Introduction
Small cell carcinomas (SCC) are a group of
neuroendocrine tumors, the majority of which arise from the lung
(1). When compared with the most
frequently reported small cell lung carcinomas (SCLCs), SCCs rarely
occur in extra-pulmonary organs and it has been reported that extra
pulmonary small cell carcinomas (ESCCs), occuring in urinary,
digestive and female reproductive system, account for only a small
amount of all SCCs (2).
Gastric SCC (GSCC), one of the typical SCC arising
from the digestive system, is a rare small cell neuroendocrine
tumor (3). However, GSCC is one of
the most aggressive malignant tumors and has an extremely poor
prognosis due to its high metastatic rate, with atypical clinical
manifestations (4). There are few
studies of cases of GSCC (5–9), with
great variability in how the tumors are treated, and little clear
guidance is currently available (10). The present study reports the case of
a 60-year-old male diagnosed with GSCC who received a combined
treatment of neoadjuvant chemotherapy, surgery and adjuvant
chemotherapy. Written informed consent was obtained from the
patient.
Case report
A 60-year-old male presented to the Nanjing
Drum-Tower Hospital (Nanjing, Jiangsu, China) with a one-year
history of epigastric distress. No other symptoms, including acid
regurgitation, eructation, nausea, emesis, diarrhea or melena, were
described. The patient had no history of hypertension or diabetes
mellitus and suffered from no infectious diseases, such as
hepatitis or tuberculosis. The patient had smoked one pack of
cigarettes per day for the past 30 years and had consumed alcohol
for ~20 years, but had no significant drug use or history of
malignancy in first-degree relatives. There was no evident
histological abnormality upon physical examination. An electronic
endoscopy revealed irregular tumor-like lesions in the cardia,
lesser curvature of the stomach and angular notch. A separate,
large, ulcerated mass with friable mucosa was observed in the
gastric cardia. Multiple biopsy specimens were obtained. The
hematoxylin and eosin-stained sections revealed densely-packed
sheets of small basophilic cells. Immunostaining was positive for
cytokeratin and the tumor was focally positive for synaptophysin
and chromogranin A. The immunohistochemical profile supported the
histological diagnosis of an SCC. No abnormality was detected on
chest radiography. A routine blood examination, liver function test
and electrocardiogram were performed. Among all the tumor markers,
the level of carbohydrate antigen (CA)-125 was 69.80 U/ml, slightly
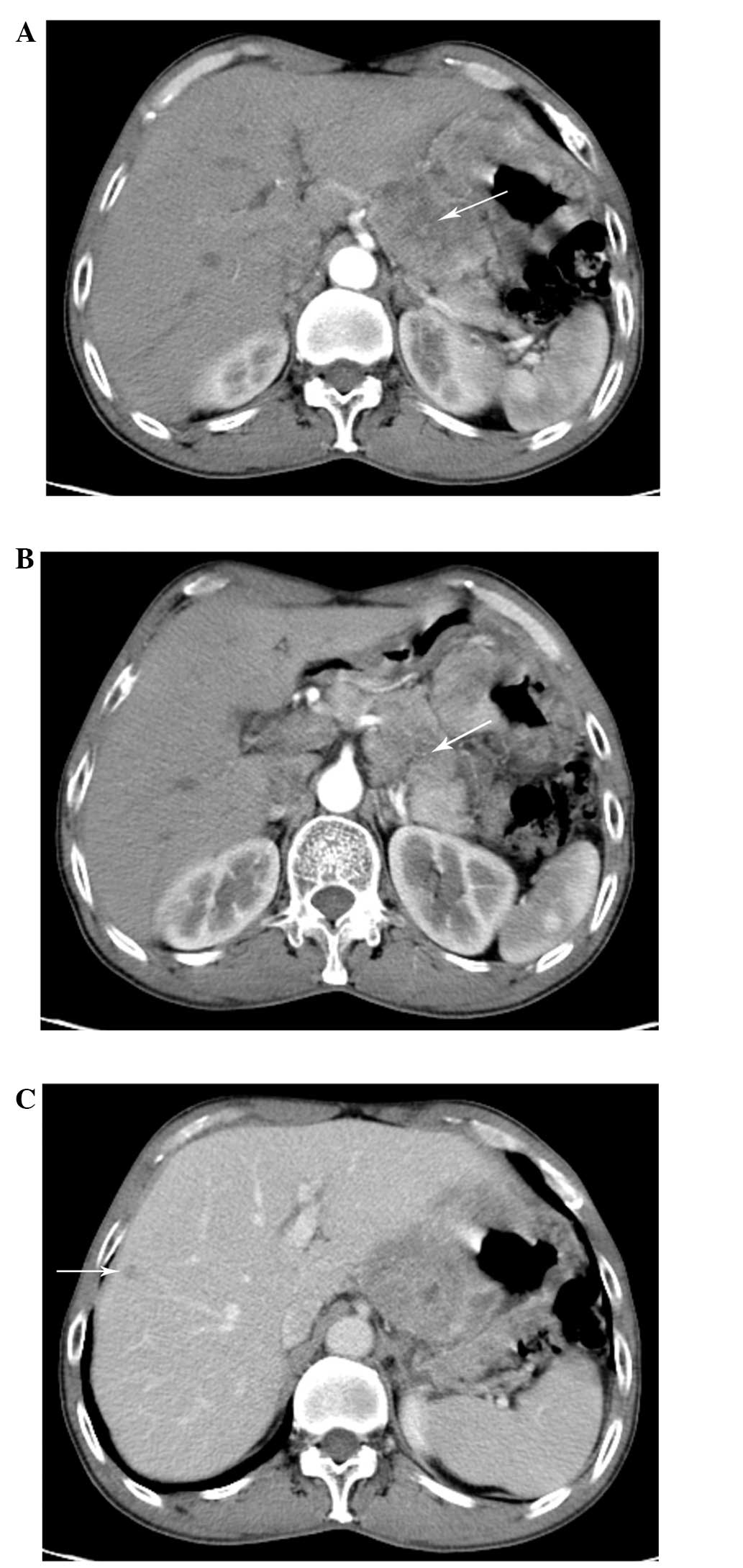
higher than the normal range of 0–35 U/ml. Computed tomography (CT)
revealed a thickening of the gastric wall and a mass (Fig. 1A), mainly in the lesser curvature of
stomach. A thickening left gastric artery entered the mass. The
mass invaded the liver and the pancreas (Fig. 1B), and the enhanced CT scan revealed
a nodular shadow of low density, with mild enhancement (Fig. 1C).
Collectively, the electronic gastroscopy (EG) and CT
findings supported a final diagnosis of metastatic GSCC. Following
a multi-disciplinary discussion between the Departments of General
Surgery and Radiation Oncology, it was decided that current
surgical methods would be of high risk and highly challenging. A
plan was developed to start the treatment of the patient using
neoadjuvant chemotherapy. Therefore, the patient underwent
treatment with irinotecan (200 mg, days 1, 21, 41 and 61) and
oxaliplatin (120 mg, days 1, 21, 41 and 61) for a four-cycle
period, without an evident adverse reaction. Following two cycles
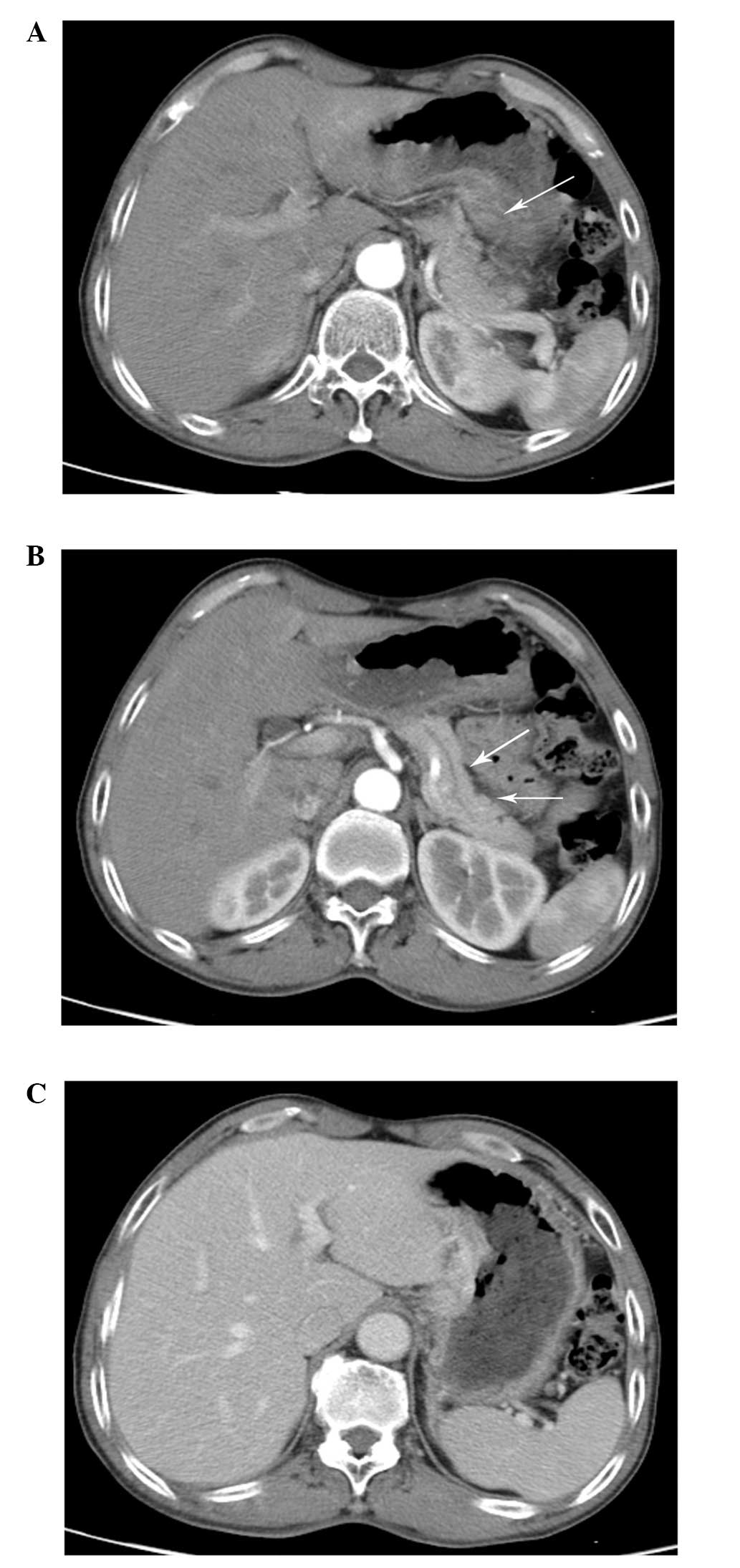
of chemotherapy, the patient achieved a partial response. The CT
scan revealed that the lesion had clearly decreased in size
(Fig. 2A and B). The enhanced CT
scan revealed that there was no evident nodule in the liver
(Fig. 2C). Levels of the CA-125
serum tumor marker dropped to within the normal range. Following
four cycles, the patient achieved another partial response, and
then another EG was performed. There were certain nodular niduses
in the gastric body and the fundus of the stomach (Fig. 3A and B). No abnormality was found in
the mucosa of the gastric antrum, neither were congestion, edema,
ulcers, bleeding or any other symptoms.
Following another multi-disciplinary discussion, the
patient underwent a D2 gastrectomy and esophagojejunal Roux-en-Y
anastomosis. There was a 5×2×1-cm mass under the cardia of the
stomach, in the lesser curvature. The serous membrane layer was
complete and there were no metastatic nodules found in the liver,
pancreas, spleen, kidneys, abdominal wall or pelvic cavity. The
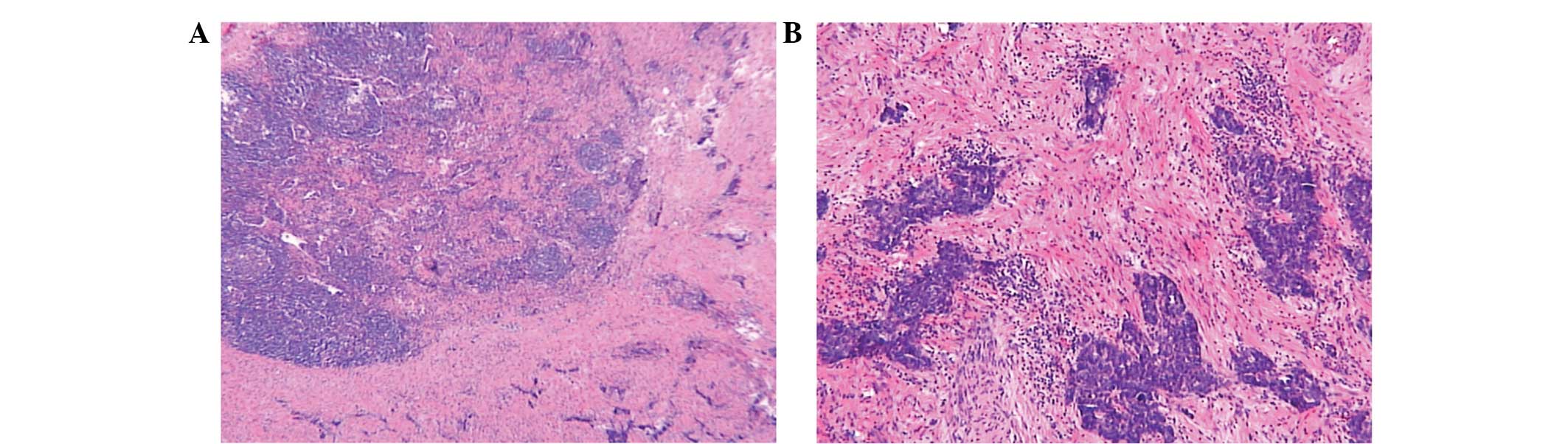
post-surgical pathology revealed that the tumor was 4×4×0.8 cm in
size, invading the serosa, nerves and vessels of the stomach. The
surgical margin was revealed to be tumor-free. Three
metastasis-positive lymph nodes were found in 27 cleaned lymph
nodes around the stomach. The pathological tumor-node-metastasis
stage was stage IV (T4aN2M1; Fig. 4A
and B). Following surgery, an enhanced CT scan was performed
and the patient received four cycles of adjuvant chemotherapy
combining irinotecan (200 mg, days 1, 21, 41 and 61) and
oxaliplatin (120 mg, days 1, 21, 41 and 61). The patient was
followed up for ~8 months and is currently alive.
Discussion
The neuroendocrine tumors of the digestive system
are typically classified by their differentiation according to the
World Health Organization classification (11) as follows: Well-differentiated,
subdivided into benign and borderline tumors;
moderately-differentiated, with low malignant potential;
poorly-differentiated, including large cell neuroendocrine
carcinoma and SSC; or mixed gland/neuroendocrine carcinoma
(12,13). SCCs are a group of neuroendocrine
tumors. SCCs are extremely rare, with the majority occurring in the
lung. Extrapulmonary SCC (EPSCC), which occurs in the bladder,
prostate, esophagus, stomach, colon, rectum and gallbladder, has
been reported domestically in China and overseas (14). EPSCC is rare and accounts for 4% of
the total number of SCCs. However, GSCC is extremely rare and
accounts for ~0.1% of all EPSCCs. In addition, according to the
literature, GSCC accounts for ~11% of all gastrointestinal SCCs
(10) and ~0.1% of all gastric
cancers.
SCC of the stomach is rarely reported. The condition
was first reported in 1976 by Matsusaka et al (17). GSCCs are large solid tumors, with
histological features similar to those of small-cell lung
carcinoma. The majority of GSCCs arise in the upper one-third of
the stomach (8,18) and tend to exhibit highly aggressive
biological behavior, with a tendency for early distant metastases.
There are reports of GSCC cases in China (19–21)
and, notably, Huang et al summarized and analyzed the
clinical features and prognosis of GSCC (22). However, the methods of GSCC therapy
are rarely mentioned in these studies. The present study reports
one of the extremely few cases of neoadjuvant chemotherapy in GSCC
patients. Chemotherapy has less effect on GSCC compared with small
cell lung cancer (SCLC) (6). GSCC
has previously been treated with regimens for SCLC, with the
combination of etoposide and cisplatin. Phase II/III studies of
irinotecan combined with cisplatin in patients with SCLC have
demonstrated the usefulness of irinotecan/carboplatin in the
chemotherapy for SCLC (18–20). Oxaliplatin, the third generation of
the platinum drugs, has a reduced gastrointestinal reaction
compared with that of cisplatin.
In the present study, following the pathological
diagnosis of GSCC, the enhanced CT scan revealed that the mass had
invaded the liver and the pancreas, with a nodular shadow of low
density in the liver and mild enhancement. Current surgical methods
were concluded to be of high risk and highly challenging following
multi-disciplinary discussion. Subsequent to four cycles of
neoadjuvant chemotherapy with irinotecan and oxaliplatin, the
enhanced CT scan revealed that the lesion was markedly decreased in
size and that there was no clear nodule in the liver. The patient
then received a D2 gastrectomy and esophagojejunal Roux-en-Y
anastomosis, followed by adjuvant chemotherapy. The present case of
combined neoadjuvant chemotherapy, surgery and adjuvant
chemotherapy highlights the comprehensive treatment of GSCCs,
particularly neoadjuvant chemotherapy, which provided the patient
with an opportunity to receive surgery. The present patient is
currently alive without disease progression and the follow-up of
this case continues.
References
|
1
|
van der Heijden HF and Heijdra YF:
Extrapulmonary small cell carcinoma. South Med J. 98:345–349.
2005.
|
|
2
|
Tanemura H, Ohshita H, Kanno A, et al: A
patient with small-cell carcinoma of the stomach with long survival
after percutaneous microwave coagulating therapy (PMCT) for liver
metastasis. Int J Clin Oncol. 7:128–132. 2002.
|
|
3
|
Frances N, Zeichner SB, Francavilla M and
Cusnir M: Gastric small-cell carcinoma found on
esophagogastroduodenoscopy: a case report and literature review.
Case Rep Oncol Med. 475961:2013.
|
|
4
|
Remick SC and Ruckdeschel JC:
Extrapulmonary and pulmonary small-cell carcinoma: tumor biology,
therapy, and outcome. Med Pediatr Oncol. 20:89–99. 1992.
|
|
5
|
Funahashi H, Miyai H, Wakasugi T, et al:
Successful combination chemotherapy with irinotecan hydrochloride
and cisplatin for primary gastric small cell carcinoma: report of a
case. World J Surg Oncol. 11:2632013.
|
|
6
|
Aoyama T, Yoshikawa T, Shirai J, et al: A
case of gastric small cell carcinoma with metastatic liver tumors
responding to surgery and chemotherapy. Gan To Kagaku Ryoho.
39:1889–1891. 2012.(In Japanese).
|
|
7
|
Takaku H, Oka K, Naoi Y, Santoh N, Setsu Y
and Mori N: Primary advanced gastric small cell carcinoma: a case
report and review of the literature. Am J Gastroenterol.
94:1402–1404. 1999.
|
|
8
|
Arai K and Matsuda M: Gastric small-cell
carcinoma in Japan: a case report and review of the literature. Am
J Clin Oncol. 21:458–461. 1998.
|
|
9
|
O’Byrne KJ, Cherukuri AK, Khan MI, et al:
Extrapulmonary small cell gastric carcinoma. A case report and
review of the literature. Acta Oncol. 36:78–80. 1997.
|
|
10
|
Brenner B, Tang LH, Klimstra DS and Kelsen
DP: Small-cell carcinomas of the gastrointestinal tract: a review.
J Clin Oncol. 22:2730–2739. 2004.
|
|
11
|
Bosman FT, Carneiro F and Hruban RH: WHO
classification of tumours of the digestive system. 3. 4th edition.
IARC Press; Lyon: 2010
|
|
12
|
Klimstra DS, Modlin IR, Coppola D, et al:
The pathologic classification of neuroendocrine tumors: a review of
nomenclature, grading, and staging systems. Pancreas. 39:707–712.
2010.
|
|
13
|
Tan EH and Tan CH: Imaging of
gastroenteropancreatic neuroendocrine tumors. World J Clin Oncol.
2:28–43. 2011.
|
|
14
|
Kim JH, Lee SH, Park J, et al:
Extrapulmonary small-cell carcinoma: a single-institution
experience. Jpn J Clin Oncol. 34:250–254. 2004.
|
|
15
|
Huang S, Zheng ZX, Xu Q and Yuan XH: The
diagnosis, treatment and prognosis evaluation of gastric small cell
carcinoma: analysis of 41 cases. Zhonghua Wai Ke Za Zhi.
51:225–229. 2013.(In Chinese).
|
|
16
|
Kusayanagi S, Konishi K, Miyasaka N, et
al: Primary small cell carcinoma of the stomach. J Gastroenterol
Hepatol. 18:743–747. 2003.
|
|
17
|
Matsusaka T, Watanabe H and Enjoji M:
Oat-cell carcinoma of the stomach. Fukuoka Igaku Zasshi. 67:65–73.
1976.
|
|
18
|
Matsui K, Kitagawa M, Miwa A, Kuroda Y and
Tsuji M: Small cell carcinoma of the stomach: a clinicopathologic
study of 17 cases. Am J Gastroenterol. 86:1167–1175. 1991.
|
|
19
|
Shao LH, Hua MR, Jun C, et al: Analysis of
clinical pathology and therapy in gastric small cell carcinomas. J
Pract Med. 23:2543–3544. 2007.
|
|
20
|
Ping Z, Ling CT and Jing L: Two case
reports of gastric small cell carcinoma and literature review. Chin
J Clin Oncol Rehabil. 8:782001.
|
|
21
|
Li X, Liao Z and Guo Y: Small cell
carcinoma of the stomach with liver and bone metastasis: a case
report. J Modern Oncol. 19:1421–1422. 2011.
|
|
22
|
Huang S, Zheng ZX, Xu Q and Yuan XH: The
diagnosis, treatment and prognosis evaluation of gastric small cell
carcinoma: analysis of 41 cases. Zhonghua Wai Ke Za Zhi.
51:225–229. 2013.(In Chinese).
|
|
23
|
Kudoh S, Fujiwara Y, Takada Y, et al:
Phase II study of irinotecan combined with cisplatin in patients
with previously untreated small-cell lung cancer. West Japan Lung
Cancer Group. J Clin Oncol. 16:1068–1074. 1998.
|
|
24
|
Boku N, Ohtsu A, Shimada Y, et al: Phase
II study of a combination of irinotecan and cisplatin against
metastatic gastric cancer. J Clin Oncol. 17:319–323. 1999.
|
|
25
|
Noda K, Nishiwaki Y, Kawahara M, et al;
Japan Clinical Oncology Group. Irinotecan plus cisplatin compared
with etoposide plus cisplatin for extensive small-cell lung cancer.
N Engl J Med. 346:85–91. 2002.
|