 95% CI | Wald | P-value |
|---|
| Gender | 0.819 | 0.127–5.274 | 0.044 | 0.834 |
| Age | 1.033 | 0.205–5.196 | 0.002 | 0.968 |
| HBsAg | 0.310 | 0.057–1.675 | 1.851 | 0.174 |
| Cirrhosis | 0.237 | 0.033–1.721 | 2.027 | 0.155 |
| Serum AFP | 0.530 | 0.062–4.494 | 0.339 | 0.560 |
| Tumor size | 0.728 | 0.181–2.923 | 0.200 | 0.655 |
| Differentiation | 15.197 | 2.039–113.291 | 7.048 | 0.008a |
| PVTT | 6.041 | 1.395–26.162 | 5.784 | 0.016a |
| Lymph node
invation | 0.032 | 0.003–0.369 | 7.627 | 0.006a |
| Encapsulation | 2.484 | 0.435–14.180 | 1.048 | 0.306 |
| Primary tumor | 3.105 | 0.395–24.435 | 1.159 | 0.282 |
| TNM stage | 75.634 | 2.757–2.075E3 | 6.554 | 0.010a |
| Gli1 mRNA | 22.298 | 2.110–235.510 | 6.663 | 0.010a |
The expression of PKM2 protein was examined by
immunohistochemistry. As shown in Fig.
1C and D, PKM2 was mainly expressed in the cytoplasm.
Immunohistochemistry indicated that the levels of PKM2 in HCC
tissues were significantly higher than that in adjacent normal
tissues (4.44±1.54 vs. 2.13±1.34; P<0.05). Furthermore, the
protein expression levels of PKM2 in three HCC cell lines (Hep3B,
HepG2 and HuH-7) and one human normal liver cell line (L-02 cell
line) were examined by western blot analysis. It was found that
PKM2 was highly expressed in the three HCC cell lines, but not in
the L-02 cell line (Fig. 1E).
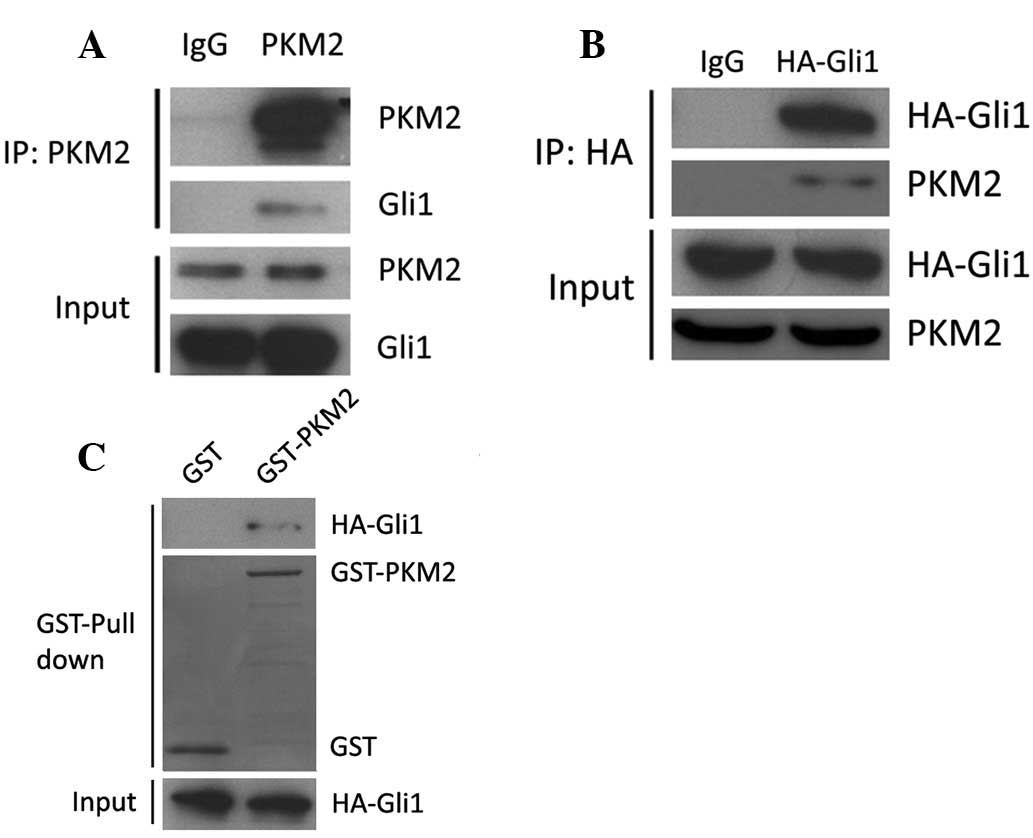
Gli1 directly interacts with PKM2
In order to investigate the functional association
between PKM2 and Gli1, we first examined the physical associations
between the two molecules using immunoprecipitation. As shown in
Fig. 2A, Gli1 protein was
precipitated by specific PKM2 antibody in 293T cell lysates. It was
also examined whether PKM2 could be precipitated by Gli1 antibody.
Due to the lack of specific Gli1 antibody for immunoprecipitation,
293T cells were transfected with HA-Gli1. The cell lysates were
subjected to incubation with HA antibody and then immunoblotted
with PKM2 antibody. It was observed that HA-Gli1 could interact
with PKM2 (Fig. 2B). To examine the
direct interaction between PKM2 and Gli1, GST-PKM2 and HA-Gli1 were
purified for the GST pull-down assay. As shown in Fig. 2C, GST-PKM2 interacted with HA-Gli1
in the 293T cells. These results indicate that PKM2 is able to
interact with Gli1 in vitro and in vivo.
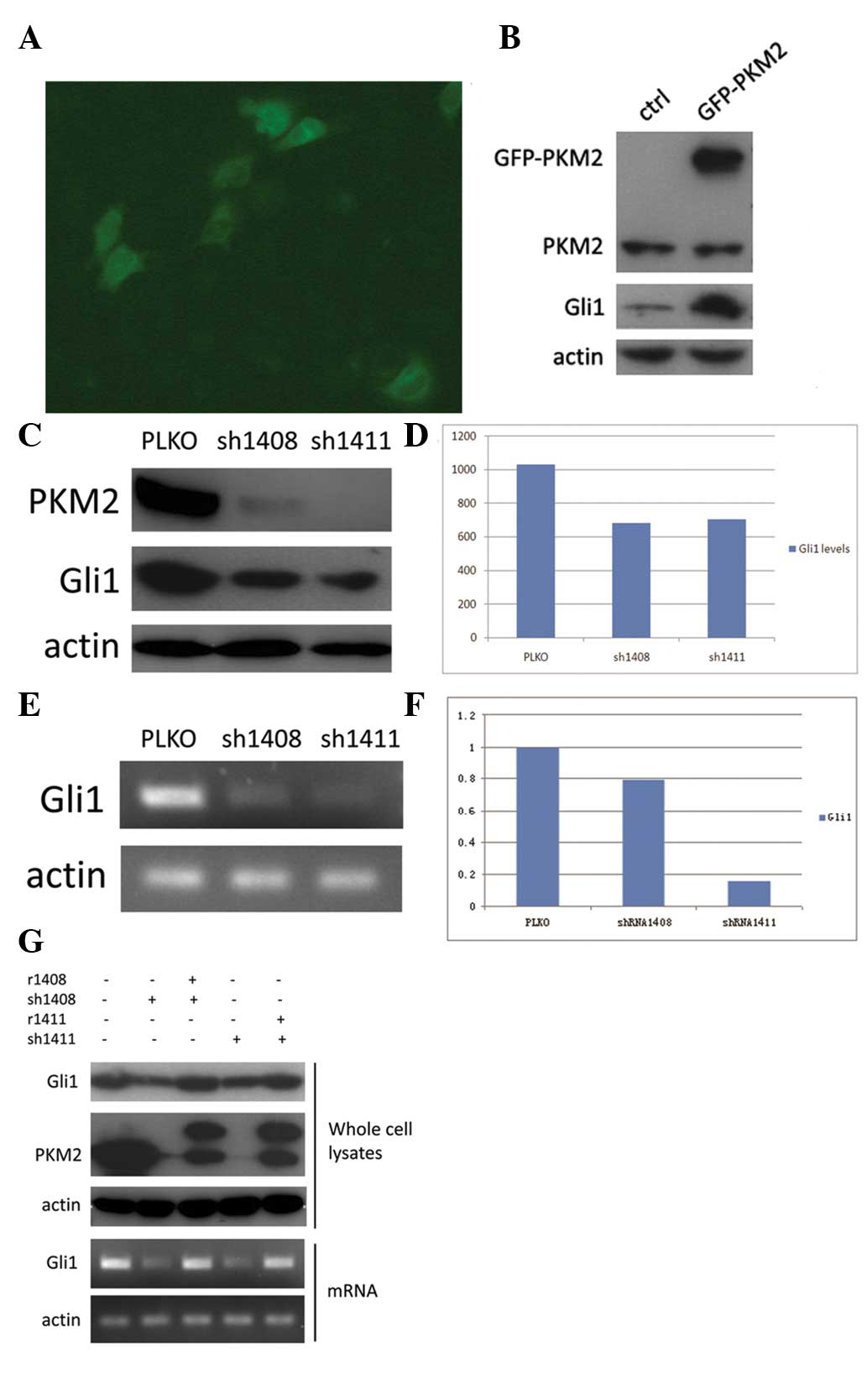
PKM2 regulates Gli1 expression
To further analyze the association between PKM2 and
Gli1, overexpression of GFP-PKM2 was induced in the HepG2 cell line
and Gli1 expression was examined (Fig.
3A). It was found that Gli1 expression was significantly
upregulated in GFP-PKM2-overexpressing cells compared with normal
HepG2 cells (Fig. 3B). As HepG2
cells normally express high levels of PKM2, we also chose to knock
down its expression. HepG2 cells were infected with recombinant
lentiviruses expressing either PKM2 shRNA 1408 or shRNA 1411, and
vector PLKO was used as control. These two distinct ‘targeted’
shRNAs (which were named 1408 and 1411) could significantly ablate
PKM2 expression in HepG2 cells (>80% of expression knocked down)
(Fig. 3C). Moreover, PKM2 knockdown
by shRNA (1408 and 1411) in HepG2 cells markedly decreased the
expression of Gli1 mRNA and protein compared with HepG2 cells
transfected with the PLKO vector. (Fig.
3C-F).
To further verify the effect of PKM2 shRNAs, which
knockdown PKM2 in HepG2 cells, the overexpression of mutated PKM2
resistant to PKM2 shRNA but can express wild-type PKM2 was induced
in HepG2 cells. It was found that the reduced Gil1 expression in
HepG2 cells with knockdown of PKM2 was completely rescued by
reconstituted expression of wild-type PKM2, and these effects were
observed at the mRNA and protein levels (Fig. 3G). These results further verify that
Gli1 is directly regulated by PKM2.
Discussion
Early hepatocellular carcinoma (HCC) is rarely
diagnosed before the middle or advanced stage (27,28).
Recently, certain HCC-associated oncogenes, such as FXR, Plk1, MDR3
and MRP, have been found to be linked with the prognosis of HCC
(29–31). However, the exact molecular
mechanism of HCC progression is unclear.
Compared with normal cells, cancer cells (including
HCC cells) show increased glycolysis and inhibition of oxidative
phosphorylation, even in the presence of sufficient oxygen (aerobic
glycolysis), which is known as the Warburg effect (19). Aerobic glycolysis is not only
conducted to increase the availability of macromolecules for
biosynthesis and cell growth, but also to contribute to
anti-apoptotic pathways. Increased glucose metabolism protects
cells from the pro-apoptotic Bcl-2 family protein, Bim, and
attenuates the degradation of the anti-apoptotic protein, Mcl-1
(32). However, some key problems
remain unclear, for example, the physiological significance of this
glucose-dependent regulation in cancer cells, and the regulatory
mechanisms of the Warburg effect remain unknown.
PKM2 has previously been known to be a key enzyme
that controls the rate-limiting step of glycolysis and plays a
central role in metabolic reprogramming during cancer progression.
PKM2 knockdown by siRNA in glioma cells has been demonstrated to
induce cell apoptosis and inhibit cell growth, cellular invasion,
metabolic activity, ATP levels and glutathione levels (33). Reduced expression of PKM2 protein in
lung tumors has been shown to inhibit tumor growth and promote
cancer cell apoptosis in vitro and in vivo (34). Christofk et al demonstrated
that mice injected with PKM1-overexpressing cells showed a delay in
tumor development compared with those injected with
PKM2-overexpressing cells (20).
Recently, PMK2 was newly characterized as a transcriptional
coactivator and protein kinase (21,35),
suggesting that PKM2 also has the ability to regulate gene
expression, cell cycle progression and metabolism in a feedback
loop. All of these findings reflect the important role of PKM2 in
tumorigenesis.
The Hh signaling pathway is essential for numerous
processes during embryonic development, including cell growth, cell
differentiation, patterning and organogenesis (36). However, aberrant Hh signaling is
observed in a variety of cancer types. Previous studies have shown
that Shh, Gli1, Smo and Patch were overexpressed in HCC, and the
Shh signaling pathway played a critical role in HCC tumorigenesis
and progression (37,38). But the molecular mechanisms of Hh
signaling in HCC remain unclear.
In the present study, we described a previously
unknown association between the Hh signaling pathway and PKM2, in
which PKM2 affects the Hh signaling pathway by regulating Gli1
transcription levels. By using PCR and immunohistochemistry, it was
demonstrated that the levels of Gli1 in HCC tissues were markedly
higher than those in adjacent normal tissues. These findings
confirmed those of the previous studies showing that Gli1
expression is aberrant in HCC, suggesting that Gli1 may be a key
marker for diagnosis (37,38). In the current study, statistical
analysis showed there was a significant correlation between Gli1
expression and tumor invasiveness. Additionally, the levels of PKM2
protein in HCC tissues were significantly higher than those in
adjacent normal tissues. These data provide a molecular basis for
improving the diagnosis and treatment of HCC patients by targeting
upregulated PKM2 and Gli1. Furthermore, in the present study,
immunoprecipitation and immunoblotting revealed a positive
correlation between PKM2 and Gli1. In addition, PKM2 overexpression
upregulated Gli1, while knockdown of PKM2 by two different shRNAs
caused a significant decrease in Gli1 expression, which could be
completely rescued by reconstituted expression of wild-type PKM2.
These results suggest that PKM2 may be an important upstream
regulator of Gli1 gene expression in HCC.
In summary, the present study has shown that PKM2 is
a regulator of Gli1 gene expression in HCC, and PKM2 may contribute
to tumorigenesis by controlling Gli1 expression. However, the exact
molecular mechanism whereby PKM2 regulates Hh signaling requires
further investigation.
References
|
1
|
Buonaguro L, Petrizzo A, Tagliamonte M,
Tornesello ML and Buonaguro FM: Challenges in cancer vaccine
development for hepatocellular carcinoma. J Hepatol. 59:897–903.
2013.
|
|
2
|
El-Serag HB: Epidemiology of viral
hepatitis and hepatocellular carcinoma. Gastroenterology.
142:1264–1273.e1. 2012.
|
|
3
|
Shariff MI, Cox IJ, Gomaa AI, Khan SA,
Gedroyc W and Taylor-Robinson SD: Hepatocellular carcinoma: current
trends in worldwide epidemiology, risk factors, diagnosis and
therapeutics. Expet Rev Gastroenterol Hepatol. 3:353–367. 2009.
|
|
4
|
Chen XP, Qiu FZ, Wu ZD, Zhang ZW, Huang ZY
and Chen YF: Long-term outcome of resection of large hepatocellular
carcinoma. Brit J Surg. 93:600–606. 2006.
|
|
5
|
Johnson RL, Rothman AL, Xie J, et al:
Human homolog of patched, a candidate gene for the basal cell nevus
syndrome. Science. 272:1668–1671. 1996.
|
|
6
|
Wilkinson SE, Furic L, Buchanan G, et al:
Hedgehog signaling is active in human prostate cancer stroma and
regulates proliferation and differentiation of adjacent epithelium.
Prostate. 73:1810–1823. 2013.
|
|
7
|
Wang DH, Clemons NJ, Miyashita T, et al:
Aberrant epithelial-mesenchymal Hedgehog signaling characterizes
Barrett’s metaplasia. Gastroenterology. 138:1810–1822. 2010.
|
|
8
|
Huang S, He J, Zhang X, et al: Activation
of the hedgehog pathway in human hepatocellular carcinomas.
Carcinogenesis. 27:1334–1340. 2006.
|
|
9
|
Ruiz i Altaba A, Sánchez P and Dahmane N:
Gli and hedgehog in cancer: tumours, embryos and stem cells. Nat
Rev Cancer. 2:361–372. 2002.
|
|
10
|
Ingham PW and McMahon AP: Hedgehog
signaling in animal development: paradigms and principles. Genes
Dev. 15:3059–3087. 2001.
|
|
11
|
Cohen MM Jr: The hedgehog signaling
network. Am J Med Genet A. 123A:5–28. 2003.
|
|
12
|
Fiaschi M, Rozell B, Bergström A and
Toftgård R: Development of mammary tumors by conditional expression
of GLI1. Cancer Res. 69:4810–4817. 2009.
|
|
13
|
Stecca B and Ruiz i Altaba A: A GLI1-p53
inhibitory loop controls neural stem cell and tumour cell numbers.
EMBO J. 28:663–676. 2009.
|
|
14
|
Lo HW, Zhu H, Cao X, Aldrich A and
Ali-Osman F: A novel splice variant of GLI1 that promotes
glioblastoma cell migration and invasion. Cancer Res. 69:6790–6798.
2009.
|
|
15
|
Goel HL, Pursell B, Chang C, et al: GLI1
regulates a novel neuropilin-2/α6β1 integrin based autocrine
pathway that contributes to breast cancer initiation. EMBO Mol Med.
5:488–508. 2013.
|
|
16
|
Nolan-Stevaux O, Lau J, Truitt ML, et al:
GLI1 is regulated through Smoothened-independent mechanisms in
neoplastic pancreatic ducts and mediates PDAC cell survival and
transformation. Genes Dev. 23:24–36. 2009.
|
|
17
|
Patil MA, Zhang J, Ho C, Cheung ST, Fan ST
and Chen X: Hedgehog signaling in human hepatocellular carcinoma.
Cancer Biol Ther. 5:111–117. 2006.
|
|
18
|
Xu QR, Zheng X, Zan XF, Yao YM, Yang W and
Liu QG: Gli1 expression and its relationship with the expression of
Shh, Vimentin and E-cadherin in human hepatocellular carcinoma. Xi
Bao Yu Fen Zi Mian Yi Xue Za Zhi. 28:536–539. 2012.(In
Chinese).
|
|
19
|
Warburg O: On the origin of cancer cells.
Science. 123:309–314. 1956.
|
|
20
|
Christofk HR, Vander Heiden MG, Harris MH,
et al: The M2 splice isoform of pyruvate kinase is important for
cancer metabolism and tumour growth. Nature. 452:230–233. 2008.
|
|
21
|
Yang W, Xia Y, Hawke D, et al: PKM2
phosphorylates histone H3 and promotes gene transcription and
tumorigenesis. Cell. 150:685–696. 2012.
|
|
22
|
Gao X, Wang H, Yang JJ, Liu X and Liu ZR:
Pyruvate kinase M2 regulates gene transcription by acting as a
protein kinase. Mol Cell. 45:598–609. 2012.
|
|
23
|
Yang W and Lu Z: Regulation and function
of pyruvate kinase M2 in cancer. Cancer Lett. 339:153–158.
2013.
|
|
24
|
Hitosugi T, Kang S, Vander Heiden MG, et
al: Tyrosine phosphorylation inhibits PKM2 to promote the Warburg
effect and tumor growth. Sci Signal. 2:ra732009.
|
|
25
|
Kim JE, Chen J and Lou Z: DBC1 is a
negative regulator of SIRT1. Nature. 451:583–586. 2008.
|
|
26
|
Kim H, Chen J and Yu X: Ubiquitin-binding
protein RAP80 mediates BRCA1-dependent DNA damage response.
Science. 316:1202–1205. 2007.
|
|
27
|
Bolondi L, Sofia S, Siringo S, et al:
Surveillance programme of cirrhotic patients for early diagnosis
and treatment of hepatocellular carcinoma: a cost effectiveness
analysis. Gut. 48:251–259. 2001.
|
|
28
|
Oka H, Kurioka N, Kim K, et al:
Prospective study of early detection of hepatocellular carcinoma in
patients with cirrhosis. Hepatology. 12:680–687. 1990.
|
|
29
|
Zhang Y, Gong W, Dai S, et al:
Downregulation of human farnesoid X receptor by miR-421 promotes
proliferation and migration of hepatocellular carcinoma cells. Mol
Cancer Res. 10:516–522. 2012.
|
|
30
|
He Z, Wu J, Dang H, Lin H, Zheng H and
Zhong D: Polo-like kinase 1 contributes to the tumorigenicity of
BEL-7402 hepatoma cells via regulation of Survivin expression.
Cancer Lett. 303:92–98. 2011.
|
|
31
|
Yu Z, Peng S, PAN H-M and WANG K-F:
Expression of multi-drug resistance-related genes MDR3 and MRP as
prognostic factors in clinical liver cancer patients.
Hepatogastroenterology. 59:1556–1559. 2012.
|
|
32
|
Zhao Y, Altman BJ, Coloff JL, et al:
Glycogen synthase kinase 3alpha and 3beta mediate a
glucose-sensitive antiapoptotic signaling pathway to stabilize
Mcl-1. Mol Cell Biol. 27:4328–4339. 2007.
|
|
33
|
Kefas B, Comeau L, Erdle N, Montgomery E,
Amos S and Purow B: Pyruvate kinase M2 is a target of the
tumor-suppressive microRNA-326 and regulates the survival of glioma
cells. Neuro Oncol. 12:1102–1112. 2010.
|
|
34
|
Shi HS, Li D, Zhang J, et al: Silencing of
pkm2 increases the efficacy of docetaxel in human lung cancer
xenografts in mice. Cancer Sci. 101:1447–1453. 2010.
|
|
35
|
Yang W, Xia Y, Ji H, et al: Nuclear PKM2
regulates β-catenin transactivation upon EGFR activation. Nature.
480:118–122. 2011.
|
|
36
|
Briscoe J and Thérond PP: The mechanisms
of Hedgehog signalling and its roles in development and disease.
Nat Rev Mol Cell Biol. 14:416–429. 2013.
|
|
37
|
Zheng X, Yao Y, Xu Q, Tu K and Liu Q:
Evaluation of glioma-associated oncogene 1 expression and its
correlation with the expression of sonic hedgehog, E-cadherin and
S100a4 in human hepatocellular carcinoma. Mol Med Rep. 3:965–970.
2010.
|
|
38
|
Chen XL, Cheng QY, She MR, et al:
Expression of sonic hedgehog signaling components in hepatocellular
carcinoma and cyclopamine-induced apoptosis through Bcl-2
downregulation in vitro. Arch Med Res. 41:315–323. 2010.
|