Introduction
Non small-cell lung cancer (NSCLC) is one of the
most commonly diagnosed types of cancer and a leading cause of
mortality worldwide (1).
Chemotherapy is a crucial strategy for advanced-stage NSCLC, and
paclitaxel (PTX) is employed as a front-line chemotherapeutic agent
in clinical oncology (2,3).
PTX is a member of the taxanes family that promotes
microtubule assembly and interferes with signal transduction
(4,5). The potent antitumor efficacy of PTX is
mediated by the direct induction of DNA damage and cell death by
apoptosis (4,6,7).
However, the efficiency of PTX-based cancer chemotherapy is
increasingly limited by the development of therapeutic resistance
(8–10). Thus, the challenge for improving
chemotherapeutic efficacy is in the development of strategies that
enhance cancer cell sensitivity to treatment.
MicroRNAs (miRNAs) are small, non-coding RNAs of ~22
nucleotides. Mature miRNAs partially bind to their target mRNAs at
complementary sites in the 3′-untranslated region (3′-UTR), and
regulate gene expression (11,12).
miRNAs are responsible for various biological and pathological
processes, including cancer development and progression (11–14).
Recent studies have reported several miRNAs that enhance
chemotherapeutic efficacy by modulating the sensitivity of cancer
cells to conventional chemotherapeutic drugs (15,16).
It has been shown that miR-221/222 confers tamoxifen resistance in
breast cancer (17); miR-137
sensitizes doxorubicin-resistant neuroblastoma cells to
doxorubicin, as shown by reduced proliferation and increased
apoptosis (18); and miR-21 confers
cisplatin resistance in gastric cancer cells (19). Other miRNAs, including miR-328
(20), miR-326 (21), and miR-34a (22), have also been shown to modulate
chemosensitivity.
We previously reported miR-7 as a key tumor
suppressor in NSCLC that could suppress cell proliferation, induce
apoptosis, inhibit cancer migration and reduce tumorigenicity in
A549 adenocarcinomic human alveolar basal cells (23). The function of miR-7 in PTX-mediated
chemotherapy for NSCLC, however, has not been investigated. Given
that our previous study demonstrated the importance of miR-7 in
NSCLC pathogenesis, the present study hypothesized that miR-7 plays
a crucial role in regulating NSCLC sensitivity to PTX.
To the best of our knowledge, this study provides
the first evidence of the potential utility of miR-7 as a
sensitizer in PTX therapy for NSCLC and thus provides a novel
molecular target for therapeutic development.
Materials and methods
Tissue samples and cell lines
Human NSCLC and matched adjacent tissues were
obtained from 20 patients at Zhongshan Hospital, Fudan University
(Shanghai, China) between 2008 and 2011. None of these patients had
received chemotherapy prior to surgery. This study was carried out
according to the World Medical Association Declaration of Helsinki
and was approved by the Medical Ethics Committee of Zhongshan
Hospital.
A549, H1395 human adenocarcinoma lymphoblasts, 95C
and 95D low and high metastatic human lung cancer cells, were
obtained from the Institute of Biochemistry and Cell Biology of the
Chinese Academy of Science (Shanghai, China). The HBE human
bronchial epithelial cell line was obtained from Xiangfu Bio
(Shanghai, China). These cell lines originated from the American
Type Culture Collection Manassas, VA, USA. A549, H1395, and HBE
were grown in Dulbecco’s modified Eagle’s medium (DMEM)
supplemented with 10% fetal bovine serum (FBS), 2 μM glutamine, 100
IU/ml penicillin, and 100 μg/ml streptomycin sulfate. 95C and 95D
cells were cultured in RPMI-1640 media, supplemented with 10% FBS,
2 μM glutamine, 100 IU/ml penicillin, and 100 μg/ml streptomycin
sulfate.
Quantitative polymerase chain reaction
(qPCR) for miRNA
Total RNA was extracted using TRIzol®
reagent (Invitrogen Life Sciences, Carlsbad, CA, USA) and
reverse-transcribed with miR-7-specific primers (Guangzhou RiboBio
Co., Ltd., Guangzhou, China). qPCR was performed using an ABI 7500
thermocycler (Applied Biosystems, Foster City, CA, USA) starting
with 1 μl cDNA and SYBR®-Green Realtime PCR Master Mix
(Toyobo Co., Ltd., Osaka, Japan). The relative amount of each miRNA
was normalized to the amount of U6. The primer sequences for U6 and
miR-7 are listed in Table I.
 | Table IPrimers used to detect the expression
of U6, miR-7, GAPDH and EGFR by quantitative polymerase chain
reaction. |
Table I
Primers used to detect the expression
of U6, miR-7, GAPDH and EGFR by quantitative polymerase chain
reaction.
| Primer | Sequence (5′-3′) |
|---|
| U6 | F:
TCAGTTTGCTGTTCTGGGTG
R: CGGTTGGCTGGAAAGGAG |
| miR-7 | F:
GGAAAGGCTCATTCGGACTA
R: ACGACGCCACCAATCACT |
| GAPDH | F:
TGCACCACCAACTGCTTAGC
R: GGCATGGACTGTGGTCATGAG |
| EGFR | F: GCG TTCGGCACG
GTGTATAA
R: GGCTTTCGGAGATGTTGCTTC |
miRNA transfection
The human miR-7 mimics (miR-7) (dsRNA
oligonucleotides), miR-7 inhibitor (single-stranded chemically
modified oligonucleotides), negative control mimic (miR-NC), and
negative control inhibitor (Ctrl inhibitor) were purchased from
RiboBio (Guangzhou RiboBio Co., Ltd). Cells were seeded in 24-well
plates (5×104 cells/well) for 24 h. When the cells were
30–50% confluent, they were transfected with miR-7 or miR-7
inhibitor (50 nmol final concentration) using
Lipofectamine® 2000 (Invitrogen Life Technologies). Six
hours after transfection, the cells were maintained in DMEM with
10% FBS for all subsequent treatments.
qPCR for EGFR
Following transfection with miR-7 or miR-NC for 24
h, A549/H1299 cells were treated with PTX (0.1 μM) for 24 h. qPCR
was performed in triplicate for each sample using the PrimerScript
RT reagent kit (Takara Bio, Inc., Dalian, China). GAPDH was used
for normalization. Primers for GAPDH and EGFR were designed using
Primer Express® (Invitrogen Life Technologies) and are
listed in Table I.
Western blotting
Proteins were extracted from A549 or 95D cells
following transfection with miR-7, miR-NC, miR-7 inhibitor, or Ctrl
inhibitor for 48 h using SDS lysis buffer (P0013G, Beyotime,
Shanghai China). Primary antibodies included anti-EGFR (1:2,000)
and anti-GAPDH (1:10,000) (Cell Signaling, Danvers, MA, USA). The
procedure for western blotting was performed as described
previously (14). Equal amounts of
protein were transferred onto polyvinylidene difluoride membranes
(Merck Millipore, Darmstadt, Germany)following resolution by
SDS-PAGE (P0012A, Beyotime). The membranes were blocked in 5%
non-fat milk and probed with the primary monoclonal rabbit
anti-human anti-EGFR (1:2,000) and monoclonal mouse anti-human
anti-GAPDH (1:10,000) antibodies (Cell Signaling). The membranes
were then washed and incubated with a polyclonal goat anti-mouse or
polyclonal goat anti-rabbit secondary antibody conjugated to
horseradish peroxidase. The specific bands were detected by
enhanced chemiluminescence (34095, Thermo Fisher Scientific,
Waltham, MA, USA)
Cell proliferation assay
Following transfection for 48 h, the cell
proliferation was measured using a Cell Counting Kit-8 assay
(CCK-8; Dojindo, Kunamoto, Japan), based upon a redox assay similar
to the MTT assay.
To analyze the effects of miRNA in combination with
PTX, the cells transfected with miR-7 or miR-NC were treated with
PTX at concentrations of 0, 10, 20, 40, 80 and 160 nM for 48 h. The
cell proliferation assays were performed using the CCK-8 assay
according to the manufacturer’s instructions.
The sensitivity of each type of cell to PTX was
evaluated using CCK-8, presented as the cell viability (%). The
concentration of PTX at which 50% of cells survived presents the
IC50 value.
Apoptosis
A549 or 95D cells were seeded in 24-well plates,
incubated for 24 h, and then transfected with miR-7, miR-NC, miR-7
inhibitor, or Ctrl inhibitor for a further 24 h. Following
transfection, the cells were treated with PTX (20 nM) for 24 h and
then collected. The level of apoptosis was analyzed by flow
cytometry and Annexin V/propidium iodide (PI) staining.
Statistical analysis
Data are presented as the mean ± standard error of
the mean, from replicate experiments (n>3). The results were
analyzed using PRISM 5.0 (GraphPad Software Inc., San Diego, CA,
USA). A Student’s t-test was used to analyze intergroup differences
for two groups, and analysis of variance was used to analyze >2
groups. P<0.05 was considered to indicate a statistically
significant difference.
Results
PTX-sensitivity of NSCLC cells is
dependent on endogenous miR-7 expression
PTX sensitivity was detected in various NSCLC cell
lines (A549, H1395, 95C, and 95D) of differing origins by in
vitro cell viability assays. Cell viability was observed to be
reduced by PTX in a dose-dependent manner (Fig. 1A). Although the trends were similar,
the sensitivity of the four NSCLC cell lines to PTX differed [half
maximal inhibitory concentration (IC50) A549: 207.8±1.38
nM; H1366: 159.6±1.42 nM; 95C: 131.3±0.74 nM; 95D: 87.94±1.41 nM),
suggesting that other factors may mediate PTX sensitivity. Our
previous screen detected miR-7 expression in these cell lines
(24). In the present study, miR-7
expression was shown to be frequently downregulated in these NSCLC
cell lines as compared with the normal lung epithelial cell line
(HBE). It was also found that miR-7 expression varied in different
cell lines, similar to the variation observed in PTX sensitivity.
It was observed that a higher expression of miR-7 in 95D cells
correlated with a higher sensitivity of this cell line to PTX,
strongly suggesting a positive association between PTX sensitivity
and endogenous miR-7 expression (Fig.
1B). Furthermore, 95D cells showed a higher apoptotic frequency
as compared with A549 cells following exposure to PTX (10.90 vs.
5.72% in early apoptosis, 22.90 vs. 12.87% in later apoptosis)
(Fig. 1C). These findings indicated
that PTX sensitivity in NSCLC cell lines is dependent on endogenous
miR-7 expression.
 | Figure 1PTX sensitivity of non-small cell lung
cancer (NSCLC) cells is positively correlated with endogenous miR-7
expression. (A) Cells were treated with different doses of PTX (0,
10, 20, 40, 80 and 160 nM) for 48 h. The cell viability was
determined using a Cell Counting kit-8 assay
(***P<0.001). (B) MiR-7 expression in NSCLC cell
lines was measured by quantitative polymerase chain reaction
(*P<0.05, **P<0.01,
***P<0.001). The sensitivity to PTX of A549, H1395,
95C and 95D cells was based on their IC50 (207.8±1.38,
159.6±1.42, 131.3±0.74 and 87.94±1.41 nM, respectively). (C)
Apoptosis of A549 and 95D cells was detected by Annexin V/PI assay
following 24-h exposure to PTX. Results are representative of 2–3
independent experiments. Error bars are the standard error of the
mean. PTX, paclitaxel; A549, adenocarcinomic human alveolar basal
epithelial cells; H1395, human adenocarcinoma lymphoblasts; 95C,
low metastatic human lung cancer cells; 95D, high metastatic human
lung cancer cells; PI, propidium iodide; IC50, half
maximal inhibitory concentration; miR-7, microRNA-7. |
Overexpression of miR-7 sensitizes NSCLC
cells to PTX
It was hypothesized that the upregulation of miR-7
increases the sensitivity of NSCLC cells to PTX. A549 cells, a line
with lower miR-7 expression, was selected as an in vitro
model. miR-7 was overexpressed in A549 cells by transfection with
miR-7 mimics, which resulted in the inhibition of A549 cell
proliferation, especially after 72 h (Fig. 2A and B). To determine whether the
increased expression of miR-7 would enhance sensitivity to PTX,
cells transfected with miR-7 or miR-NC mimics were treated with PTX
and viability was measured using the CCK-8 assay. Pre-treatment
with miR-7 mimics enhanced PTX-mediated suppression of A549 cell
viability, most notably at lower doses of PTX. It was observed that
20 nM PTX combined with miR-7 mimics was as effective as 80 nM PTX
treatment alone (Fig. 2C). These
data showed that the overexpression of miR-7 enabled reduction of
the dose of PTX used for treatment.
 | Figure 2Overexpression of miR-7 in A549
adenocarcinomic human alveolar basal epithelial cells, increases
cellular sensitivity to PTX. (A) MiR-7 expression in A549 cells,
following transfection with miR-7 or miR-NC, was determined by
quantitative polymerase chain reaction (***P<0.001).
(B) Cell viability of A549 cells was performed by a Cell Counting
kit-8 (CCK-8) assay following transfection with miR-7 mimics or
miR-NC (*P<0.05, ***P<0.001). (C and D)
A549 cells transfected with miR-7 mimics or miR-NC were treated
with PTX for 48 h. Cell viability and apoptosis were evaluated by
the CCK-8 and Annexin V/PI assays (*P<0.05,
**P<0.01, ***P<0.001). Results are
representative of 2–3 independent experiments. Error bars are the
standard error of the mean. PTX, paclitaxel; miR-7, microRNA-7;
miR-NC, microRNA-negative control mimic; PI, propidium iodide. |
Apoptosis is the predominant mechanism of
PTX-induced toxicity. An Annexin V/PI assay was therefore used to
measure the apoptotic frequency in miR-7-transfected A549 cells
treated with PTX (20 nM). In comparison to PTX treatment alone, PTX
and miR-7 overexpression induced apoptosis in A549 cells (11.57 vs.
7.63% in early apoptosis and 24.65 vs. 11.53% in later apoptosis)
(Fig. 2D). Therefore, miR-7
expression sensitized the cells to PTX-induced apoptosis, thus
enhancing its cytotoxic effect.
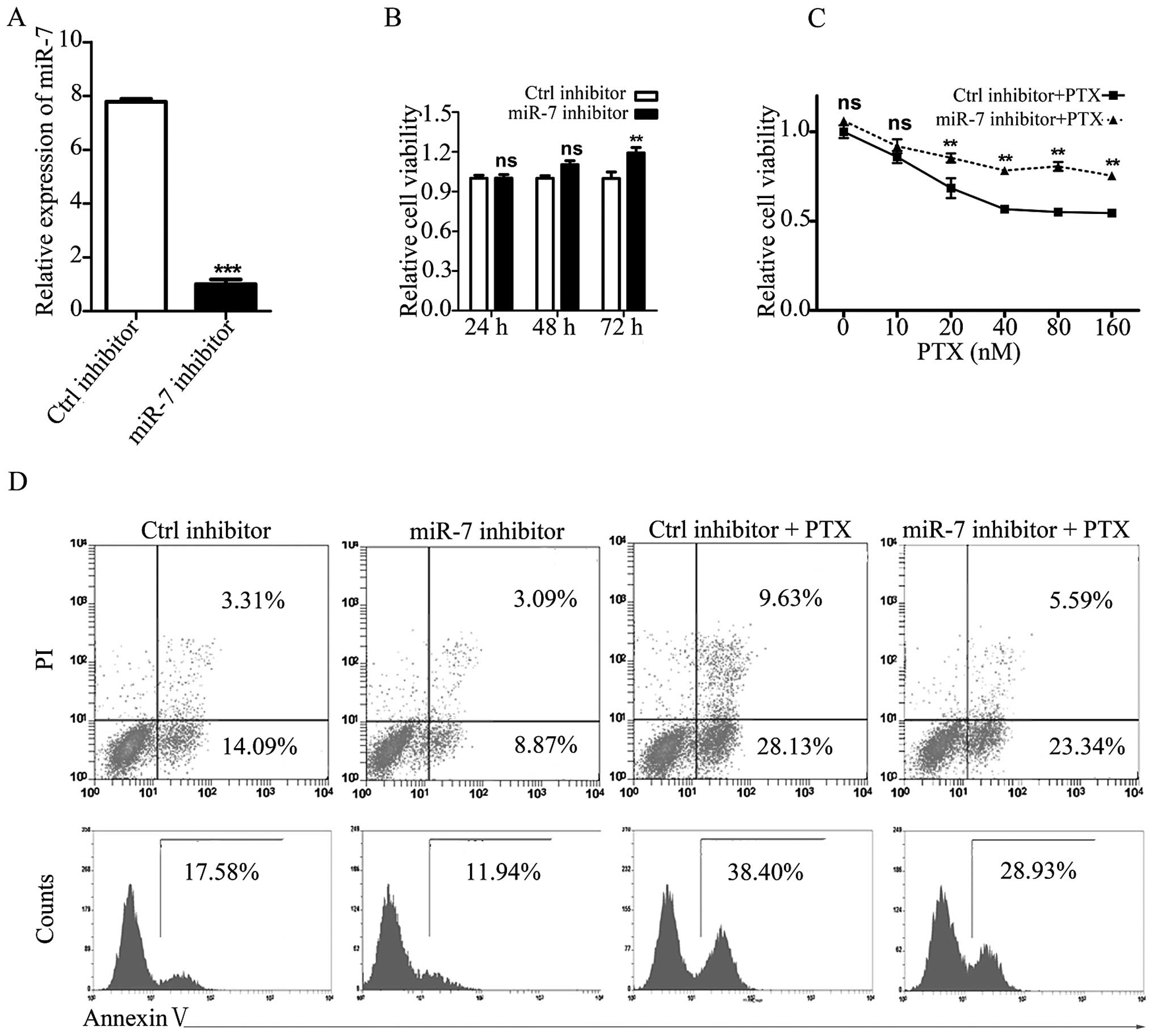
Inhibition of miR-7 promotes NSCLC cell
resistance to PTX
To investigate the role of miR-7 in PTX sensitivity,
the high miR-7-expressing PTX-sensitive NSCLC cell line, 95D, was
used as an in vitro model. MiR-7 inhibitor was used to knock
down endogenous miR-7 (Fig. 3A).
Following 72-h knockdown, the proliferation of 95D cells was
observed to be increased (Fig.
3B).
Following transfection with the miR-7 or control
inhibitor, 95D cells were treated with PTX at various
concentrations. As expected, the combined treatment of 95D cells
with an miR-7 inhibitor and PTX restored the PTX-mediated
suppression of cell viability and apoptosis (Fig. 3C and D). Therefore, both the gain-
and loss-of-function experiments indicated an important function
for miR-7 in the PTX sensitivity of NSCLC cells.
miR-7-enhanced PTX sensitivity in NSCLC
cells is mediated by EGFR
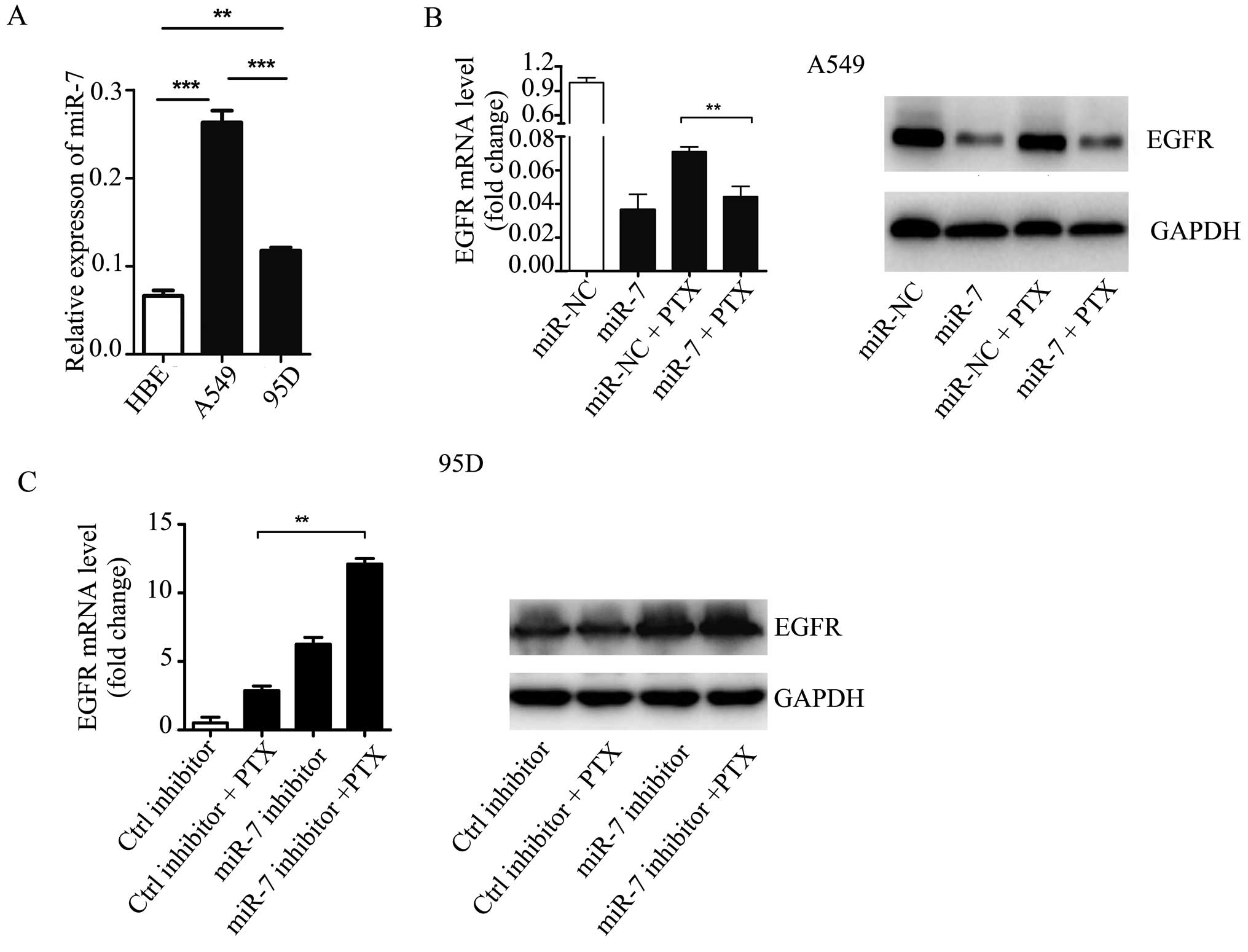
Given the function of EGFR in NSCLC development and
apoptosis, it was hypothesized that EGFR is involved in
miR-7-mediated NSCLC cell sensitivity to PTX. qPCR showed that EGFR
was upregulated in NSCLC A549 and 95D cells as compared with HBE
cells (Fig. 4A). Furthermore, EGFR
expression was significantly higher in A549 cells as compared with
95D cells. An inverse correlation was observed between the
endogenous miR-7 expression levels and PTX sensitivities.
The expression of EGFR was then examined in A549
cells treated with miR-7 mimics, PTX, or both, by qPCR and western
blotting. Overexpression of miR-7 inhibited EGFR expression in A549
cells. In comparison to PTX plus miR-NC, the cells treated with PTX
following pretreatment with miR-7 mimics exhibited a significantly
lower EGFR expression, at the mRNA and protein level (Fig. 4B). Conversely, the inhibition of
miR-7 was associated with an increased mRNA and protein expression
of EGFR in 95D cells treated with PTX (Fig. 4C). These data indicate that EGFR may
mediate the molecular mechanism of NSCLC sensitization to PTX by
miR-7.
Discussion
Accumulating reports have indicated that
chemotherapeutic treatments alter miRNA profiles in cancer
(15,25,26).
Several microRNAs, including miR-203b (27), miR-21 (28), and miR-137 (18) may also contribute to
chemotherapeutic efficacy. In the present study, miR-7 was
identified as an important molecule in PTX treatment. It was first
shown that PTX sensitivity is dependent on endogenous miR-7
expression in NSCLC cell lines. Overexpression of miR-7 sensitized
NSCLC cells to PTX, mainly by promoting PTX-induced cell apoptosis.
Furthermore, administration of lower doses of PTX following
pretreatment with miR-7 mimics, produced similar antitumor effects
as compared with higher doses of PTX treatment alone (Fig. 2C). This finding suggests that miR-7
may allow for a reduction in the PTX doses used in chemotherapy and
help address the problem of therapeutic resistance in NSCLC.
MiR-7 is a putative tumor suppressor in a large
range of solid tumors, and is often downregulated in NSCLC
(29). Results of the present study
have shown that the expression of miR-7 varied in different NSCLC
cell lines, since these cell lines originated from different
individuals. The interindividual variability of miR-7 expression
was confirmed (data not shown). In NSCLC, miR-7 has been reported
to suppress tumorigenesis by targeting a number of important
proto-oncogenes, including EGFR, IRS1 (insulin receptor substrate
1), IRS2, RAF1 (v-raf-1 murine leukemia viral oncogene homologue
1), and PAK1 (p21/CDC42/RAC1-activated kinase 1), and by inhibiting
EGFR/AKT pathway activation (30–33).
Additionally, inhibition of cell proliferation is associated with
EGFR downregulation following transfection of miR-7 into NSCLC
cells. Combined treatment with PTX and miR-7 mimics reduced EGFR
expression at the mRNA and protein levels. By contrast, the miR-7
inhibitor increased EGFR expression in PTX-treated cells. These
data indicate that miR-7-mediated enhancement of A549
chemosensitivity to PTX may occur through EGFR targeting.
The present study has focused on EGFR since it was
previously identified as a critical target of miR-7 in many solid
tumors, including NSCLC, and it contributes to tumor progression
and poor prognosis (30,32,34).
EGFR also functions in chemotherapeutic resistance and radiation
tolerance in tumor cells, in which multiple downstream pathways of
EGFR, including RAS-RAF-MAK-MAKP, PI3K/AKT, and STAT, are activated
(31,33,35–38).
Lee et al (38) reported
that the overexpression of miR-7 increases the radiosensitivity of
various human cancers by directly suppressing the activation of
EGFR-PI3K-AKT. The present study has demonstrated that EGFR
functions in miR-7-enhanced chemosensitivity to PTX, and a similar
signaling pathway downstream of EGFR may be involved. In summary,
our findings have improved the understanding of the role of miR-7
in promoting chemosensitivity of cancer cells to PTX, and has
enhanced existing knowledge on miRNAs in modulating
chemotherapeutic efficacy. The identification of miR-7 as a
potential sensitizer in PTX therapy provides a fundamental basis
for new approaches in the development of novel and PTX therapeutic
strategies.
Acknowledgements
This study was supported by the National Science
Foundation of China (grant nos. 81072408, 81273215, 81372313 and
81272259), the Major Research Plan of the National Natural Science
Foundation of China (91229110), the China Postdoctoral Science
Foundation (213M530001), Specialized Research Fund for the Doctoral
Program of Higher Education (20120071110046) ‘985 Third Project’
Key Discipline, Interdisciplinary Outstanding Doctoral Research
Grant program and the Graduates Creation Foundation of Fudan
University (EZF101321).
References
|
1
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010.
|
|
2
|
Gu J, Ding JY, Lu CL, et al:
Overexpression of CD88 predicts poor prognosis in non-small-cell
lung cancer. Lung Cancer. 81:259–265. 2013.
|
|
3
|
Edelman MJ: Novel taxane formulations and
microtubule-binding agents in non-small-cell lung cancer. Clin Lung
Cancer. 10:S30–S34. 2009.
|
|
4
|
Morales-Cano D, Calviño E, Rubio V, et al:
Apoptosis induced by paclitaxel via Bcl-2, Bax and caspases 3 and 9
activation in NB4 human leukaemia cells is not modulated by ERK
inhibition. Exp Toxicol Pathol. 65:1101–1108. 2013.
|
|
5
|
Kavallaris M: Microtubules and resistance
to tubulin-binding agents. Nat Rev Cancer. 10:194–204. 2010.
|
|
6
|
Wan YF, Guo XQ, Wang ZH, Ying K and Yao
MH: Effects of paclitaxel on proliferation and apoptosis in human
acute myeloid leukemia HL-60 cells. Acta Pharmacol Sin. 25:378–384.
2004.
|
|
7
|
Xu R, Sato N, Yanai K, et al: Enhancement
of paclitaxel-induced apoptosis by inhibition of mitogen-activated
protein kinase pathway in colon cancer cells. Anticancer Res.
29:261–270. 2009.
|
|
8
|
Kastl L, Brown I and Schofield AC: Altered
DNA methylation is associated with docetaxel resistance in human
breast cancer cells. Int J Oncol. 36:1235–1241. 2010.
|
|
9
|
Le XF and Bast RC Jr: Src family kinases
and paclitaxel sensitivity. Cancer Biol Ther. 12:260–269. 2011.
|
|
10
|
Modok S, Mellor HR and Callaghan R:
Modulation of multidrug resistance efflux pump activity to overcome
chemoresistance in cancer. Curr Opin Pharmacol. 6:350–354.
2006.
|
|
11
|
Lujambio A and Lowe SW: The microcosmos of
cancer. Nature. 482:347–355. 2012.
|
|
12
|
Volinia S, Calin GA, Liu CG, et al: A
microRNA expression signature of human solid tumors defines cancer
gene targets. Proc Natl Acad Sci USA. 103:2257–2261. 2006.
|
|
13
|
Jiang P, Liu R, Zheng Y, et al: MiR-34a
inhibits lipopolysaccharide-induced inflammatory response through
targeting Notch1 in murine macrophages. Exp Cell Res.
318:1175–1184. 2012.
|
|
14
|
Zheng Y, Xiong S, Jiang P, et al:
Glucocorticoids inhibit lipopolysaccharide-mediated inflammatory
response by downregulating microRNA-155: a novel anti-inflammation
mechanism. Free Radic Biol Med. 52:1307–1317. 2012.
|
|
15
|
Haenisch S and Cascorbi I: miRNAs as
mediators of drug resistance. Epigenomics. 4:369–381. 2012.
|
|
16
|
Schoof CR, Botelho EL, Izzotti A and
Vasques Ldos R: MicroRNAs in cancer treatment and prognosis. Am J
Cancer Res. 2:414–433. 2012.
|
|
17
|
Miller TE, Ghoshal K, Ramaswamy B, et al:
MicroRNA-221/222 confers tamoxifen resistance in breast cancer by
targeting p27Kip1. J Biol Chem. 283:29897–29903. 2008.
|
|
18
|
Takwi AA, Wang YM, Wu J, et al: miR-137
regulates the constitutive androstane receptor and modulates
doxorubicin sensitivity in parental and doxorubicin-resistant
neuroblastoma cells. Oncogene. 2013.
|
|
19
|
Yang SM, Huang C, Li XF, Yu MZ, He Y and
Li J: miR-21 confers cisplatin resistance in gastric cancer cells
by regulating PTEN. Toxicology. 306:162–168. 2013.
|
|
20
|
Pan YZ, Morris ME and Yu AM: MicroRNA-328
negatively regulates the expression of breast cancer resistance
protein (BCRP/ABCG2) in human cancer cells. Mol Pharmacol.
75:1374–1379. 2009.
|
|
21
|
Liang Z, Wu H, Xia J, et al: Involvement
of miR-326 in chemotherapy resistance of breast cancer through
modulating expression of multidrug resistance-associated protein 1.
Biochem Pharmacol. 79:817–824. 2010.
|
|
22
|
Kastl L, Brown I and Schofield AC:
miRNA-34a is associated with docetaxel resistance in human breast
cancer cells. Breast Cancer Res Treat. 131:445–454. 2012.
|
|
23
|
Xiong S, Zheng Y, Jiang P, Liu R, Liu X
and Chu Y: MicroRNA-7 inhibits the growth of human non-small cell
lung cancer A549 cells through targeting BCL-2. Int J Biol Sci.
7:805–814. 2011.
|
|
24
|
Xiong S, Zheng Y, Jiang P, Liu R, Liu X,
Qian J, Gu J, Chang L, Ge D and Chu Y: PA28gamma emerges as a novel
functional target of tumor suppressor microRNA-7 in non-small-cell
lung cancer. Br J Cancer. 110:353–362. 2014.
|
|
25
|
Nordentoft I, Birkenkamp-Demtroder K,
Agerbæk M, et al: miRNAs associated with chemo-sensitivity in cell
lines and in advanced bladder cancer. BMC Med Genomics.
5:402012.
|
|
26
|
Tian W, Chen J, He H and Deng Y: MicroRNAs
and drug resistance of breast cancer: basic evidence and clinical
applications. Clin Transl Oncol. 15:335–342. 2013.
|
|
27
|
Zhang Y, Hu H, Song L, Cai L, Wei R and
Jin W: Epirubicin-mediated expression of miR-302b is involved in
osteosarcoma apoptosis and cell cycle regulation. Toxicol Lett.
222:1–9. 2013.
|
|
28
|
Costa PM, Cardoso AL, Nóbrega C, et al:
MicroRNA-21 silencing enhances the cytotoxic effect of the
antiangiogenic drug sunitinib in glioblastoma. Hum Mol Genet.
22:904–918. 2013.
|
|
29
|
Duncavage E, Goodgame B, Sezhiyan A,
Govindan R and Pfeifer J: Use of microRNA expression levels to
predict outcomes in resected stage I non-small cell lung cancer. J
Thorac Oncol. 5:1755–1763. 2010.
|
|
30
|
Rai K, Takigawa N, Ito S, et al: Liposomal
delivery of MicroRNA-7-expressing plasmid overcomes epidermal
growth factor receptor tyrosine kinase inhibitor-resistance in lung
cancer cells. Mol Cancer Ther. 10:1720–1727. 2011.
|
|
31
|
Kefas B, Godlewski J, Comeau L, et al:
microRNA-7 inhibits the epidermal growth factor receptor and the
Akt pathway and is down-regulated in glioblastoma. Cancer Res.
68:3566–3572. 2008.
|
|
32
|
Webster RJ, Giles KM, Price KJ, et al:
Regulation of epidermal growth factor receptor signaling in human
cancer cells by microRNA-7. J Biol Chem. 284:5731–5741. 2009.
|
|
33
|
Chou YT, Lin HH, Lien YC, et al: EGFR
promotes lung tumorigenesis by activating miR-7 through a
Ras/ERK/Myc pathway that targets the Ets2 transcriptional repressor
ERF. Cancer Res. 70:8822–8831. 2010.
|
|
34
|
Merlo V, Longo M, Novello S and Scagliotti
GV: EGFR pathway in advanced non-small cell lung cancer. Front
Biosci (Schol Ed). 3:501–517. 2011.
|
|
35
|
Petitprez A and Larsen AK: Irinotecan
resistance is accompanied by upregulation of EGFR and Src signaling
in human cancer models. Curr Pharm Des. 19:958–964. 2013.
|
|
36
|
Rekhtman N, Paik PK, Arcila ME, et al:
Clarifying the spectrum of driver oncogene mutations in
biomarker-verified squamous carcinoma of lung: lack of EGFR/KRAS
and presence of PIK3CA/AKT1 mutations. Clin Cancer Res.
18:1167–1176. 2012.
|
|
37
|
Tanaka K, Babic I, Nathanson D, et al:
Oncogenic EGFR signaling activates an mTORC2-NF-kappaB pathway that
promotes chemotherapy resistance. Cancer Discov. 1:524–538.
2011.
|
|
38
|
Lee KM, Choi EJ and Kim IA: microRNA-7
increases radiosensitivity of human cancer cells with activated
EGFR-associated signaling. Radiother Oncol. 101:171–176. 2011.
|