Introduction
Lung cancer is the most common cause of
cancer-associated mortality worldwide (1). Non-small cell lung carcinoma (NSCLC)
is a major type of lung cancer. Out of all the NSCLCs,
adenocarcinoma is the most common histological type (2). The introduction of the epidermal
growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) and
the approval of their clinical use has provided novel insights into
the treatment of advanced NSCLC (3,4). EGFR
mutation is a validated predictive marker for response and
progression-free survival when using EGFR-TKIs during first-line
therapy in advanced lung adenocarcinoma (4–6).
Soda et al reported that a minority of lung
tumors harbored a small inversion within chromosome 2p, giving rise
to echinoderm microtubule-associated protein-like 4
(EML4)-anaplastic lymphoma kinase (ALK), a transformation fusion
gene (7). The epidemiological
characteristics exhibit prevalence in 5% of adenocarcinomas. The
presence of the EML4-ALK fusion is associated with younger, male
patients who have no smoking history or a light smoking habit
(8–11). Common features of lung carcinoma
harboring the ALK-fusion gene include the absence of lepidic growth
and marked nuclear pleomorphism, a solid or acinar growth pattern,
a substantial amount of extracellular mucus and the presence of
mucus cells (12). In addition, a
solid signet-ring cell pattern and a mucinous cribriform pattern
are observed at least focally in the majority of cases. Tumors with
EML4-ALK translocations appear to be exclusive of EGFR and KRAS
mutations (8,11,13).
The first ALK inhibitor to be used in a clinical trial was
crizotinib, which is a dual inhibitor for ALK and MET kinase
(14). The response rate for
crizotinib in patients with ALK-rearranged NSCLCs in the trial was
revealed to be 57%, with a disease control rate of up to 90%
(10). Therefore, it is necessary
to develop a feasible method of detecting ALK rearrangement.
In the present study, cases harboring ALK
rearrangement were selected on the basis of previously documented
characteristic features, including adenocarcinoma histology and
mucin production. Using this cohort, the correlation between two
different immunohistochemistry (IHC) procedures was examined,
including the intercalated antibody-enhanced polymer (iAEP) method
with antibody 5A4 (Nichirei Biosciences, Inc., Tokyo, Japan) and
the fully automated Bond-Max system (Leica Biosystems Newcastle,
Ltd., Newcastle Upon Tyne, UK) with rabbit monoclonal antibody D5F3
(Cell Signaling Technology, Inc., Danvers, MA, USA), and
fluorescence in situ hybridization (FISH) for ALK.
Materials and methods
Materials and study design
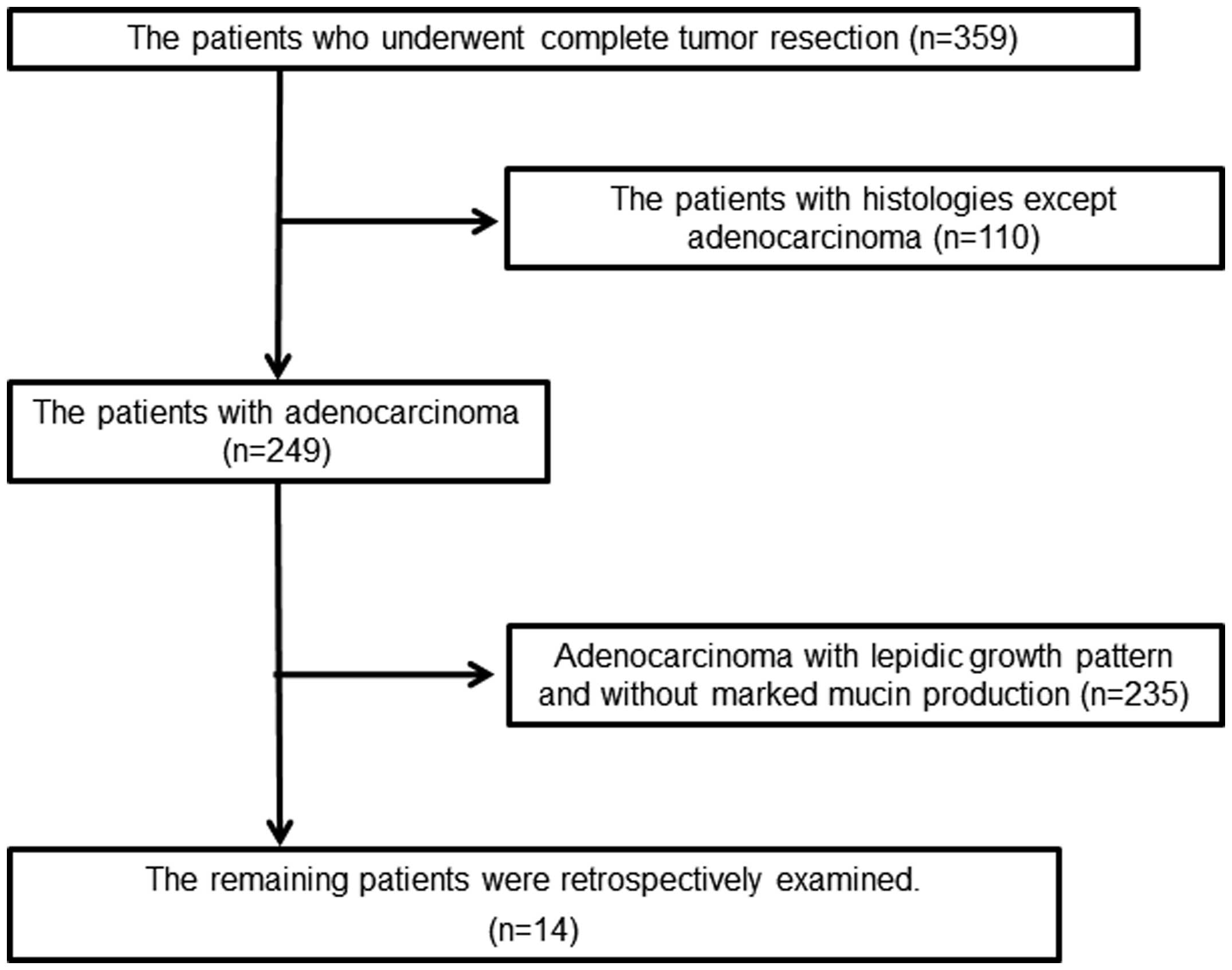
The present retrospective study examined 359
patients with primary lung carcinoma whose tumors had been
completely surgically removed at the Department of Surgery, Kurume
University (Kurume, Fukuoka, Japan), between 2002 and 2011. Out of
the 359 patients, 110 patients who were not histologically
diagnosed with adenocarcinoma were excluded. The remaining 249
patients were histologically diagnosed with adenocarcinoma. Out of
the 249 cases, 14 cases were selected due to the presence of marked
mucin production (Fig. 1). The
present study was approved by the ethical committee of Kurume
University (no. 104). Written informed consent was obtained from
the paitents.
Immunohistochemistry
IHC for ALK was performed on paraffin-embedded
sections by two different procedures. Two antibody preparations
specific for the intracellular region of ALK were used, namely 5A4
(Nichirei Biosciences, Inc.) and D5F3 (Cell Signaling Technology,
Inc.). The paraffin-embedded tissue samples were cut to a 4-μm
thickness, examined on a coated slide glass and labeled with the
antibodies as aforementioned. IHC using clone 5A4 was performed
with the ALK detection kit, according to the manufacturer’s
instructions (Nichirei Biosciences, Inc.). This kit applies an iAEP
method (15). IHC with clone D5F3
(rabbit monoclonal antibody; 1:200) was performed on the fully
automated Bond-Max system (Leica Biosystems Newcastle, Ltd.) using
onboard heat-induced antigen retrieval with ER2 for 20 min and a
refine polymer detection system (Leica Biosystems Newcastle, Ltd.).
The histological specimens were incubated with the primary antibody
for 14 min at room temperature and DAB was used as the chromogen in
all IHC experiments.
The immunoreactive distribution was graded into five
levels according to the distribution of immunoreactive tumor cells:
0, when there were no positive cells; 1+, when the area covered by
immunoreactive cells was 1–25%; 2+, when the area was 26–50%; 3+,
when the area was 51–75%; and 4+, when the area was >76%. The
staining intensity for ALK was graded into four levels following
the procedure of a previous study (16): 0, no staining; 1+, faint cytoplasmic
staining; 2+, moderate, smooth cytoplasmic staining; and 3+,
intense granular cytoplasmic staining. The total score was obtained
from the immunoreactive distribution multiplied by the staining
intensity score.
FISH for ALK rearrangement
To identify ALK rearrangements, FISH was performed
on formalin-fixed, paraffin embedded tumors using a break-apart
probe for ALK (Vysis LSI ALK Dual Color Probe; Abbott Molecular,
Des Plaines, IL, USA). FISH for ALK locus rearrangement was
considered positive if ≥14% of the tumor cells counted exhibited a
split signal. The criteria for probe signal interpretation in ≥100
interphase nuclei were as follows: i) Separated green and orange
signals or single red signals identified the cells with rearranged
ALK; and ii) overlapping of red and green signals (yellowish)
indicated the cells in which ALK was not rearranged.
Status of the EGFR tyrosine kinase
domain
Genomic DNA was extracted from paraffin-embedded
tissues using a QIAamp DNA Micro kit (Qiagen Inc., Valencia, CA,
USA). Polymerase chain reaction (PCR) was performed using the
TaqMan Mutation Detection Assay (Applied Biosystems Life
Technologies, Carlsbad, CA, USA) using StepOneTM Real Time PCR
System and Mutation DetectorTM Software version 1.0 (Applied
Biosystems Life Technologies), according to the manufacturer’s
instructions. To identify the EGFR mutation, the following primers
were used: Hs00000228_mu, Hs00000157_mu, and Hs00000102_mu. The PCR
solution (Applied Biosystems Life Technologies) consisted of 10 μl
TaqMan® Genotyping Master Mix, 2 μl genomic DNA, 6 μl nuclease-free
water and 2 μl TaqMan Mutation Detection Assay. The PCR conditions
were as follows: One cycle at 95°C for 10 min, five cycles at 92°C
for 15 sec and 1 min at 58°C, 40 cycles at 92°C for 15 sec and 1
min at 60°C.
Statistical analysis
The association between cases with and without ALK
rearrangement was examined by Student t-test or χ2 test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
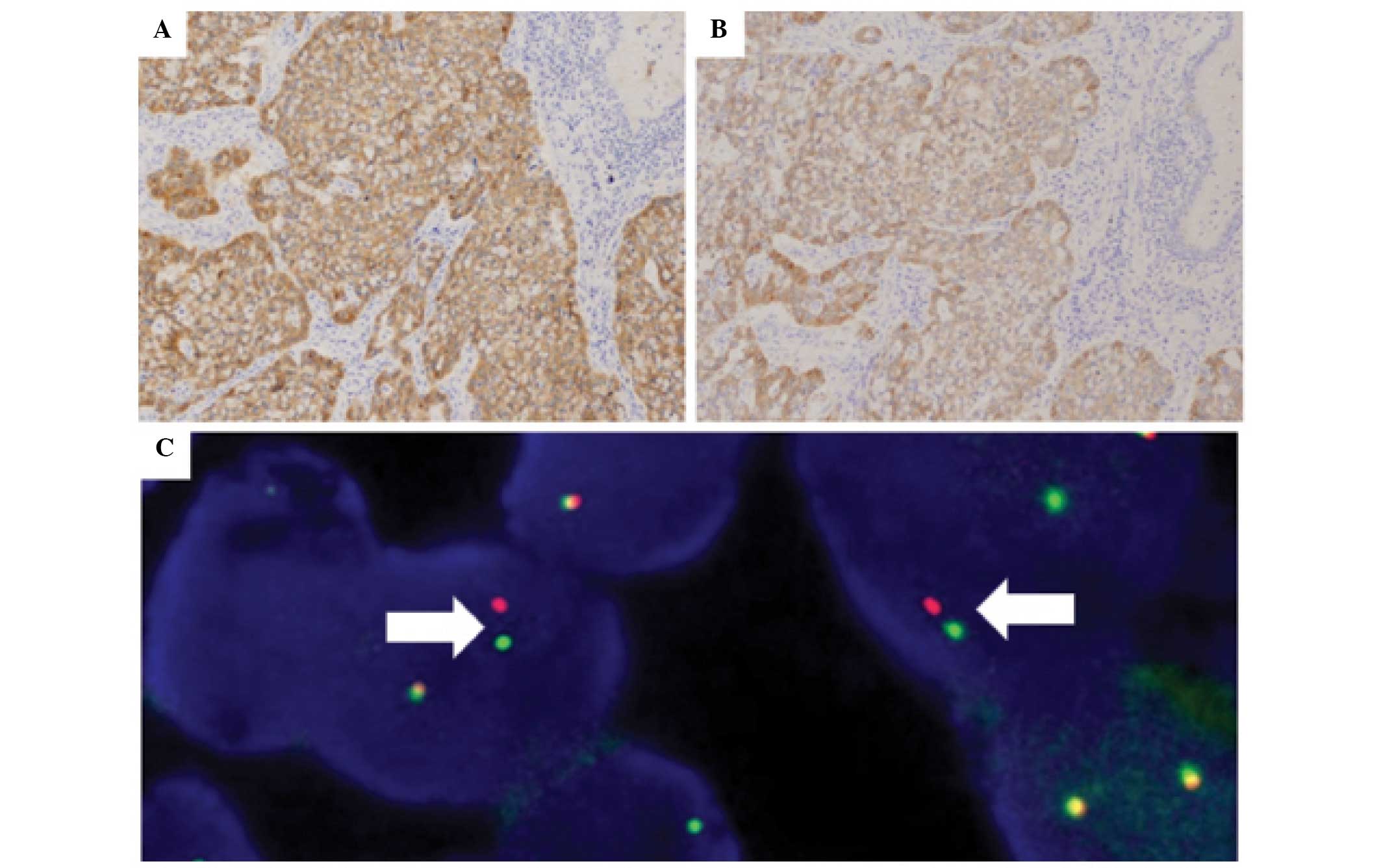
Eight cases of ALK-positive lung carcinoma were
found by IHC. FISH revealed that seven out of eight (87.5%) cases
possessed ALK rearrangement (Fig.
2A–C). The clinicopathological findings are shown in Table I. The IHC scores of the two
different antibodies almost correlated with each other (Table II), but there was no statistical
difference. The ALK-positive area was widely distributed in each
method. The distribution score was 4 (>76%) or >3 (>51%)
in both methods. However, tumor components exhibiting a solid
signet ring cell pattern demonstrated a weaker cytoplasmic signal
in D5F3 with the Bond-Max system in four cases (Fig. 3A and B).
 | Table IClinicopathological features of
adenocarcinoma with and without ALK rearrangement. |
Table I
Clinicopathological features of
adenocarcinoma with and without ALK rearrangement.
| Feature | ALK+
(n=7) | ALK−
(n=7) | P-value |
|---|
| Age, years (±SD) | 59.4±8.9 | 59.3±12.0 | 0.98 |
| Gender (M:F), n | 4:3 | 0:7 | 0.018 |
| Median smoking habit,
BI | 0 | 0 | 1 |
| Histomorphology,
% |
| Any papillary
pattern | 28.6 | 43.9 | 0.53 |
| Any acinar
pattern | 85.7 | 100.0 | 0.30 |
| Mucinous
cribriforma | 57.1 | 0.0 | 0.018 |
| Any solid
pattern | 85.7 | 87.5 | 1 |
| Solid signet ring
cella | 85.7 | 14.3 | 0.0075 |
 | Table IIImmunohistochemical stain score of the
two antibodies and procedures. |
Table II
Immunohistochemical stain score of the
two antibodies and procedures.
| Case | ALK 5A4 with
iAEPa | ALK D5F3 with
Bond-Max systema |
|---|
| 1 | 12 (3×4) | 12 (3×4) |
| 2 | 12 (3×4) | 12 (3×4) |
| 3 | 8 (2×4) | 6 (2×3) |
| 4 | 8 (2×4) | 8 (2×4) |
| 5 | 12 (3×4) | 12 (3×4) |
| 6 | 8 (2×4) | 6 (2×3) |
| 7 | 9 (3×3) | 8 (2×4) |
| Average | 9.9 | 9.1 |
In order to screen effectively, the cases with
adenocarcinoma histology with mucin production were focused on and
14 cases were selected. Out of the 14 cases, seven cases were
identified as ALK-positive lung carcinoma. All cases demonstrated
the previously described characteristic histological patterns, such
as a mucinous cribriform and/or solid signet ring cell pattern
(Table I). One case, which had
characteristic histological patterns of ALK-positive lung
carcinoma, was identified by IHC as possessing ALK expression, but
FISH demonstrated that the carcinoma lacked ALK rearrangement. This
case exhibited neither split signals for ALK nor normal signals.
ALK-positive lung carcinoma was significantly predominant for male
patients in this study. However, these findings may be non-specific
for ALK-positive lung carcinoma due to the small sample size.
EGFR mutation was not found in any of the seven
ALK-positive lung carcinomas.
Discussion
An ideal method for determining the presence of
ALK-rearrangement has yet to be established. However, according to
the Food and Drug Administration, it is necessary to confirm
ALK-rearrangement by FISH in order to use the ALK inhibitor,
crizotinib (17). Although FISH
analysis is essential for the clinical usage of crizotinib in the
United States, a previous study has demonstrated that initial
screening by FISH alone does not detect all cases with ALK-positive
lung carcinoma (8). In addition,
the interpretation of FISH for ALK in NSCLC tends to be difficult,
as ALK-positive lung carcinoma possesses an intrachromosomal
rearrangement, resulting in a relatively close separation of the
break-apart probes (16).
Discordances between IHC and FISH have been thoroughly investigated
in HER2/neu-positive breast carcinoma. The discordances between IHC
and FISH are reported to be in the range of 10–20% (18–20).
This may result from delayed or prolonged fixation, errors in IHC
interpretation, HER2/neu antibody reagent limitations and the
different antibodies used (20), a
lack of interlaboratory standardization and reproducibility in the
interpretation of the results (21)
or genetic heterogeneity, which can contribute to positive IHC and
negative FISH tests (22,23). At present, as there is no definitive
recommendation from the laboratories performing IHC and FISH for
ALK rearrangement in NSCLC, it is necessary to develop simple and
accurate screening systems. Therefore, the present study focused on
IHC for ALK rearrangement using two different antibodies and
procedures. Previous studies have reported that IHC is a reliable
screening tool for ALK-positive lung carcinoma (15,24–26).
In the present study, it was demonstrated that the IHC score for
ALK rearrangement using rabbit monoclonal antibody D5F3 with the
Bond-Max system was similar to that of antibody 5A4 with the iAEP
method. The combination of the D5F3 antibody and the Bond-Max
system is simple and much cheaper than the iAEP method. This
combination could also be suitable for the screening of
ALK-positive lung cancer. Additionally, the D5F3 antibody could
detect numerous variants of EML4-ALK or an unknown oncogenic fusion
(27). In the present study, as the
distribution scores of ALK in each method were relatively high, IHC
for ALK may have low heterogeneity, suggesting that using IHC for
ALK could be useful in limited tissue samples, such as in biopsy
specimens or cytology, for the screening of ALK-positive lung
carcinoma (28). Recently,
Takamochi et al also described the expression of ALK on IHC
as homogeneous (29). By contrast,
Selinger et al reported that tissue microarray samples from
the same tumor demonstrated heterogeneity of IHC for ALK when
exhibiting weak or faint staining (30). Although explanations for these
discrepancies remain elusive, the different samples and IHC
procedures utilized in each study may be associated. In the present
study, tumor components exhibiting a solid signet ring cell pattern
demonstrated a slightly weak cytoplasmic signal, which may be
attributed to abundant cytoplasmic mucin. As this component is
known to be one of the characteristic histological findings in
ALK-positive lung carcinoma, an awareness of marked mucin
production is necessary to avoid an underestimation of the
proportion of ALK-rearranged cells. Therefore, the assessment of
IHC for ALK in limited tissue samples should be performed with
care, particularly when the IHC signal is weak in a solid signet
ring cell component.
Among the eight cases in the present study that were
confirmed to exhibit ALK expression by IHC, seven cases were
demonstrated to possess ALK rearrangement by FISH. The sensitivity
of FISH for ALK was 87.5%. This sensitivity was lower than that of
previous studies. The one case in which FISH did not confirm ALK
rearrangement possessed high IHC scores for ALK expression and
demonstrated characteristic histological patterns. A few studies
have documented that all cases demonstrating a strong intensity of
ALK on IHC were also revealed to have ALK rearrangement by FISH
(16,28). The precise reasons for the
discrepancy observed in the present study remain elusive. However,
the case that lacked ALK rearrangement according to FISH was >10
years old. Neither a split signal for ALK nor a normal signal could
be detected in this case. This may have resulted from degeneration
of the DNA or from delayed or prolonged fixation. Thus, the ALK
test should be performed promptly in accordance with the College of
American Pathologists, International Association for the Study of
Lung Cancer and Association for Molecular Pathology (CAP/IASLC/AMP)
guidelines (31).
Although 14 cases were enrolled in the present study
on the basis of the presence of characteristic histological
patterns, any case with adenocarcinoma should not be excluded from
the possibility of ALK-positive lung carcinoma without IHC or FISH
for ALK rearrangement, in accordance with the CAP/IASLC/AMP
guidelines (31). None of the
ALK-positive lung carcinomas harbored coexisting EGFR mutations in
the present study. These findings are consistent with those of
previous studies, demonstrating that ALK positive lung carcinoma is
exclusive of EGFR mutations (8,11,13).
In conclusion, a combination of the methodologies of
IHC and FISH could be suitable for screening for ALK-positive lung
carcinoma. The IHC for ALK, using the rabbit monoclonal antibody
D5F3 and the Bond-Max system, demonstrated similar results to those
of the iAEP method and showed low heterogeneity. As the present
study is on a small scale, further expanded studies using larger
cohorts should be conducted in order to confirm the validity of
screening for AKL-positive lung carcinoma using the D5F3
antibody.
References
|
1
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108.
2005.
|
|
2
|
Travis WD, Brambilla E, Noguchi M, et al:
International Association for the Study of Lung Cancer/American
Thoracic Society/European Respiratory Society international
multidisciplinary classification of lung adenocarcinoma. J Thorac
Oncol. 6:244–285. 2011.
|
|
3
|
Fukuoka M, Yano S, Giaccone G, et al:
Multi-institutional randomized phase II trial of gefitinib for
previously treated patients with advanced non-small-cell lung
cancer (The IDEAL 1 Trial). J Clin Oncol. 21:2237–2246. 2003.
|
|
4
|
Pao W, Miller V, Zakowski M, et al: EGF
receptor gene mutations are common in lung cancers from ‘never
smokers’ and are associated with sensitivity of tumors to gefitinib
and erlotinib. Proc Natl Acad Sci USA. 101:13306–13311. 2004.
|
|
5
|
Azzoli CG, Baker S Jr, Temin S, et al:
American Society of Clinical Oncology: American Society of Clinical
Oncology clinical practice guideline update on chemotherapy for
stage IV non-small-cell lung cancer. J Clin Oncol. 27:6251–6266.
2009.
|
|
6
|
Lynch TJ, Bell DW, Sordella R, et al:
Activating mutations in the epidermal growth factor receptor
underlying responsiveness of non-small-cell lung cancer to
gefitinib. N Engl J Med. 350:2129–2139. 2004.
|
|
7
|
Soda M, Choi YL, Enomoto M, et al:
Identification of the transforming EML4-ALK fusion gene in
non-small-cell lung cancer. Nature. 448:561–566. 2007.
|
|
8
|
Rodig SJ, Mino-Kenudson M, Dacic S, et al:
Unique clinicopathologic features characterize ALK-rearranged lung
adenocarcinoma in the western population. Clin Cancer Res.
15:5216–5223. 2009.
|
|
9
|
Shaw AT, Yeap BY, Mino-Kenudson M, et al:
Clinical features and outcome of patients with non-small-cell lung
cancer who harbor EML4-ALK. J Clin Oncol. 27:4247–4253. 2009.
|
|
10
|
Kwak EL, Bang YJ, Camidge DR, et al:
Anaplastic lymphoma kinase inhibition in non-small-cell lung
cancer. N Engl J Med. 363:1693–1703. 2010.
|
|
11
|
Takahashi T, Sonobe M, Kobayashi M, et al:
Clinicopathologic features of non-small-cell lung cancer with
EML4-ALK fusion gene. Ann Surg Oncol. 17:889–897. 2010.
|
|
12
|
Yoshida A, Tsuta K, Nakamura H, et al:
Comprehensive histologic analysis of ALK-rearranged lung
carcinomas. Am J Surg Pathol. 35:1226–1234. 2011.
|
|
13
|
Wong DW, Leung EL, So KK, et al:
University of Hong Kong Lung Cancer Study Group: The EML4-ALK
fusion gene is involved in various histologic types of lung cancers
from nonsmokers with wild-type EGFR and KRAS. Cancer.
115:1723–1733. 2009.
|
|
14
|
Christensen JG, Zou HY, Arango ME, et al:
Cytoreductive antitumor activity of PF-2341066, a novel inhibitor
of anaplastic lymphoma kinase and c-Met, in experimental models of
anaplastic large-cell lymphoma. Mol Cancer Ther. 6:3314–3322.
2007.
|
|
15
|
Takeuchi K, Choi YL, Togashi Y, et al:
KIF5B-ALK, a novel fusion oncokinase identified by an
immunohistochemistry-based diagnostic system for ALK-positive lung
cancer. Clin Cancer Res. 15:3143–3149. 2009.
|
|
16
|
Yi ES, Boland JM, Maleszewski JJ, et al:
Correlation of IHC and FISH for ALK gene rearrangement in non-small
cell lung carcinoma: IHC score algorithm for FISH. J Thorac Oncol.
6:459–465. 2011.
|
|
17
|
FDA. FDA summary of safety and
effectiveness data. http://www.accessdata.fda.gov/cdrh_docs/pdf11/p110012b.pdf.
Accessed August 26, 2011
|
|
18
|
Baselga E, Torrelo A, Drolet BA, Zambrano
A, Alomar A and Esterly NB: Familial nonmembranous aplasia cutis of
the scalp. Pediatr Dermatol. 22:213–217. 2005.
|
|
19
|
Elkin EB, Weinstein MC, Winer EP, Kuntz
KM, Schnitt SJ and Weeks JC: HER-2 testing and trastuzumab therapy
for metastatic breast cancer: a cost-effectiveness analysis. J Clin
Oncol. 22:854–863. 2004.
|
|
20
|
Gouvêa AP, Milanezi F, Olson SJ, Leitao D,
Schmitt FC and Gobbi H: Selecting antibodies to detect HER2
overexpression by immunohistochemistry in invasive mammary
carcinomas. Appl Immunohistochem Mol Morphol. 14:103–108. 2006.
|
|
21
|
Roche PC, Suman VJ, Jenkins RB, et al:
Concordance between local and central laboratory HER2 testing in
the breast intergroup trial N9831. J Natl Cancer Inst. 94:855–857.
2002.
|
|
22
|
Vance GH, Barry TS, Bloom KJ, et al:
College of American Pathologists: Genetic heterogeneity in HER2
testing in breast cancer: panel summary and guidelines. Arch Pathol
Lab Med. 133:611–612. 2009.
|
|
23
|
Allred DC and Swanson PE: Testing for
erbB-2 by immunohistochemistry in breast cancer. Am J Clin Pathol.
113:171–175. 2000.
|
|
24
|
Conklin CM, Craddock KJ, Have C, Laskin J,
Couture C and Ionescu DN: Immunohistochemistry is a reliable
screening tool for identification of ALK rearrangement in
non-small-cell lung carcinoma and is antibody dependent. J Thorac
Oncol. 8:45–51. 2013.
|
|
25
|
Mino-Kenudson M, Chirieac LR, Law K, et
al: A novel, highly sensitive antibody allows for the routine
detection of ALK-rearranged lung adenocarcinomas by standard
immunohistochemistry. Clin Cancer Res. 16:1561–1571. 2010.
|
|
26
|
Park HS, Lee JK, Kim DW, et al:
Immunohistochemical screening for anaplastic lymphoma kinase (ALK)
rearrangement in advanced non-small cell lung cancer patients. Lung
Cancer. 77:288–292. 2012.
|
|
27
|
Li Y, Pan Y, Wang R, et al: ALK-rearranged
lung cancer in Chinese: a comprehensive assessment of
clinicopathology, IHC, FISH and RT-PCR. PLoS One. 8:e690162013.
|
|
28
|
Kawahara A, Akiba J, Abe H, et al:
Eml4-alk-positive lung adenocarcinoma with signet-ring cells. Diagn
Cytopathol. 42:460–463. 2014.
|
|
29
|
Takamochi K, Takeuchi K, Hayashi T, Oh S
and Suzuki K: A rational diagnostic algorithm for the
identification of ALK rearrangement in lung cancer: a comprehensive
study of surgically treated Japanese patients. PLoS One.
8:e697942013.
|
|
30
|
Selinger CI, Rogers TM, Russell PA, et al:
Testing for ALK rearrangement in lung adenocarcinoma: a multicenter
comparison of immunohistochemistry and fluorescent in situ
hybridization. Mod Pathol. 26:1545–1553. 2013.
|
|
31
|
Lindeman NI, Cagle PT, Beasley MB, et al:
Molecular testing guideline for selection of lung cancer patients
for EGFR and ALK tyrosine kinase inhibitors: guideline from the
College of American Pathologists, International Association for the
Study of Lung Cancer, and Association for Molecular Pathology. Arch
Pathol Lab Med. 137:828–860. 2013.
|