Introduction
Vascular endothelial growth factor (VEGF) promotes
angiogenesis in hepatocellular carcinoma (HCC). VEGF is upregulated
in HCC as compared with surrounding non-HCC tissues (1); this upregulation has been correlated
with advanced stage and poor outcome in HCC (2). Sorafenib, a multikinase inhibitor
administered orally to HCC patients, targets the VEGF receptor,
platelet-derived growth factor receptor and c-kit (3). Sorafenib treatment has been
demonstrated to significantly prolong the survival times of HCC
patients: 10.7 months as compared with 7.9 months in a placebo
group (4).
However, one limitation of sorafenib treatment is
the resistance of HCC to the reagent. Phosphatidyl-inositol (PI) 3
kinase and mitogen-activated protein (MAP) kinase are predominant
downstream signaling pathways of VEGF that regulate cell
proliferation (5). Although
sorafenib inhibits the MAP kinase signaling pathway (6), the PI3 kinase signaling pathway is not
affected, thereby resulting in HCC resistance (7). Another limitation of sorafenib is
toxicity, which negatively affects the patient’s quality of life.
For example, a high rate of dermatological adverse effects has been
reported (4,8). However, administering a combination of
sorafenib and other molecular targeting agents is expected to
improve the efficacy and relieve particular adverse effects of the
drug. For example, liver-specific microRNA-122 sensitizes tumors to
the antitumor effects of sorafenib (9). However, a major limitation of microRNA
is that the effects depend on transfection efficiency;
untransfected cells are not affected. Therefore, small molecule
inhibitors are desirable as these inhibitors affect the majority of
cells.
Insulin-like growth factor (IGF)-1 is a hormone that
is expressed abundantly in the fetus and exerts an important role
in fetal growth and development. Inhibiting the IGF-1 signaling
pathway in cancer therapy may have no adverse effects, since IGF-1
concentrations are reduced following birth (10). Picropodophyllin (PPP) is a specific
inhibitor of the IGF-1 receptor (IGF-1R), which is involved in
tumor cell growth (11,12). PPP has been shown to successfully
suppress the proliferation of HCC and hepatoblastoma cells
(13,14).
Therefore, in the present study, the proliferation
and motility of HCC cells that had been treated with a combination
of PPP and sorafenib were analyzed. Normal human umbilical vein
endothelial cells (HUVECs) were also used to assess angiogenesis
following drug treatment.
Materials and methods
Cell culture
HLF and PLC/PRF/5 HCC lines were purchased from the
RIKEN cell bank (RIKEN Life Science Center, Tsukuba, Japan) and
cultured in Dulbecco’s modified Eagle’s medium (DMEM;
Sigma-Aldrich, St. Louis, MO, USA) supplemented with 10% fetal
bovine serum (Life Technologies, Grand Island, NY, USA). HUVECs
(Lonza, Basel, Switzerland) were cultured in EGM™-2 BulletKit™
(Lonza) following the manufacturer’s instructions. The cultured
cells were incubated in 5% carbon dioxide at 37°C in a humidified
chamber. Hematoxylin and eosin (H&E) staining was performed on
cells grown in four-well chambers (Becton Dickinson, Franklin
Lakes, NJ, USA) after 48 h of incubation.
Cell proliferation assay
The HUVEC cells were trypsinized, harvested and
plated onto 96-well flat-bottom plates (Asahi Techno Glass,
Funabashi, Japan) at a density of 1,000 cells per well. Following
24 h of culture, sorafenib (JS Research Chemicals Trading e.Kfm,
Wedel, Germany) or PPP (Wako Pure Chemical Industries, Ltd., Osaka,
Japan) was added to the medium. After 72 h of incubation, a
3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium
inner salt (MTS) assay was performed following the manufacturer’s
instructions (Promega Corporation, Madison, WI, USA). The MTS was
bio-reduced by the cells into a colored formazan product, the
absorbance of which was analyzed at a wavelength of 490 nm using an
iMark microplate reader (Bio-Rad, Hercules, CA, USA).
Scratch assay
The HUVEC cells were injured using a sterile 200-μm
pipette tip at 24 h after plating into four-well chambers; the
cells were then stained with H&E after 48 h (15). The distance between the scratched
line and the growing edge of the cells was measured at five
points.
Statistical analysis
One-way analysis of variance was utilized for
statistical analysis using JMP10.0.2 (SAS Institute, Cary, NC,
USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
MTS assay
The synergistic suppression of cell proliferation by
PPP and sorafenib was analyzed using an MTS assay. The
proliferation of HLF cells following treatment with 10 μM sorafenib
and 0, 0.02, 0.06, 0.2 and 0.6 μM PPP was suppressed to 61.4±1.0,
44.3±14.0, 19.2±12.8, 20.9±6.3 and 10.3±6.7%, respectively, of the
proliferation observed in the untreated control cells (Fig. 1A). Similarly, the proliferation of
PLC/PRF/5 cells was suppressed to 44.2±0.1, 26.3±6.9, 25.5±8.3,
18.1±6.2 and 14.2±8.6% of control cell proliferation following
treatment with 10 μM sorafenib and 0, 0.02, 0.06, 0.2 and 0.6 μM
PPP, respectively (Fig. 1B). The
proliferation of HUVECs was suppressed to 19.8±0.1, 15.6±2.9,
8.7±5.7, 3.5±1.8 and 5.4±3.8% control cell proliferation using the
same respective treatments (Fig.
1C). Therefore, PPP inhibited cell proliferation in a
dose-dependent manner.
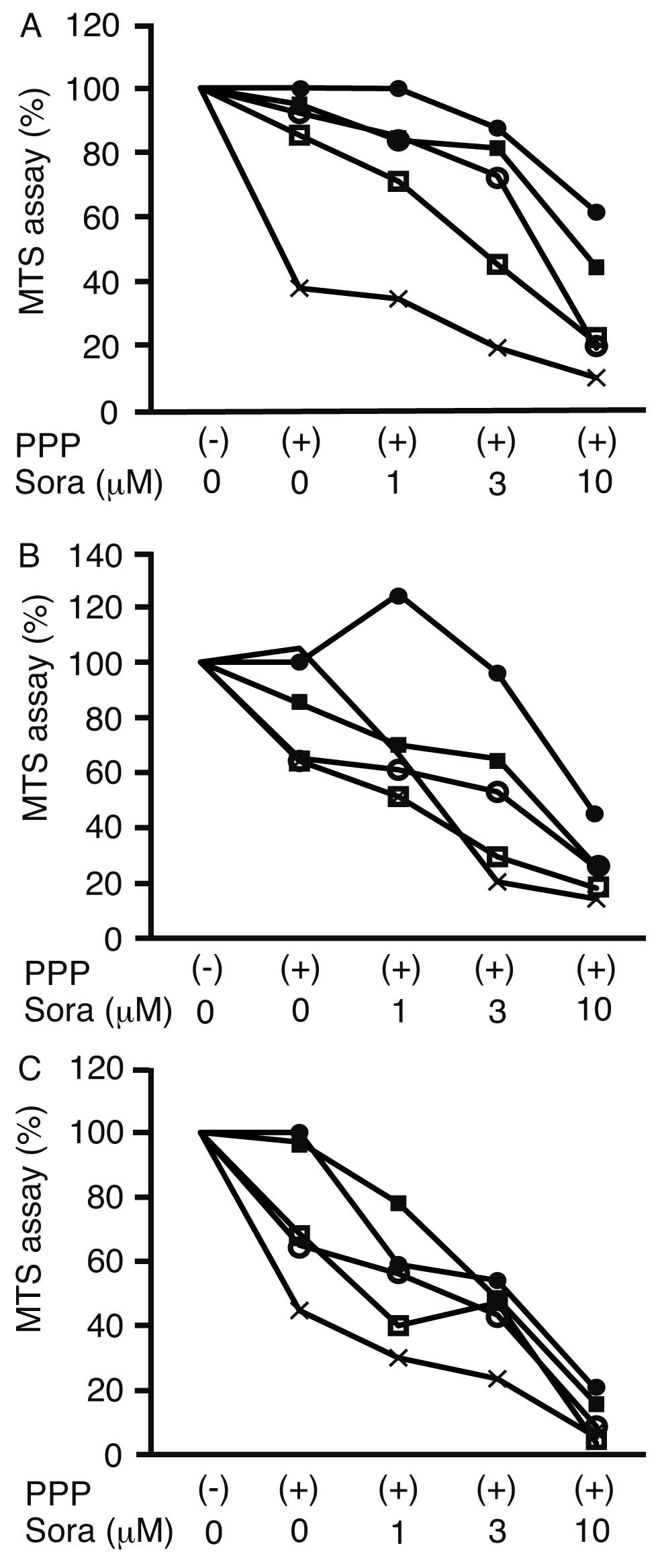 | Figure 1Cell proliferation assay. A
3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium
inner salt assay was performed following the addition of
picropodophyllin (PPP) and/or sorafenib (Sora) to (A) HLF, (B)
PLC/PRL/F and (C) normal human umbilical vein endothelial cells,
and the cell proliferation rate is presented as the percentage of
the untreated cell proliferation rate. ●, 0 μM PPP; ■, 0.02 μM PPP;
○, 0.06 μM PPP; □, 0.2 μM PPP; ×, 0.6 μM PPP; (−), without PPP;
(+), with PPP, n=3. |
Tables I and
II show the raw data obtained from
the MTS assays in HLF and PLC/RRF/5 cells, respectively. The
combination of sorafenib and PPP suppressed cell proliferation more
efficiently than 10 μM sorafenib alone. The initial aim was to
reduce the concentration of sorafenib and, therefore, the effects
of 3 μM sorafenib were analyzed. The combination of 3 μM sorafenib
and 0.2 or 0.6 μM PPP suppressed the proliferation of HLF and
PLC/PRF/5 cells more effectively than 10 μM sorafenib alone.
Therefore, the combination of 3 μM sorafenib and 0.2 μM PPP was
used for subsequent experiments.
 | Table ICell proliferation assay in HLF
cells. |
Table I
Cell proliferation assay in HLF
cells.
| Sorafenib (μM) |
|---|
|
|
|---|
| 0 | 1 | 3 | 10 |
|---|
| PPP (μM) |
| 0.00 | 100 | 99.8±7.8 | 87.5±5.6 | 61.6±1.0 |
| 0.02 | 94.9±5.2 | 83.7±12.4 | 81.3±9.5 | 44.3±14.0a |
| 0.06 | 92.2±7.9 | 85.4±7.2 | 72.7±10.3 | 19.2±7.8a |
| 0.20 | 85.3±12.4 | 71.3±15.3 | 45.2±6.2a | 20.9±6.3a |
| 0.60 | 37.8±2.9a | 34.5±8.1a | 19.3±8.7a | 10.0±6.7a |
 | Table IICell proliferation assay in PLC/PRF/5
cells. |
Table II
Cell proliferation assay in PLC/PRF/5
cells.
| Sorafenib (μM) |
|---|
|
|
|---|
| 0 | 1 | 3 | 10 |
|---|
| PPP (μM) |
| 0.00 | 100 | 124.6±30.1 | 95.8±10.7 | 44.2±0.1 |
| 0.02 | 85.2±9.5 | 69.7±9.8 | 64.6±13.2 | 26.3±6.9a |
| 0.06 | 65.2±6.9 | 61.0±13.2 | 53.4±12.5 | 25.5±8.3a |
| 0.20 | 105.4±7.2 | 66.7±11.4 | 20.4±9.7a | 14.2±8.6a |
| 0.60 | 64.2±8.1 | 51.3±4.1 | 29.5±6.9a | 18.1±6.2a |
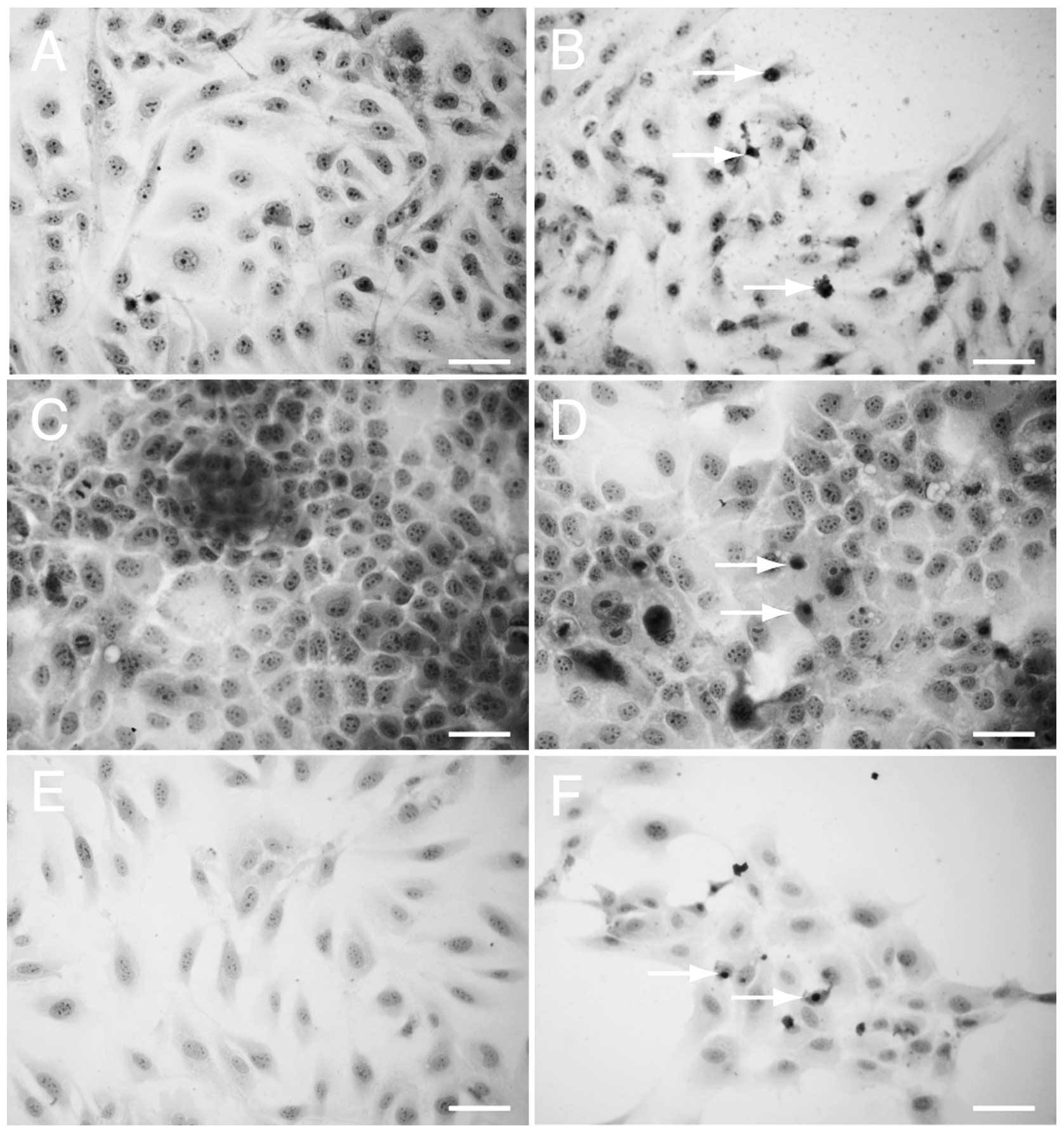
H&E staining
HLF (Fig. 2A and B),
PLC/PRF/5 (Fig. 2C and D) and HUVEC
(Fig. 2E and F) cells were stained
with H&E to assess the morphological changes following drug
treatment. The cells that had been treated with 3 μM sorafenib and
0.2 μM PPP exhibited pyknotic nuclei, which is a characteristic of
apoptotic cells (Fig. 2B, D and F).
Pyknotic nuclei were not observed in the untreated cells (Fig. 2A, C and E).
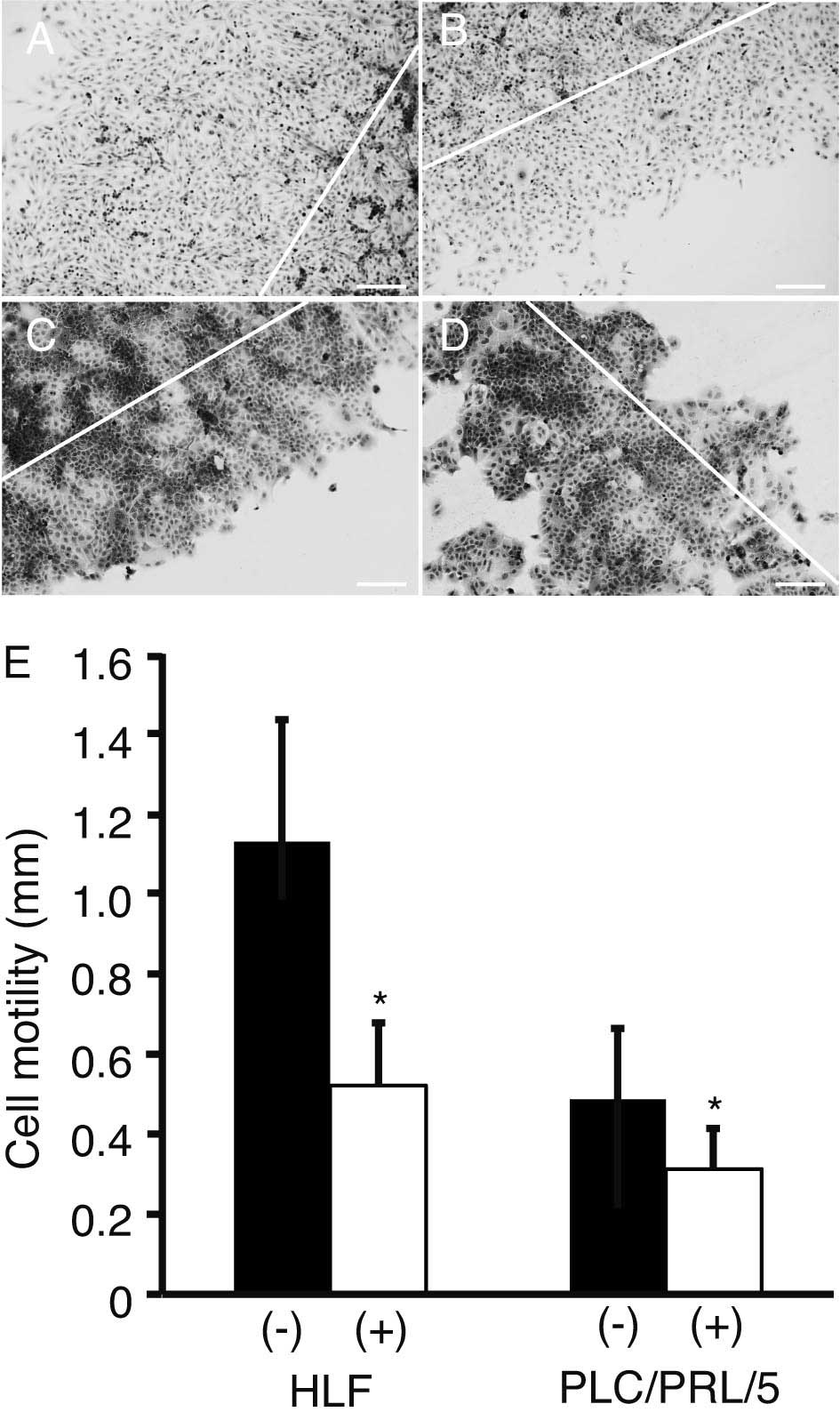
Scratch assay
The motility of HLF and PLC/PRF/5 cells was analyzed
using a scratch assay (Fig. 3A–D).
The distance between the scratched line and the growing edge of the
cells was measured for cells with (Fig.
3B and D) or without (Fig. 3A and
C) treatment with 3 μM sorafenib and 0.2 μM PPP. Cell motility
was significantly suppressed by the treatment, as compared with
that of the control (P<0.05; Fig.
3E).
Discussion
In the present study, PPP enhanced sorafenib-induced
suppression of proliferation and motility in HCC cells. NVP-AEW541,
another IGF-1R inhibitor, and sorafenib were previously shown to
suppress cell proliferation and induce apoptosis synergistically
(16). These data suggest that
IGF-1R inhibitors and sorafenib suppress cell proliferation
synergistically. Sorafenib upregulates IGF-1R and increases Akt
(Ser473) phosphorylation (17,18).
This suggests that sorafenib may activate signaling pathways
downstream of IGF-1R; thus, treating HCC cells with IGF-1R
inhibitors and sorafenib is feasible.
Cell motility may be used to indicate invasion and
metastasis (19). The invasiveness
of HCC cells has previously been revealed to be suppressed by PPP
and sorafenib individually (14,20).
However, to the best of our knowledge, the effects of the two drugs
in combination have not been examined. In the present study, the
effect of a combination of PPP and sorafenib on cell motility was
assessed. The data clearly indicate that PPP and sorafenib
suppressed the motility of HCC cells synergistically. This suggests
that the combination of these two drugs may inhibit HCC metastasis
to distant organs.
Suppressing angiogenesis is a predominant mechanism
of the antitumor effects of sorafenib (21). Similarly, inhibiting IGF-1R was
shown to suppress the proliferation of HUVECs and induce apoptosis
(22). The present study clearly
demonstrated that the combination of sorafenib and PPP markedly
suppressed proliferation and induced apoptosis in HUVECs. This
suggests that the combination of PPP and sorafenib may more
effectively suppress angiogenesis.
The combination of 1 μM NVP-AEW541 and 10 μM
sorafenib has been previously demonstrated to suppress cell
proliferation more effectively than 10 μM sorafenib alone (16). However, the combination of
NVP-AEW541 and concentrations of sorafenib lower than10 μM has not
been investigated. In the present study, the combination of 0.2 μM
PPP and 3 μM sorafenib decreased cell proliferation more
efficiently than 10 μM sorafenib alone. This suggests that using
co-treatment with an IGF-1R inhibitor may allow the effective dose
of sorafenib to be reduced, which may lower the risk of adverse
effects. Nevertheless, the combination of PPP and sorafenib may
cause different adverse events. Thus, future studies that analyze,
through western blotting, the signaling pathways that are altered
by co-treatment are required.
In conclusion, in the present study, PPP enhanced
sorafenib-induced suppression of proliferation and motility in HCC
cells. Therefore, the combination of PPP and sorafenib may exert
antitumor and antiangiogenic effects.
Acknowledgements
This study was supported in part by a Research
Grant-in-Aid for Scientific Research (grant no. 23591002) from the
Japan Society for the Promotion of Science.
References
|
1
|
El-Assal ON, Yamanoi A, Soda Y, et al:
Clinical significance of microvessel density and vascular
endothelial growth factor expression in hepatocellular carcinoma
and surrounding liver: possible involvement of vascular endothelial
growth factor in the angiogenesis of cirrhotic liver. Hepatology.
27:1554–1562. 1998.
|
|
2
|
Chao Y, Li CP, Chau GY, et al: Prognostic
significance of vascular endothelial growth factor, basic
fibroblast growth factor, and angiogenin in patients with
resectable hepatocellular carcinoma after surgery. Ann Surg Oncol.
10:355–362. 2003.
|
|
3
|
Shin JW and Chung YH: Molecular targeted
therapy for hepatocellular carcinoma: current and future. World J
Gastroenterol. 19:6144–6155. 2013.
|
|
4
|
Llovet JM, Ricci S, Mazzaferro V, et al:
SHARP Investigators Study Group: Sorafenib in advanced
hepatocellular carcinoma. N Engl J Med. 359:378–390. 2008.
|
|
5
|
Villanueva A and Llovet JM: Targeted
therapies for hepatocellular carcinoma. Gastroenterology.
140:1410–1426. 2011.
|
|
6
|
Liu L, Cao Y, Chen C, et al: Sorafenib
blocks the RAF/MEK/ERK pathway, inhibits tumor angiogenesis, and
induces tumor cell apoptosis in hepatocellular carcinoma model
PLC/PRF/5. Cancer Res. 66:11851–11858. 2006.
|
|
7
|
Zhai B and Sun XY: Mechanisms of
resistance to sorafenib and the corresponding strategies in
hepatocellular carcinoma. World J Hepatol. 5:345–352. 2013.
|
|
8
|
Zhang X, Yang XR, Huang XW, et al:
Sorafenib in treatment of patients with advanced hepatocellular
carcinoma: a systematic review. Hepatobiliary Pancreat Dis Int.
11:458–466. 2012.
|
|
9
|
Bai S, Nasser MW, Wang B, et al:
MicroRNA-122 inhibits tumorigenic properties of hepatocellular
carcinoma cells and sensitizes these cells to sorafenib. J Biol
Chem. 284:32015–32027. 2009.
|
|
10
|
Brown AL, Graham DE, Nissley SP, et al:
Developmental regulation of insulin-like growth factor II mRNA in
different rat tissues. J Biol Chem. 261:13144–13150. 1986.
|
|
11
|
Girnita A, Girnita L, del Prete F, et al:
Cyclolignans as inhibitors of the insulin-like growth factor-1
receptor and malignant cell growth. Cancer Res. 64:236–242.
2004.
|
|
12
|
Tomizawa M, Shinozaki F, Sugiyama T, et
al: Insulin-like growth factor I receptor involvement in
proliferation of NOR-P1 cells in serum-free media. J Cell Biochem.
113:2714–2720. 2012.
|
|
13
|
Tomizawa M and Saisho H: Signaling pathway
of insulin-like growth factor-II as a target of molecular therapy
for hepatoblastoma. World J Gastroenterol. 12:6531–6535. 2006.
|
|
14
|
Tomizawa M and Yokosuka O:
Picropodophyllin suppresses the proliferation and invasion of
hepatocellular carcinoma under serum starvation. Mol Med Rep.
1:685–688. 2008.
|
|
15
|
Pennisi PA, Barr V, Nunez NP, Stannard B
and Le Roith D: Reduced expression of insulin-like growth factor I
receptors in MCF-7 breast cancer cells leads to a more metastatic
phenotype. Cancer Res. 62:6529–6537. 2002.
|
|
16
|
Ou DL, Lee BS, Chang YC, et al:
Potentiating the efficacy of molecular targeted therapy for
hepatocellular carcinoma by inhibiting the insulin-like growth
factor pathway. PLoS One. 8:e665892013.
|
|
17
|
Huynh H, Ngo VC, Koong HN, et al: AZD6244
enhances the anti-tumor activity of sorafenib in ectopic and
orthotopic models of human hepatocellular carcinoma (HCC). J
Hepatol. 52:79–87. 2010.
|
|
18
|
Gedaly R, Angulo P, Hundley J, et al:
PKI-587 and sorafenib targeting PI3K/AKT/mTOR and Ras/Raf/MAPK
pathways synergistically inhibit HCC cell proliferation. J Surg
Res. 176:542–548. 2012.
|
|
19
|
Tomizawa M, Kondo F and Kondo Y: Growth
patterns and interstitial invasion of small hepatocellular
carcinoma. Pathol Int. 45:352–358. 1995.
|
|
20
|
Tomizawa M, Shinozaki F, Sugiyama T, et
al: Sorafenib suppresses the cell cycle and induces the apoptosis
of hepatocellular carcinoma cell lines in serum-free media. Exp
Ther Med. 1:863–866. 2010.
|
|
21
|
Xiong YQ, Sun HC, Zhang W, et al: Human
hepatocellular carcinoma tumor-derived endothelial cells manifest
increased angiogenesis capability and drug resistance compared with
normal endothelial cells. Clin Cancer Res. 15:4838–4846. 2009.
|
|
22
|
Bid HK, London CA, Gao J, et al: Dual
targeting of the type 1 insulin-like growth factor receptor and its
ligands as an effective antiangiogenic strategy. Clin Cancer Res.
19:2984–2994. 2013.
|