Introduction
In developed countries where the aging population is
increasing, cancer is one of the most prominent illnesses in terms
of public welfare and health. One in four mortalities in the United
States, for example, is due to cancer (1). In the United States, the incidence of
CRC has increased significantly in recent years, based on changing
lifestyles. CRC is one of the most prominent causes of mortality
from neoplastic disease in Japan. Distant metastases, such as liver
or lung metastases, are the major cause of mortality in CRC
(2). The ability to identify the
genes responsible for CRC development and progression and the
ability to understand the associated clinical significance are
vital for diagnosing and treating CRC sufficiently. The
characterization of key molecules has great potential with regard
to the generation of novel approaches for the treatment of CRC.
The annexins are a family of well-conserved
proteins, characterized by the ability to interact with membrane
phospholipids in a Ca2+-dependent manner (3). The structures of all annexins contain
type II Ca2+ sites that are located in the protein core
domains, built of four or eight homologous segments known as
annexin repeats (4,5). In breast cancer, ANXA9 has been
reported as a gene that is associated with the relapse in bone
(6). However, the association
between ANXA9 expression and CRC remains unknown.
The aim of the present study was to analyze the
correlation between ANXA9 expression levels in the CRC
tissues of patients and the clinicopathological factors, and to
investigate the possible functions of the gene in the tumorigenesis
and metastasis of CRC.
Materials and methods
Clinical tissue samples
In total, 100 patients (61 males and 39 females)
with CRC were registered and underwent curative surgery for
resection of CRC and distant metastases, if present, at the Medical
Institute of Bioregulation at Kyushu University (Beppu, Ohita,
Japan) and the Department of Gastroenterological Surgery, Osaka
University Graduate School of Medicine (Suita, Osaka, Japan)
between 1994 and 2003. None of the patients received chemotherapy
or radiotherapy prior to surgery. Primary CRC specimens and
adjacent normal colorectal mucosa samples were obtained from the
patients following the receipt of written informed consent. This
study was approved by the ethics committee of Osaka University
Graduate School of Medicine (Osaka, Japan). The surgical specimens
were fixed in formalin, processed through graded ethanol and
embedded in paraffin. The sections were stained with hematoxylin
and eosin, and Elastica van Gieson (Merck Millipore, Billerica, MA,
USA) stains, and the degree of histological differentiation,
lymphatic invasion and venous invasion was examined. Additionally,
sections from all specimens were frozen in liquid nitrogen
immediately after resection and kept at −80°C until RNA extraction.
Subsequent to surgery, the patients underwent follow-up blood
examinations to assess the tumor markers, serum carcinoembryonic
antigen (CEA) and cancer antigen (CA19-9), and imaging modalities,
such as abdominal ultrasonography, computed tomography and chest
X-rays, were performed every 3 to 6 months. Post-operatively, the
stage III and IV patients received 5-fluorouracil-based
chemotherapy for six months [mFOLFOX6; (oxaliplatin, 85
mg/m2, 5-fluorouracil 2800 mg/m2, for 2
weeks, for 12 courses), UFT (300 mg/m2/day × 28 days/5
weeks, for 5 courses), capecitabine (2500 mg/m2/day × 14
days/3 weeks, for 8 courses), or TS-1 (80 mg/m2/day × 28
days/6 weeks, for 4 courses)], whereas the stage I and II patients
principally received no chemotherapy. All therapies were performed
according to the Japanese Society for Cancer of the Colon and
Rectum guidelines (7).
Clinicopathological factors were assessed according to the tumor
node metastasis (TNM) classification of the International Union
Against Cancer (8).
RNA preparation and expression
analysis
Total RNA was prepared using TRIzol reagent
(Invitrogen, Carlsbad, CA, USA) or with DNase using a modified acid
guanidium-thiocyanate-phenol-choroform procedure (9). Reverse transcription was performed
with SuperScriptII (Life Technologies, Carlsbad, CA, USA) or by the
methods reported previously (10).
A 242-bp ANXA9 fragment was amplified. Two human
ANXA9 oligonucleotide primers for the polymerase chain
reaction (PCR) were designed as follows: Forward,
5′-TGAGCCCAATTACCAAGTCC-3′ and reverse, 5′-GTTCAGCCAAACACGGAAAT-3′.
The forward primer is located in exon 13 and the reverse primer in
exon 14. A PCR kit (TaKaRa Ex Taq; Takara, Kyoto, Japan) on GeneAMP
PCR System 9600 (PE Applied Biosystems, Foster City, CA, USA) was
used to perform 35 cycles of PCR with the following parameters:
95°C for 40 sec, 45°C for 40 sec and 72°C for 60 sec. An 8-μl
aliquot of each reaction mixture was size-fractionated in a 1.5%
agarose gel and visualized with ethidium bromide staining. To
ensure that the RNA was not degraded, a PCR assay with primers
specific for the glyceraldehyde-3-phosphate dehydrogenase
(GAPDH) gene was performed for 1 min at 95°C, 1 min at 56°C
and 1 min at 72°C for 30 cycles. The GAPDH primers were as
follows: Forward, 5′-TTGGTATCGTGGAAGGACTCA-3′ and reverse,
5′-TGTCATCATATTGGCAGGTT-3′, and produced a 270-bp amplicon.
Complementary DNA from the Human Reference Total RNA (Clontech,
Palo Alto, CA, USA) was studied concurrently as a source of
positive controls. For quantitative assessment, reverse
transcription-quantitative PCR (RT-qPCR) was performed using a
LightCycler FastStart DNA Master SYBR Green I kit (Roche
Diagnostics, Tokyo, Japan) for cDNA amplification of ANXA9
and GAPDH. The amplification protocol consisted of 35 cycles
of denaturation at 95°C for 10 sec, annealing at 60°C for 10 sec
and elongation at 72°C for 10 sec. The products were then subjected
to a temperature gradient from 55°C to 95°C with continuous
fluorescence monitoring to produce a melting curve of the products.
The expression ratios of the ANXA9 mRNA copies in the tumor
and normal tissues were calculated following normalization against
GAPDH mRNA expression.
Statistical analysis
The ANXA9 expression levels between the CRC
and normal colorectal mucosa (normal tissue) samples, and the
association between ANXA9 expression and the
clinicopathological factors were analyzed with the χ2
test. Kaplan-Meier survival curves were plotted and compared with
the generalized log-rank test. Univariate and multivariate analyses
to identify prognostic factors were performed using Cox’s
proportional hazard regression model. The values in the in
vitro assays were analysed with Wilcoxon’s rank test. All tests
were analyzed with JMP software (SAS Institute, Cary, NC, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Expression of ANXA9 in clinical tissue
specimens
RT-qPCR analysis was performed with primary CRC
tissues and samples from adjacent normal colorectal regions.
ANXA9 expression was calculated as ANXA9/GAPDH
expression for each tumor or normal tissue sample (Fig. 1). The mean expression level in the
tumor tissues was found to be larger than that of the normal
tissues, and there was a significant difference between the tumor
and normal tissues subsequent to dividing the samples into two
groups according to the mean expression value of the tumor and
normal tissues (P=0.047) (Table I).
In the following analyses, ANXA9 expression normalized by
GAPDH expression in the tumor tissue was calculated
following division by ANXA9 expression level in the normal
tissue.
 | Table IANXA9 mRNA expression in
primary CRC specimens and normal colorectal mucosa samples. |
Table I
ANXA9 mRNA expression in
primary CRC specimens and normal colorectal mucosa samples.
| ANXA9
expression | Primary CRC | Normal mucosa | P-value |
|---|
| <Mean | 41 | 55 | 0.047* |
| ≥Mean | 59 | 45 | |
Expression of ANXA9 and
clinicopathological characteristics
For the clinicopathological evaluation, experimental
samples were divided into two groups according to the expression
status. Patients with values >1 (ANXA9 expression level
in the tumor tissue was larger than that of the corresponding
normal tissue) were assigned to the high expression group and the
others were assigned to the low expression group.
Clinicopathological factors associated with the ANXA9
expression status of the 100 patients are summarized in Table II. The data indicated that
ANXA9 expression was not significantly correlated with these
clinicopathological factors.
 | Table IIClinicopathological factors of 100
colorectal cancer patients with high (n=56) and low (n=44) levels
of ANXA9 mRNA expression. |
Table II
Clinicopathological factors of 100
colorectal cancer patients with high (n=56) and low (n=44) levels
of ANXA9 mRNA expression.
| Factors | Low expression, n
(%) | High expression, n
(%) | P-value |
|---|
| Age, years |
| <68 | 25 (56.8) | 27 (48.2) | 0.392 |
| ≥68 | 19 (43.2) | 29 (51.8) | |
| Gender |
| Male | 24 (54.5) | 37 (66.1) | 0.244 |
| Female | 20 (45.5) | 19 (33.9) | |
| Histological
grade |
| Well-moderate | 41 (93.2) | 55 (98.2) | 0.202 |
| Poor | 3 (6.8) | 1 (1.8) | |
| Tumor size, mm |
| <30 | 8 (18.2) | 9 (16.1) | 0.780 |
| ≥30 | 36 (81.8) | 47 (83.9) | |
| Tumor invasion |
| Tis | 4 (9.1) | 3 (5.4) | 0.316 |
| T1 | 7 (15.9) | 6 (10.7) | |
| T2 | 9 (20.4) | 6 (10.7) | |
| T3 | 19 (43.2) | 28 (50.0) | |
| T4 | 5 (11.4) | 13 (23.2) | |
| Lymph node
metastasis |
| N0 | 31 (70.5) | 33 (58.9) | 0.233 |
| N1–2 | 13 (29.5) | 23 (41.1) | |
| Lymphatic
invasion |
| Absent | 20 (45.5) | 26 (46.4) | 0.824 |
| Present | 24 (54.5) | 30 (53.6) | |
| Venous invasion |
| Absent | 33 (75.0) | 42 (75.0) | 1.000 |
| Present | 11 (25.0) | 14 (25.0) | |
| Metastasis |
| M0 | 38 (86.4) | 50 (89.3) | 0.655 |
| M1 | 6 (13.6) | 6 (10.7) | |
| UICC stage |
| 0-I | 17 (38.6) | 13 (23.2) | 0.221 |
| II | 13 (29.5) | 18 (32.1) | |
| IIIA | 4 (9.1) | 14 (25.0) | |
| IIIB | 4 (9.1) | 5 (8.9) | |
| IV | 6 (13.6) | 6 (10.7) | |
Association between ANXA9 expression and
prognosis
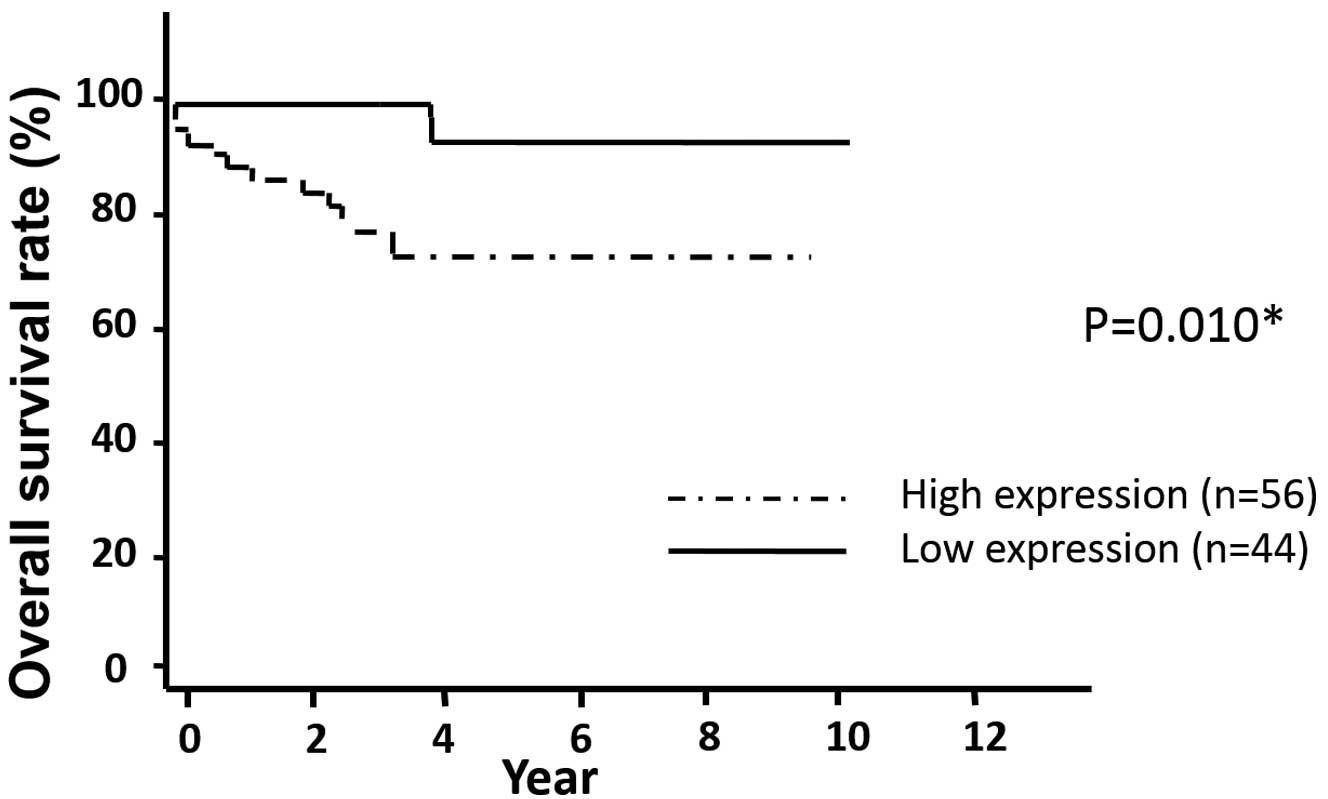
The data shows that the post-operative overall
survival rate was significantly lower in the patients in the high
expression group compared with that in the low expression group
(P=0.010) (Fig. 2). The median
follow-up time was 3.83 years. Table
III shows the results of the univariate and multivariate
analyses for factors associated with overall survival. The
univariate analysis showed that age (P=0.010), tumor invasion
(P=0.002), lymph node metastasis (P=0.001), lymphatic invasion
(P=0.012), venous invasion (P=0.026), metastasis (P<0.001) and
ANXA9 expression (P=0.004) were significantly correlated
with overall survival. The multivariate regression analysis
indicated that the ANXA9 high expression group (hazard
ratio, 3.13; 95% confidence interval, 1.32–12.86; P=0.007), tumor
invasion (hazard ratio, 2.21; 95% confidence interval, 1.09–5.02;
P=0.026) and distant metastasis (hazard ratio, 10.82; 95%
confidence interval, 3.08–35.36; P=0.001) were independent
predictors of overall survival.
 | Table IIIUnivariate and multivariate analyses
for overall survival (Cox’s proportional hazards regression
model). |
Table III
Univariate and multivariate analyses
for overall survival (Cox’s proportional hazards regression
model).
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|---|
| Factors | HR | 95% CI | P-value | HR | 95% CI | P-value |
| Age, years
(<68/≥68) | 5.43 | 1.42–35.40 | 0.010* | 2.58 | 0.52–18.92 | 0.252 |
| Gender
(male/female) | 1.96 | 0.58–8.84 | 0.288 | | | |
| Histological grade
(poor/well-moderate) | 0.01 | 0.00–3.02 | 0.495 | | | |
| Tumor size, mm
(≥30/<30) | 1.13 | 0.58–2.90 | 0.731 | | | |
| Tumor invasion
(T4/Tis-3) | 2.47 | 1.39–4.54 | 0.002* | 2.21 | 1.09–5.02 | 0.026* |
| Lymph node
metastasis (N1–2/N0) | 6.88 | 2.04–31.12 | 0.001* | 2.07 | 0.45–10.76 | 0.342 |
| Lymphatic invasion
(present/absent) | 5.31 | 1.39–34.63 | 0.012* | 1.49 | 0.29–10.93 | 0.640 |
| Venous invasion
(present/absent) | 3.78 | 1.17–12.14 | 0.026* | 2.77 | 0.77–10.59 | 0.116 |
| Distant metastasis
(M1/M0) | 10.82 | 3.08–35.36 | <0.001* | 16.64 | 2.92–132.64 | 0.001* |
| ANXA9
expression (high/low) | 3.00 | 1.32–12.86 | 0.004* | 3.13 | 1.30–13.69 | 0.007* |
Discussion
The annexin gene family was discovered in 1984 and
the members were isolated in the presence of calcium, which served
as a substrate for the epidermal growth factor receptor/kinase
(11,12). ANXA9, initially termed annexin 31,
is a protein that is believed to function in the organization and
regulation of membrane/cytoskeleton linkage (13). The gastrointestinal cancer cell
line, HepG2, expresses ANXA9 protein, which can be detected by
A9-specific antibodies (3). The
expression of annexin has been reported in Xenopus,
Drosophilia, Dictyostelium, Caenorhabditis
elegans, Neurospora, Giardia and all plants
(14–16). However, the role of annexin in
cancer has not been clearly defined and the biological analysis
remains incomplete.
The present study showed that ANXA9
expression is an independent prognostic factor for CRC. This
suggests that tumor malignancy correlates with ANXA9
expression and that this may also affect the values of other
prognostic factors in multivariate analysis, such as distant
metastasis, which was significant in the univariate analysis.
ANXA9 expression was a significant prognostic factor
reflecting overall survival and distant metastasis. To the best of
our knowledge, the present study is the first to show ANXA9
as a statistically significant predictor for CRC prognosis
following curative resection, as well as other reported factors
(17). The present results suggest
that an ANXA9-dependent pathway may be involved in the
progression of CRC.
The prediction of recurrence and metastases
following curative surgical resection aid the determination of the
necessity for intensive follow-up and adjuvant CRC therapy
(18–20). While certain patients respond well
to CRC treatment, others do not, therefore individualized
predictions and strategies with higher precision are required for
treating metastasis (21). In the
present study, the clinicopathological analysis revealed that CRC
patients with low levels of ANXA9 expression showed an
improved prognosis for overall survival compared with those
patients with high levels of expression. The data indicates that
ANXA9 is a presumptive novel predictor of CRC prognosis.
Several adjuvant chemotherapies are valuable in
specific stages of CRC and indicate the usefulness of less invasive
surgery for the disease (17–20,22–28).
For these cases, an informative prognostic marker, which is
independent from the traditional TNM classification and contributes
to diagnoses and treatments, is extremely important. The present
data indicate the candidacy of ANXA9. While improved
pre-operative and post-operative treatments, such as chemotherapy
and radiotherapy combined with surgery, have contributed to the
reduction of recurrences of CRC, half of all cases eventually
metastasize despite systemic chemotherapy followed by surgery
(29). Adjuvant chemotherapy for
CRC is preferred in highly suspicious cases of recurrence. In these
cases, ANXA9 analysis may aid in the predictions and
treatment of patients with a poor prognosis.
References
|
1
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010.
|
|
2
|
Stein U and Schlag PM: Clinical,
biological, and molecular aspects of metastasis in colorectal
cancer. Recent Results Cancer Res. 176:61–80. 2007.
|
|
3
|
Goebeler V, Ruhe D, Gerke V and Rescher U:
Atypical properties displayed by annexin A9, a novel member of the
annexin family of Ca(2+) and lipid binding proteins. FEBS Lett.
546:359–364. 2003.
|
|
4
|
Hawkins TE, Merrifield CJ and Moss SE:
Calcium signaling and annexins. Cell Biochem Biophys. 33:275–296.
2000.
|
|
5
|
Gerke V and Moss SE: Annexins: from
structure to function. Physiol Rev. 82:331–371. 2002.
|
|
6
|
Smid M, Wang Y, Klijn JG, et al: Genes
associated with breast cancer metastatic to bone. J Clin Oncol.
24:2261–2267. 2006.
|
|
7
|
Watanabe T, Itabashi M, Shimada Y, et al:
Japanese Society for Cancer of the Colon and Rectum: Japanese
Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2010
for the treatment of colorectal cancer. Int J Clin Oncol. 17:1–29.
2012.
|
|
8
|
Sobin LH and Fleming ID: TNM
Classification of Malignant Tumors, fifth edition (1997). Union
Internationale Contre le Cancer and the American Joint Committee on
Cancer. Cancer. 80:1803–1804. 1997.
|
|
9
|
Mimori K, Mori M, Shiraishi T, et al:
Clinical significance of tissue inhibitor of metalloproteinase
expression in gastric carcinoma. Br J Cancer. 76:531–536. 1997.
|
|
10
|
Mori M, Staniunas RJ, Barnard GF, et al:
The significance of carbonic anhydrase expression in human
colorectal cancer. Gastroenterology. 105:820–826. 1993.
|
|
11
|
Fava RA and Cohen S: Isolation of a
calcium-dependent 35-kilodalton substrate for the epidermal growth
factor receptor/kinase from A-431 cells. J Biol Chem.
259:2636–2645. 1984.
|
|
12
|
Huang KS, Wallner BP, Mattaliano RJ, et
al: Two human 35 kd inhibitors of phospholipase A2 are related to
substrates of pp60v-src and of the epidermal growth factor
receptor/kinase. Cell. 46:191–199. 1986.
|
|
13
|
Morgan RO, Bell DW, Testa JR and Fernandez
MP: Human annexin 31 genetic mapping and origin. Gene. 227:33–38.
1999.
|
|
14
|
Morgan RO and Fernández MP: Molecular
phylogeny of annexins and identification of a primitive homologue
in Giardia lamblia. Mol Biol Evol. 12:967–979. 1995.
|
|
15
|
Morgan RO and Fernandez MP: Annexin gene
structures and molecular evolutionary genetics. Cell Mol Life Sci.
53:508–515. 1997.
|
|
16
|
Braun EL, Kang S, Nelson MA and Natvig DO:
Identification of the first fungal annexin: analysis of annexin
gene duplications and implications for eukaryotic evolution. J Mol
Evol. 47:531–543. 1998.
|
|
17
|
André T, Quinaux E, Louvet C, et al: Phase
III study comparing a semimonthly with a monthly regimen of
fluorouracil and leucovorin as adjuvant treatment for stage II and
III colon cancer patients: final results of GERCOR C96.1. J Clin
Oncol. 25:3732–3738. 2007.
|
|
18
|
Wolpin BM and Mayer RJ: Systemic treatment
of colorectal cancer. Gastroenterology. 134:1296–1310. 2008.
|
|
19
|
Kornmann M, Formentini A, Ette C, et al:
Prognostic factors influencing the survival of patients with colon
cancer receiving adjuvant 5-FU treatment. Eur J Surg Oncol.
34:1316–1321. 2008.
|
|
20
|
Bathe OF, Dowden S, Sutherland F, et al:
Phase II study of neoadjuvant 5-FU + leucovorin + CPT-11 in
patients with resectable liver metastases from colorectal
adenocarcinoma. BMC Cancer. 4:322004.
|
|
21
|
Sadanandam A, Lyssiotis CA, Homicsko K, et
al: A colorectal cancer classification system that associates
cellular phenotype and responses to therapy. Nat Med. 19:619–625.
2013.
|
|
22
|
Iijima M, Kano Y, Nohno T and Namba M:
Cloning of cDNA with possible transcription factor activity at the
G1-S phase transition in human fibroblast cell lines. Acta Med
Okayama. 50:73–77. 1996.
|
|
23
|
Hansen WJ, Cowan NJ and Welch WJ:
Prefoldin-nascent chain complexes in the folding of cytoskeletal
proteins. J Cell Biol. 145:265–277. 1999.
|
|
24
|
Hodgson G, Hager JH, Volik S, et al:
Genome scanning with array CGH delineates regional alterations in
mouse islet carcinomas. Nat Genet. 29:459–464. 2001.
|
|
25
|
Lacy AM, García-Valdecasas JC, Delgado S,
et al: Laparoscopy-assisted colectomy versus open colectomy for
treatment of non-metastatic colon cancer: a randomised trial.
Lancet. 359:2224–2229. 2002.
|
|
26
|
Weeks JC, Nelson H, Gelber S, et al:
Clinical Outcomes of Surgical Therapy (COST) Study Group:
Short-term quality-of-life outcomes following laparoscopic-assisted
colectomy vs open colectomy for colon cancer: a randomized trial.
JAMA. 287:321–328. 2002.
|
|
27
|
Clinical Outcomes of Surgical Therapy
Study Group. A comparison of laparoscopically assisted and open
colectomy for colon cancer. N Engl J Med. 350:2050–2059. 2004.
|
|
28
|
Jayne DG, Guillou PJ, Thorpe H, et al: UK
MRC CLASICC Trial Group: Randomized trial of laparoscopic-assisted
resection of colorectal carcinoma: 3-year results of the UK MRC
CLASICC Trial Group. J Clin Oncol. 25:3061–3068. 2007.
|
|
29
|
Koshariya M, Jagad RB, Kawamoto J, et al:
An update and our experience with metastatic liver disease.
Hepatogastroenterology. 54:2232–2239. 2007.
|