Perspective
As a relatively recently identified alkylating
(methylating) agent, temozolomide (TMZ) has become a focus of
attention, most notably in malignant glioma and melanoma treatment
(1,2). Resistance to TMZ occurs following
prolonged treatment and therefore poses a major therapeutic
challenge. A key mechanism of the resistance to TMZ is the
overexpression of O6-methylguanine-DNA methyl
transferase (MGMT) (3). MGMT
repairs the TMZ-induced DNA lesion, O6MeG, by removing
the methyl group from guanine to a cysteine residue (4). Suppressing MGMT activity, therefore,
could enhance the cytotoxicity of TMZ against melanoma and
glioblastoma multiforme (4).
In previous years, we have focused our research on
oncolytic virotherapy. Oncolytic viruses exhibit selective
replication and lysis in tumor cells, while also amplifying the
expression and functions of therapeutic gene in the tumor
microenvironment (5). Two main
strategies are used for oncolytic adenovirus generation. One
strategy is the deletion of the viral element that is required for
replication of the virus in normal cells, but is dispensable in
tumor cells, such as ONYX-015 or ZD55 with E1B-55K gene deletion
(6,7). The other strategy is the use of a
tumor-specific promoter to drive the gene that is required for
viral replication (8). In clinical
trials, the E1B 55-kDa-deleted oncolytic virus, ONYX-015, or the
ONYX-015 derivative, H101, have exhibited encouraging anticancer
activity when combined with chemotherapy (9).
RNA interference (RNAi) technology is able to
downregulate targeted genes and has been evaluated as a potential
therapeutic strategy in human cancer therapy (10). The knockdown of DNA repair genes by
small interfering RNA (siRNA) and virally delivered short hairpin
RNA (shRNA), can sensitize various cancer cells to chemotherapeutic
agents in vitro (11). A
previous study has shown that the use of siRNA to transiently
transfect nasopharyngeal carcinoma cells and glioma cells results
in the inhibition of MGMT gene expression and increased sensitivity
to bis-chloroethylnitrosourea (12). Similarly, a study by Kato et
al (13) revealed that the
transduction of TMZ-resistant glioma cells with a LipoTrust™
liposome, which contains siRNA to inhibit MGMT gene expression,
enhanced the sensitivity of the glioma cells to TMZ.
Zheng et al (14,15)
focused on the production of several shRNA constructs using an
oncolytic virus for delivery. Examples of these constructs included
siRNAs against Ki67 and hTERT, which were observed to act as
antiproliferative and apoptotic inducers in cancer cells. shRNA
delivery via armed oncolytic viruses has potential for enhancing
antitumor efficacy as a consequence of synergism between viral
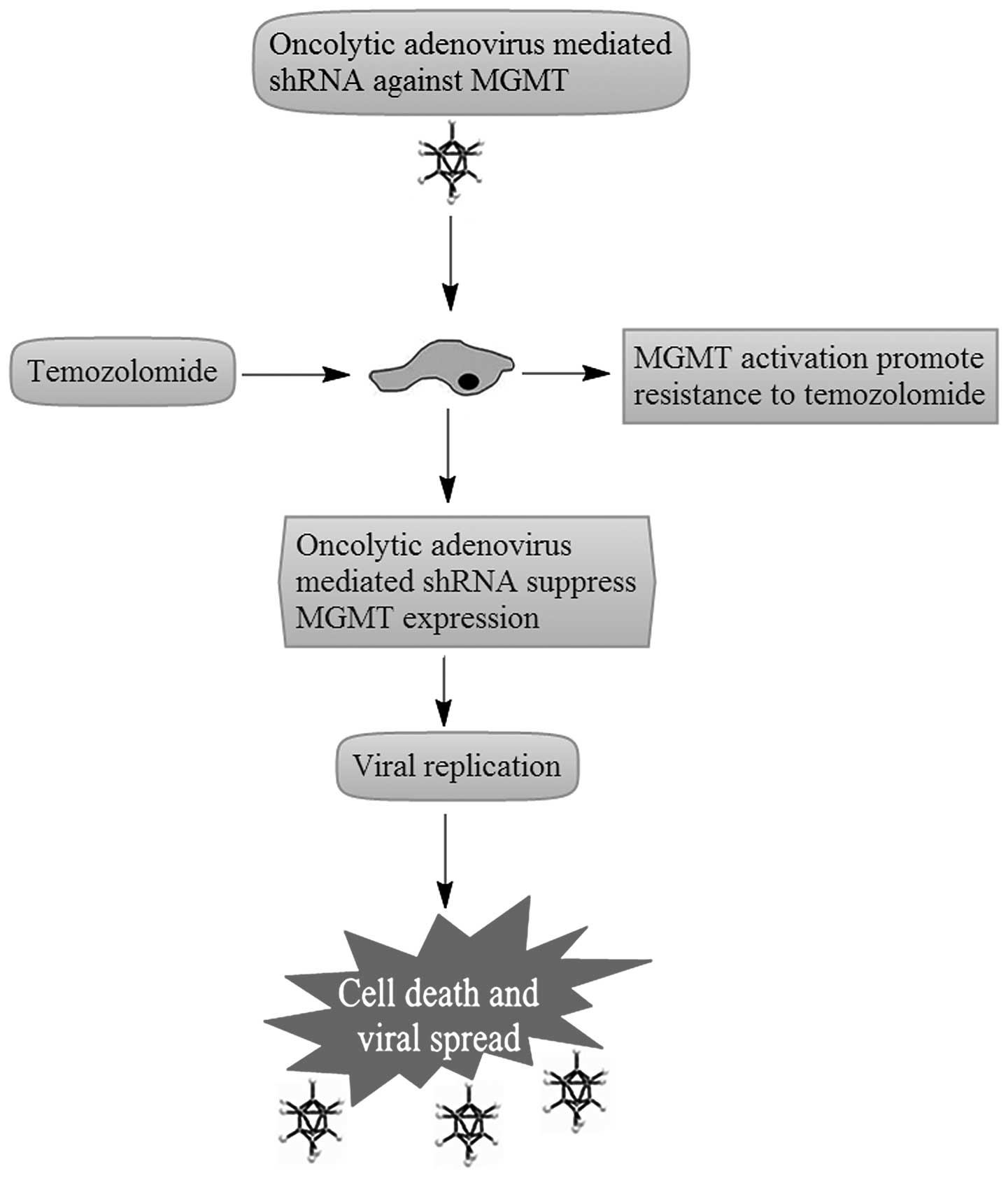
replication and oncolysis and shRNA antitumor responses (11). When conveying shRNA, oncolytic
viruses are expected to effect a marked reduction in the tumor MGMT
level, which should result in an increase in the cytotoxicity of
TMZ (Fig. 1).
We hypothesize that the effects of the oncolytic
virus-mediated RNAi of MGMT activity may enhance the cytotoxicity
of TMZ in tumors for the following reasons: Firstly, the use of
armed oncolytic viruses to deliver shRNA combines the advantages of
gene therapy and virotherapy. The inserted shRNA can target the DNA
repair protein, MGMT, in tumor cells and multiply by several 100-
to several 1,000-fold in parallel with viral replication. The
oncolytic adenovirus-armed shRNA targeting MGMT also offers the
advantage of enhancing shRNA-mediated antitumor responses through
its intrinsic oncolytic activity (10). Secondly, as a delivery agent that
couples shRNA expression with viral replication, oncolytic
adenoviruses can minimize the effects of off-target activity in
normal cells, and facilitate, sustain and regenerate shRNA
expression within the tumor microenvironment (15). Thirdly, as oncolytic adenovirus
vectors and chemotherapeutic agents act by different mechanisms,
there is a synergistic or additive effect rather than
cross-resistance on the death of tumor cells (5).
The combination of these advantages and
possibilities suggest that using oncolytic adenoviruses to deliver
therapeutic shRNA targeting MGMT protein may be a powerful
technique for overcoming resistance to TMZ in human cancers. This
may result in a significantly enhanced antitumor outcome through
MGMT-knockdown and viral oncolysis.
Acknowledgements
This study was supported by a grant from the
National Natural Science Foundation of China (grant no.
81372916).
References
|
1
|
O’Reilly SM, Newlands ES, Glaser MG, et
al: Temozolomide: a new oral cytotoxic chemotherapeutic agent with
promising activity against primary brain tumours. Eur J Cancer.
29A:940–942. 1993.
|
|
2
|
Bleehen NM, Newlands ES, Lee SM, et al:
Cancer Research Campaign phase II trial of temozolomide in
metastatic melanoma. J Clin Oncol. 13:910–913. 1995.
|
|
3
|
Plummer ER, Middleton MR, Jones C, et al:
Temozolomide pharmacodynamics in patients with metastatic melanoma:
DNA damage and activity of repair enzymes
O6-alkylguanine alkyltransferase and poly(ADP-ribose)
polymerase-1. Clin Cancer Res. 11:3402–3409. 2005.
|
|
4
|
Fan CH, Liu WL, Cao H, et al:
O6-methylguanine DNA methyltransferase as a promising
target for the treatment of temozolomide-resistant gliomas. Cell
Death Dis. 4:e8762013.
|
|
5
|
Jiang G, Xin Y, Zheng JN and Liu YQ:
Combining conditionally replicating adenovirus-mediated gene
therapy with chemotherapy: a novel antitumor approach. Int J
Cancer. 129:263–274. 2011.
|
|
6
|
Bischoff JR, Kirn DH, Williams A, et al:
An adenovirus mutant that replicates selectively in p53-deficient
human tumor cells. Science. 274:373–376. 1996.
|
|
7
|
Heise C, Hermiston T, Johnson L, et al: An
adenovirus E1A mutant that demonstrates potent and selective
systemic anti-tumoral efficacy. Nat Med. 6:1134–1139. 2000.
|
|
8
|
Rodriguez R, Schuur ER, Lim HY, et al:
Prostate attenuated replication competent adenovirus (ARCA) CN706:
a selective cytotoxic for prostate-specific antigen-positive
prostate cancer cells. Cancer Res. 57:2559–2563. 1997.
|
|
9
|
Garber K: China approves world’s first
oncolytic virus therapy for cancer treatment. J Natl Cancer Inst.
98:298–300. 2006.
|
|
10
|
Chu L, Gu J, Sun L, et al: Oncolytic
adenovirus-mediated shRNA against Apollon inhibits tumor cell
growth and enhances antitumor effect of 5-fluorouracil. Gene Ther.
15:484–494. 2008.
|
|
11
|
Jiang G, Li LT, Xin Y, et al: Strategies
to improve the killing of tumors using temozolomide: targeting the
DNA repair protein MGMT. Curr Med Chem. 19:3886–3892
|
|
12
|
Kuo CC, Liu JF and Chang JY: DNA repair
enzyme, O6-methylguanine DNA methyltransferase,
modulates cytotoxicity of camptothecin-derived topoisomerase I
inhibitors. J Pharmacol Exp Ther. 316:946–954. 2006.
|
|
13
|
Kato T, Natsume A, Toda H, et al:
Efficient delivery of liposome-mediated MGMT-siRNA reinforces the
cytotoxity of temozolomide in GBM-initiating cells. Gene Ther.
17:1363–1371. 2010.
|
|
14
|
Zheng JN, Pei DS, Mao LJ, et al:
Inhibition of renal cancer cell growth in vitro and in vivo with
oncolytic adenovirus armed short hairpin RNA targeting Ki-67
encoding mRNA. Cancer Gene Ther. 16:20–32. 2009.
|
|
15
|
Zheng JN, Pei DS, Sun FH, et al:
Inhibition of renal cancer cell growth by oncolytic adenovirus
armed short hairpin RNA targeting hTERT gene. Cancer Biol Ther.
8:84–91. 2009.
|