Introduction
Colorectal cancer (CRC), which is the second most
common cancer in each gender globally, has relatively high local
and distant recurrence rates (1).
Although radical surgery and post-operative chemotherapy are
currently the main treatment for patients with CRC, recurrence
following an apparently curative resection remains common, with
reported relapse rates of up to 40% (2). Therefore, accurate early detection of
recurrence and metastasis is critical to improving the survival
rate of CRC patients. Serum carcinoembryonic antigen (CEA)
measurement is the most widely accepted method for determining
recurrent CRC (3). Measurements of
serum CEA and periodic abdominal ultrasound and computed tomography
(CT) are useful for detecting recurrent HCC in the early stages.
However, rising CEA levels often occur even when conventional
imaging studies and clinical examinations are normal. Accordingly,
a more sensitive means for localizing tumor foci would aid in the
management of such patients.
18F-fluorodeoxyglucose (FDG) positron
emission tomography (PET)/CT is a functional imaging tool that can
provide anatomical and functional information, and has been used
for the staging and restaging of several cancers (4). 18F-FDG PET/CT has also been
used in patients with CRC for staging and restaging. It has been
shown in several studies that 18F-FDG PET/CT is a
sensitive imaging tool in the detection of CRC recurrence in
patients with elevated CEA levels (5–8).
The present study aimed to evaluate the association
between the diagnostic value of PET/CT in patients with
post-operative recurrent and metastatic CRC, and the different
levels of CEA.
Materials and methods
Patients
Between August 2010 and May 2013, a total of 105
patients (67 males and 38 females) with suspected recurrence of
CRC, as observed histologically, by elevated CEA levels or by
conventional imaging, were studied using FDG-PET. The mean age was
48 years (33±67 years). All the patients were confirmed with colon
cancer or CRC by pathology, consisting of 83 cases of
adenocarcinoma, 5 of mucinous adenocarcinoma, 9 of signet-ring cell
carcinoma and 8 of carcinoid tumors. PET/CT was performed in all
patients at 2–42 months post-resection. Baseline and follow-up CEA
levels were available in 105 patients for comparison. The CEA
levels were measured within the time of the FDG PET/CT study.
According to a study by Tan et al (9), these patients were grouped into two
categories depending on the CEA level. The CEA level was determined
using an electrochemiluminescence immunoassay (Siemens Healthcare
Diagnostics, Inc., New York, NY, USA). Values of <5 ng/ml were
considered normal. In total, 68 patients with a CEA level of ≥5
ng/ml were included in the CEA-positive group, while the remaining
37 patients were included in the CEA-negative group. Written
informed consent was obtained from all patients. This study was
approved by the ethics committee of Shandong Cancer Hospital and
Institute (Jinan, China).
18F-FDG PET scans
All patients fasted for 4–6 h prior to PET scan,
which was performed using a Discovery LS PET/CT scanner (GE
Healthcare, Buckinghamshire, UK). The imaging agent
18F-FDG, with a radiochemical purity of >95%, was
produced by using a circular accelerator and synthesized
automatically by the automated synthesis modules of GE Healthcare.
Serum glucose levels were monitored immediately prior to the
injection of the 18F-FDG; the blood glucose level was
<7 mmol/l in all cases. Intravenous 18F-FDG was
administered at ~5.5 MBq/kg of body weight, and following a 45 to
60-min uptake period, the PET/CT scan was performed. In the PET/CT
system, CT acquisition was performed on spiral dual-slice CT, with
a slice thickness of 4 mm and a pitch of 1. The image was acquired
using a 512×512-pixel matrix and a pixel size of 1 mm. Subsequent
to CT, two dimensional (2D) PET acquisition was performed for 2–3
min per bed position. PET data were acquired using a 128×128-pixel
matrix, with a slice thickness of 1.5 mm. CT-based attenuation
correction of the emission images was used. PET images were
reconstructed by iterative method ordered subset expectation
maximization (2 iterations and 8 subsets). Following CT
acquisition, the table was moved toward the field of view of the
PET, and PET acquisition of the same axial range was begun with the
patient in the same position on the table. Following completion of
PET acquisition, the reconstructed attenuation-corrected PET
images, CT images and fused images of matching pairs of PET and CT
images were available for review in axial, coronal and sagittal
planes, as well as in maximum intensity projections, 2D cine
mode.
Data analysis
All 18F-FDG PET scans were visually
assessed by two experienced nuclear medicine physicians familiar
with the clinical information. On image analysis, the PET/CT
findings were interpreted as positive if the focal area of FDG
uptake in the lesions was greater than that of the surrounding
tissue, except for the physiological uptake of the body (such as
the gastrointestinal tract and bladder) on the same plane of the CT
images. Any questionable lesions that were detected by PET/CT, but
that had no change in location and/or morphology, were considered
to be positive if the disease had higher FDG uptake during the
delayed scanning. PET findings were interpreted as negative if the
FDG uptake was equal to or less than that of the surrounding
tissue. In cases of disagreement, a final decision was made by
consensus. Maximum standardized uptake values were calculated for
suspected lesions. The gold standard was therefore determined
either on the basis of histology or on a 6-month follow-up
[significant tumor progression according to the Response Evaluation
Criteria In Solid Tumors (10,11)]
Statistical analysis
SPSS version 19.0 (SPSS, Inc., Chicago, IL, USA) was
used for the statistical analysis. According to the different
levels of CEA, the χ2 test was carried out for
comparison of the detection value of 18F-FDG PET/CT in
the patients with CRC recurrence and metastasis. P<0.05 was
considered to indicate a statistically significant difference.
Results
Of the 105 CRC patients who underwent surgery, 87
cases were confirmed with local recurrence or metastasis by
histopathology or 6-month clinical follow-up. In these 87 cases,
there were 28 cases of liver metastasis, 25 of lung metastases, 30
of local recurrence and metastasis (intra-abdominal organs and
tissues, excluding the liver, pelvis and abdominal wall) and 12 of
metastasis at other sites (such as the bones, neck, mediastinal
lymph nodes and adrenal gland). Cases with multiple metastases were
present in this count.
PET/CT diagnosis of recurrence and
metastasis in CRC patients
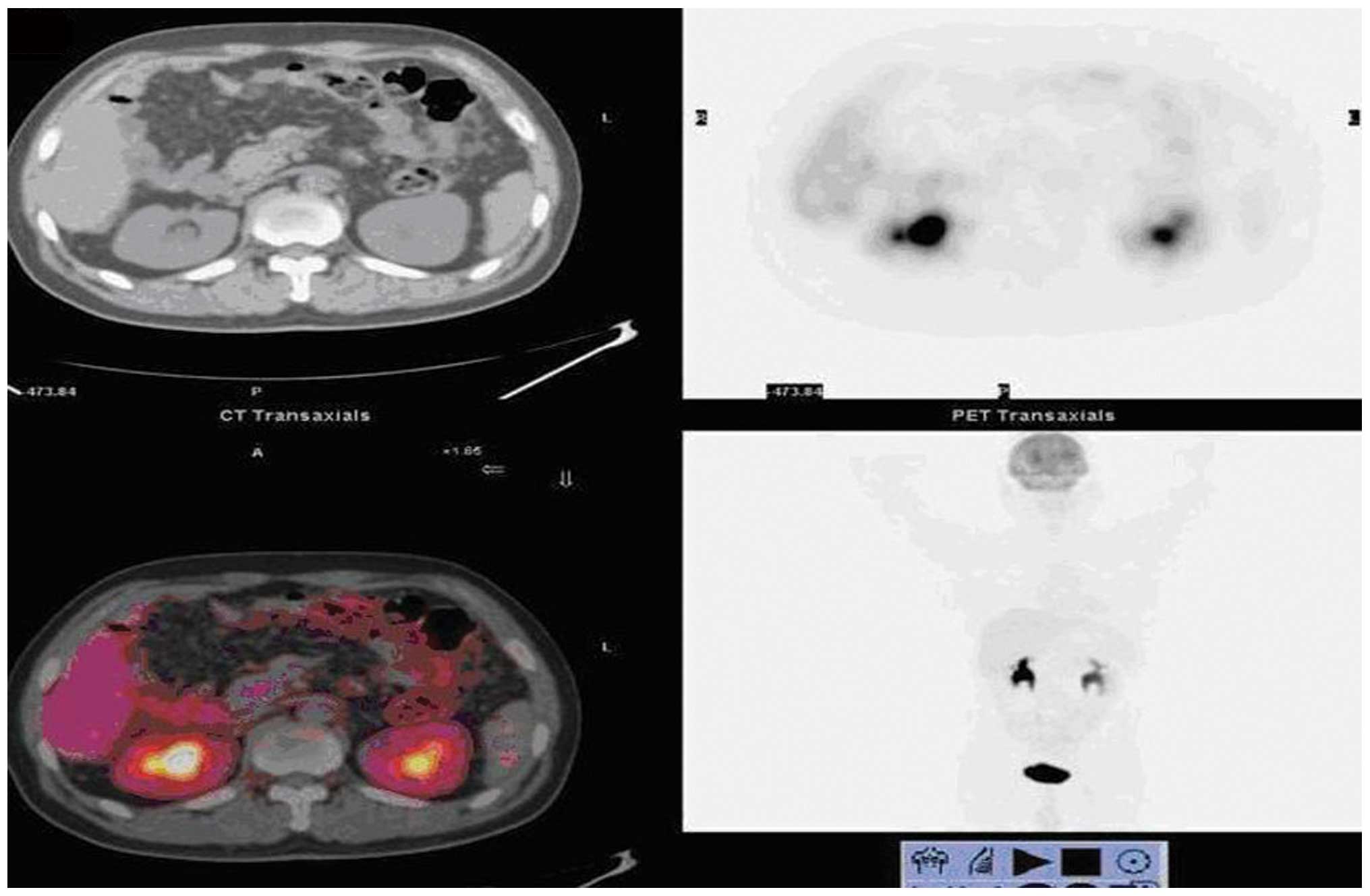
A total of 85 patients were diagnosed as
true-positives by PET/CT, including one case diagnosed as
anastomotic recurrence and right pelvic lymph node metastasis. In
this case, anastomosis was proven by inflammatory changes upon
biopsy, the high-uptake in the right pelvic lymph node was proven
to be a true-positive lesion and the left lobe of the thyroid was
found to contain malignant lesions during PET/CT examination; the
diagnosis of papillary thyroid carcinoma was confirmed by biopsy
(Fig. 1). A total of 18
true-negative cases and 2 false-negative cases were found by
PET/CT, including one 18F-FDG uptake-negative case,
which exhibited severe symptoms of intestinal obstruction and
underwent a laparotomy, which confirmed peritoneal metastasis
(Fig. 2); another case with
retroperitoneal lymph node metastasis was diagnosed as
false-negative, as the lymph nodes were too small and due to low
uptake. However, this patient was found to exhibit retroperitoneal
lymph node metastasis during color Doppler ultrasound examination
and lung metastases subsequent to the 6-month clinical follow-up.
The sensitivity, accuracy and negative predictive values of
monitoring the recurrence and metastasis of patients with CRC by
PET/CT were 97.7% (85/87), 97.1% (102/105) and 90.0% (18/20),
respectively.
Detection rate of CEA-positive and
-negative groups compared by PET/CT
Of the 105 patients, 87 were confirmed with
recurrence and metastasis by histopathology or 6-month clinical
follow-up. In the 68 cases of the CEA-positive group, recurrence
and metastasis was correctly detected by PET/CT in 58 cases, with a
detection rate of 85.3% (58/68). In the 37 cases of the
CEA-negative group, 28 cases were correctly detected, with a
detection rate of 75.7% (28/37). There was no significant
difference (P=0.221) between the PET/CT detection rates for
recurrence and metastasis between the two groups of patients.
Diagnostic value of CEA levels in CRC
patients for recurrence and metastasis
In the 68 CEA-positive patients, 59 cases were
eventually diagnosed with recurrence and metastasis. In the 37
CEA-negative patients, 28 cases were eventually diagnosed with
recurrence and metastasis. The sensitivity of the CEA levels for
monitoring the recurrence and metastasis of the patients with CRC
was 67.8% (59/87).
Discussion
CRC is one of the most common cancer entities
worldwide. Surgical resection is the optimal treatment for CRC,
which is a highly curable disease if detected in its early stages,
while recurrence following apparently curative resection remains
common, with reported relapse rates of up to 40% (12). Suspected recurrence is present in
the first two years of follow-up in ~60% of patients who undergo
first curative surgery for CRC (13). Early and accurate detection of such
recurrences is not only key to the minimization of subsequent
metastatic spread and to the planning of radical surgery, but also
to improving the survival rate and outcome of patients to a certain
extent.
Serum CEA is a tumor cell adhesion molecule and a
useful tumor marker for relapse detection, prognosis estimation and
therapy monitoring in CRC patients. Elevated serum CEA levels are
detected in two-thirds of patients with CRC. It is possible that
tumor recurrence and metastasis may be found prior to the changes
in tumor morphology through monitoring the level of CEA. On
average, conventional methods localize disease relapse 3–9 months
after the elevation of the CEA levels has been documented (14). However, CEA levels are also
increased in smokers, inflammatory bowel disease, pancreatitis,
liver disease and in patients with epithelial tumors at other
sites, but a normal CEA level cannot rule out tumor recurrence and
metastasis. The tumor location, distribution, size and other
factors affect the CEA level. A prospective study by Moertel et
al (15) demonstrated only a
25% post-operative recurrence rate in CRC patients with elevated
CEA levels. In this study, 24 patients developed recurrence and
metastasis in the CEA-negative group, while 4 patients did not
develop recurrence and metastasis in the CEA-positive group.
Conventional imaging modalities, including CT, magnetic resonance
and echography, have limited sensitivity and specificity for the
detection of recurrent CRC (16).
In general, structural or anatomical imaging modalities encounter
difficulties in identifying tumors in the distorted anatomy
following surgery and radiotherapy (17,18).
The distinction between scar and viable tumor tissue is
particularly problematic in previously treated abdominal regions.
However, conventional imaging modalities also have certain
limitations in monitoring lymph node metastasis, as they cannot
distinguish whether enlarged lymph nodes are caused by metastasis,
nor analyze certain smaller nodes and lymph node metastases
(19,20).
18F-FDG PET/CT is a hybrid imaging
modality that can provide anatomical and functional information,
and has been used for the staging and restaging of several cancers
(21). In recent years, it has been
used increasingly to identify recurrent disease. 18F-FDG
PET/CT can not only display the morphology, density and anatomical
changes in the lesion, but can also provide chemical information
and metabolic functions at the molecular level (22). 18F-FDG is the most
commonly used imaging agent in PET/CT imaging, and its distribution
in the body reflects the level of glucose metabolism in vivo
(23). FDG preferentially accumulates in malignant tumors and
metastatic lesions due to the increased rate of glucose
consumption, secondary to the increased rate of glycolysis and cell
membrane glucose transporters.
A previous study has shown that PET/CT is extremely
accurate for the detection of local and/or distant recurrent
disease in CRC patients, with high specificity and sensitivity
(24). In this study, the
specificity and sensitivity of monitoring the recurrence and
metastasis of patients with CRC using PET/CT was higher than that
using the CEA level. Certain studies have shown higher diagnostic
performances of FDG-PET/CT in comparison with other conventional
imaging modalities in the detection of CRC recurrence, particularly
in the case of locoregional recurrence and lymph nodes metastases
(25). Schmidt et al
(26) reported that the diagnostic
accuracy of FDG-PET/CT was higher than that of whole-body (WB)-MRI
in the follow-up of patients suffering from CRC. The overall
diagnostic accuracy for PET-CT was recorded as 91% (sensitivity,
86%; specificity, 96%) and that for WB-MRI was recorded as 83%
(sensitivity, 72%; specificity, 93%), respectively. Simó et
al (27) studies assessed the
effect of PET/CT detection of recurrent disease on the management
of patients with CRC and showed that FDG-PET had a significant
impact on the management of patients with suspected recurrence.
In conclusion, PET/CT has a higher diagnostic value
for monitoring the recurrence and metastasis of patients with CRC.
In the CRC patients with normal or elevated CEA levels, PET/CT
showed high specificity and sensitivity for recurrence and
metastasis, and is therefore an ideal method for monitoring the
status of the disease.
References
|
1
|
Desch CE, Benson AB III, Somerfield MR, et
al; American Society of Clinical Oncology. Colorectal cancer
surveillance: 2005 update of an American Society of Clinical
Oncology practice guideline. J Clin Oncol. 23:8512–8519. 2005.
|
|
2
|
Elias D, Sideris L, Pocard M, et al:
Results of R0 resection for colorectal liver metastases associated
with extrahepatic disease. Ann Surg Oncol. 11:274–280. 2004.
|
|
3
|
Lin JK, Lin CC, Yang SH, et al: Early
postoperative CEA level is a better prognostic indicator than is
preoperative CEA level in predicting prognosis of patients with
curable colorectal cancer. Int J Colorectal Dis. 26:1135–1141.
2011.
|
|
4
|
Dirisamer A, Halpern BS, Flöry D, et al:
Performance of integrated FDG-PET/contrast-enhanced CT in the
staging and restaging of colorectal cancer: comparison with PET and
enhanced CT. Eur J Radiol. 73:324–328. 2010.
|
|
5
|
Zhang C, Chen Y, Xue H, et al: Diagnostic
value of FDG-PET in recurrent colorectal carcinoma: a
meta-analysis. Int J Cancer. 124:167–173. 2009.
|
|
6
|
Zhang Y, Feng B, Zhang GL, et al: Value of
18F-FDG PET-CT in surveillance of postoperative
colorectal cancer patients with various carcinoembryonic antigen
concentrations. World J Gastroenterol. 20:6608–6614. 2014.
|
|
7
|
Panagiotidis E, Datseris IE, Rondogianni
P, et al: Does CEA and CA 19–9 combined increase the likelihood of
18F-FDG in detecting recurrence in colorectal patients with
negative CeCT? Nucl Med Commun. 35:598–605. 2014.
|
|
8
|
Makis W, Kurzencwyg D and Hickeson M:
18F-FDG PET/CT superior to serum CEA in detection of colorectal
cancer and its recurrence. Clin Imaging. 37:1094–1097. 2013.
|
|
9
|
Tan E, Gouvas N, Nicholls RJ, et al:
Diagnostic precision of carcinoembryonic antigen in the detecetion
of recurrence of colorectal cancer. Surg Oncol. 18:15–24. 2009.
|
|
10
|
Ding Q, Cheng X, Yang L, et al: PET/CT
evaluation of response to chemotherapy in non-small cell lung
cancer: PET response criteria in solid tumors (PERCIST) versus
response evaluation criteria in solid tumors (RECIST). J Thorac
Dis. 6:677–683. 2014.
|
|
11
|
Litière S, de Vries EG, Seymour L, et al;
RECIST Committee. The components of progression as explanatory
variables for overall survival in the Response Evaluation Criteria
in Solid Tumours 1.1 database. Eur J Cancer. 50:1847–1853.
2014.
|
|
12
|
Esteves FP, Schuster DM and Halkar RK:
Gastrointestinal tract malignancies and positron emission
tomography: an overview. Semin Nucl Med. 36:169–181. 2006.
|
|
13
|
Nielsen HJ, Jess P, Aldulaymi BH, et al:
Early detection of recurrence after curative resection for
colorectal cancer - obstacles when using soluble biomarkers? Scand
J Gastroenterol. 48:326–333. 2013.
|
|
14
|
Liu FY, Chen JS, Changchien CR, et al:
Utility of 2-fluoro-2-deoxy-D-glucose positron emission tomography
in managing patients of colorectal cancer with unexplained
carcinoembryomic antigen elevation at different levels. Dis Colon
Rectum. 48:1900–1912. 2005.
|
|
15
|
Moertel CG, Fleming TR, Macdonald JS, et
al: An evaluation of the carcinoembryonic antigen (CEA) test for
monitoring patients with resected colon cancer. JAMA. 270:943–947.
1993.
|
|
16
|
Zerhouni EA, Rutter C, Hamilton SR, et al:
CT and MR imaging in the staging of colorectal carcinoma: report of
the Radiology Diagnostic Oncology Group II. Radiology. 200:443–451.
1996.
|
|
17
|
Ozkan E, Soydal C, Araz M, et al: The role
of 18F-FDG PET/CT in detecting colorectal cancer recurrence in
patients with elevated CEA levels. Nucl Med Commun. 33:395–402.
2012.
|
|
18
|
Chiewvit S, Jiranantanakorn T,
Apisarnthanarak P, et al: Detection of recurrent colorectal cancer
by 18F-FDG PET/CT comparison with contrast enhanced CT scan. J Med
Assoc Thai. 96:703–708. 2013.
|
|
19
|
Peng NJ, Hu C, King TM, et al: Detection
of resectable recurrences in colorectal cancer patients with
2-[18F]fluoro-2-deoxy-D-glucose-positron emission
tomography/computed tomography. Cancer Biother Radiopharm.
28:479–487. 2013.
|
|
20
|
Lu YY, Chen JH, Chien CR, et al: Use of
FDG-PET or PET/CT to detect recurrent colorectal cancer in patients
with elevated CEA: a systematic review and meta-analysis. Int J
Colorectal Dis. 28:1039–1047. 2013.
|
|
21
|
Lee JE, Kim SW, Kim JS, et al: Prognostic
value of 18-fluorodeoxyglucose positron emission
tomography-computed tomography in resectable colorectal cancer.
World J Gastroenterol. 18:5072–5077. 2012.
|
|
22
|
Choi EK, Yoo IeR, Park HL, et al: Value of
Surveillance (18)F-FDG PET/CT in Colorectal Cancer: Comparison with
Conventional Imaging Studies. Nucl Med Mol Imaging. 46:189–195.
2012.
|
|
23
|
Sanli Y, Kuyumcu S, Ozkan ZG, et al: The
utility of FDG-PET/CT as an effective tool for detecting recurrent
colorectal cancer regardless of serum CEA levels. Ann Nucl Med.
26:551–558. 2012.
|
|
24
|
Maas M, Rutten IJ, Nelemans PJ, et al:
What is the most accurate whole-body imaging modality for
assessment of local and distant recurrent disease in colorectal
cancer? A meta-analysis: imaging for recurrent colorectal cancer.
Eur J Nucl Med Mol Imaging. 38:1560–1571. 2011.
|
|
25
|
Mittal BR, Senthil R, Kashyap R, et al:
18F-FDG PET-CT in evaluation of postoperative colorectal cancer
patients with rising CEA level. Nucl Med Commun. 32:789–793.
2011.
|
|
26
|
Schmidt GP, Baur-Melnyk A, Haug A, et al:
Whole-body MRI at 1.5 T and 3 T compared with FDG-PET-CT for the
detection of tumor recurrence in patients with colorectal cancer.
Eur Radiol. 19:1366–1378. 2009.
|
|
27
|
Simó M, Lomeña F, Setoain J, et al:
FDG-PET improves the management of patients with suspected
recurrence of colorectal cancer. Nucl Med Commun. 23:975–982.
2002.
|