Introduction
Gallbladder cancer (GBC) is the most aggressive type
of biliary tract cancer (BTC) and exhibits the shortest median
survival time worldwide. Complete surgical resection offers the
only chance of complete remission; however, GBC is characterized by
local invasion, extensive regional lymph node metastasis, vascular
encasement and distant metastases. Therefore, only 10% of patients
present with early-stage disease are considered to be candidates
for surgery (1). Therefore,
chemotherapy serves as the primary treatment in the majority of GBC
cases. Previous studies have found that patients with metastatic
GBC who receive palliative therapy have a median survival time of
approximately six months (2,3).
Therefore, more effective chemotherapies are required for the
management of GBC.
S-1 is a novel orally administered drug composed of
a combination of the 5-fluorouracil (5-FU) prodrug, tegafur (FT),
5-chloro-2,4-dihydroxypyridine (CDHP) and oteracil potassium (OXO)
in a 1:0.4:1 molar concentration ratio (4). Based on the results obtained from
randomized phase III trials, S-1 has become a key drug in the
treatment of advanced gastric cancer in Japan and is considered to
be the standard option for chemotherapy (5,6).
Furthermore, gemcitabine and S-1 have also been approved for
clinical use in the treatment of BTC by the Ministry of Health,
Labour and Welfare in August 2007 (7).
The current study presents a case of GBC in which
the patient underwent successful surgical curative resection
following a single dose of S-1. The focus of this study was the
candidate characteristics that affect the therapeutic efficacy of
S-1-based chemotherapy. In particular, the gene expression involved
in the S-1 metabolic pathway was investigated by analyzing
dihydropyrimidine dehydrogenase (DPD), thymidylate synthase
(TS) and orotate phosphoribosyltransferase (OPRT)
gene expression. The patient provided full written informed consent
prior to the initiation of the study.
Case report
A 60-year-old female was admitted to Asahikawa City
Hospital (Asahikawa, Japan) with a primary complaint of jaundice in
2008. The patient’s medical history was unremarkable and, with the
exception of the jaundice, the physical examination revealed no
abnormalities. The results from the laboratory tests indicated
abnormal values for total bilirubin (7.9 mg/dl; normal range,
0.2–1.0 mg/dl), serum glutamic oxaloacetic transaminase (124 IU/l;
normal range, 6–40 IU/l) and serum glutamic pyruvic transaminase
(228 IU/l; normal range, 6–37 IU/l). On admission, tumor marker
levels were as follows: Carcinoembryonic antigen, 21.1 ng/ml (upper
normal limit, 4.9 ng/ml) and carbohydrate antigen 19–9, 107.6 IU/ml
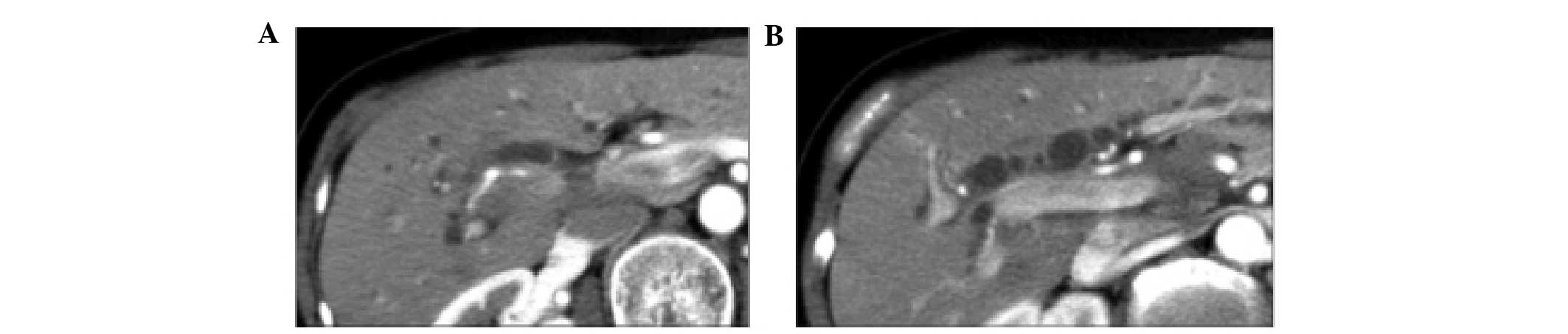
(upper normal limit, 39 IU/ml). An abdominal computed tomography
(CT) scan revealed the presence of advanced GBC, which had invaded
the liver, as well as regional lymph node metastasis and perineural
invasion of the common hepatic and celiac arteries (Fig. 1). The tumor was considered to be
inoperable due to the presence of perineural invasion of the common
hepatic and celiac arteries. With informed consent from the
patient, chemotherapy with S-1 was initiated at a dose of 100 mg
twice daily for four weeks, followed by a 14-day rest period, for a
total of 25 cycles.
Clinical course of treatment
The patient was followed up every six weeks for a
total of 36 months. The tumor marker levels decreased after two
months(Fig. 2), and a CT scan
demonstrated a clear reduction in size in the regions of the liver
that were invaded by the GBC, as well as those affected by lymph
node metastasis and perineural invasion (Fig. 3). A partial response was maintained
for over 30 months and, subsequently, surgical resection with
curative intent was planned for 36 months following the initiation
of treatment with S-1.
The patient underwent percutaneous transhepatic
portal embolization and, three weeks following this, a right
lobectomy with extrabiliary duct resection and lymphadenectomy was
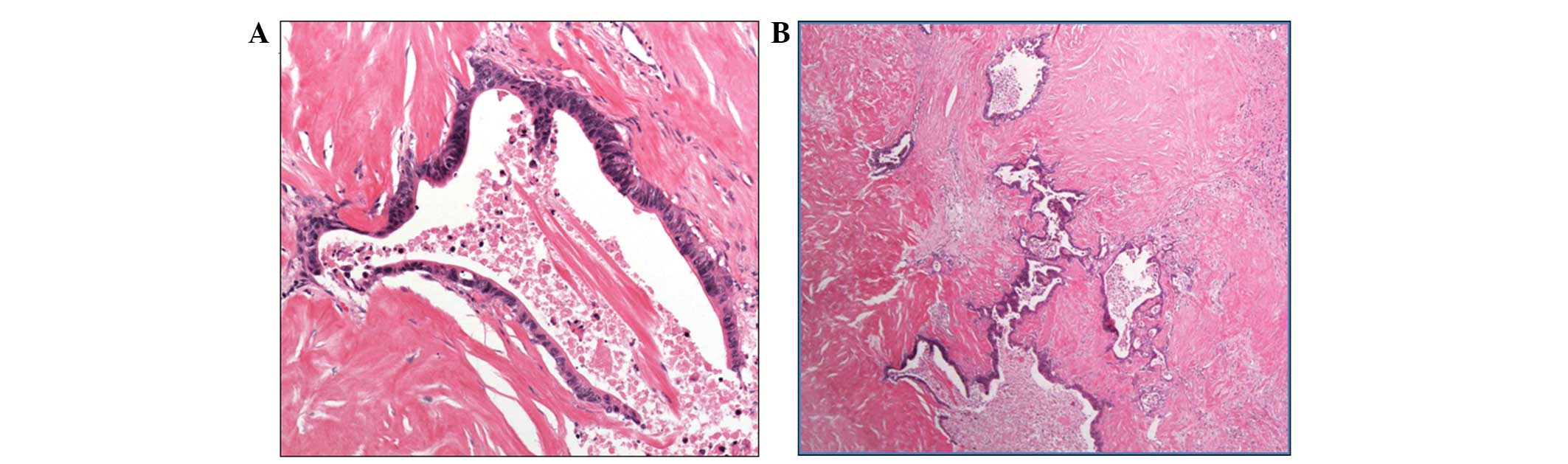
performed. The pathological findings of the tumor were compatible
with a diagnosis of adenocarcinoma of the gallbladder. The tumor
had directly invaded the liver and cancer cells were found in the
perineural area; however there was no metastatic lesion in the
liver and no regional lymph node metastasis. All surgical margins
were negative (Fig. 4) and a
pathological R0 resection was achieved. The postoperative course
was uneventful, and the patient was discharged 25 days following
surgery.
Intratumoral gene expression levels of
DPD, OPRT and TS
The resected gallbladder specimen was analyzed to
determine the intratumoral gene expression levels of DPD, OPRT and
TS, which encode the corresponding key enzymes that are involved in
the metabolism of 5-FU (8,9). The expression levels of these genes
were all measured by Response Genetics (Los Angels, CA, USA) using
the Danenburg Tumor Profile method and laser capture
microdissection, as described previously (10,11).
The specimen exhibited high intratumoral DPD gene expression
levels compared with those observed in the BTC cases who were
non-responders of S-1 treatment (Table
I).
 | Table IIntratumoral DPD, TS and
OPRT gene expression in biliary tract cancer patients. |
Table I
Intratumoral DPD, TS and
OPRT gene expression in biliary tract cancer patients.
| Author (ref.) | TS | DPD | OPRT |
|---|
| Present case | 3.26 | 8.21 | 0.91 |
| Kitajima et al
(14) | 1.88 | 8.21 | 0.91 |
| Case 1 | 14.42 | 3.16 | 1.35 |
| Case 2 | 2.73 | 2.78 | 0.73 |
Discussion
The symptoms of GBC are vague and non-specific;
therefore, GBC is often detected at an advanced or metastatic
stage. The most effective treatment for GBC is surgical resection;
however, the majority of GBCs are unresectable at the time of
diagnosis (1,12,13)
and, hence, numerous GBC patients undergo chemotherapy (14).
In colorectal cancer, primary systemic chemotherapy
appears to be a promising approach to the management of patients
with initially unresectable liver metastases, as it leads to a
reduction in the lesion size, which facilitates the surgical
resection in a high proportion of cases (15). Patients who are able to undergo
complete resection following chemotherapy tend to achieve improved
outcomes (15–17).
A review of the literature identified only three
case reports with regard to surgical resection following
chemotherapy for unresectable GBC (Table II) (14,18,19).
Two of the four cases survived for >1 year without recurrence
(one survived for a year and no information is available for one
case). Compared with unresectable GBC cases, the patients reported
in these cases had a good prognosis. In the present case, surgery
was performed three years following treatment with S-1. To date, no
evidence of recurrence has been observed for 30 months following
surgery.
 | Table IISurgical resection following
chemotherapy for unresectable gallbladder cancer. |
Table II
Surgical resection following
chemotherapy for unresectable gallbladder cancer.
| Author (ref.) | Regime | Time to surgery after
chemotherapy, months | Regime after
surgery | Prognosis |
|---|
| Kitajima et al
(14) | S-1 | 8 | Unknown | Unknown |
| Morimoto et al
(18) | Gem | 12 | Gem | No recurrence for 20
months |
| Takita et al
(19) | Gem + S-1 | 9 | S-1 | No recurrence for 12
months |
| Present case | S-1 | 36 | S-1 | No recurrence for 30
months |
The novel antitumor drug S-1 contains a prodrug of
5-FU and was developed based on the biochemical effects of CDHP, a
DPD inhibitor, and OXO, an OPRT inhibitor, in the small intestine.
The principal roles of these modulators are to inhibit the
degradation of 5-FU and to protect against 5-FU-induced
gastrointestinal toxicity. TS is a major target of 5-FU, which
inhibits DNA synthesis. High TS activity in cancer tissue is
considered to reduce the efficacy of 5-FU and it is likely that the
DPD mRNA level is also a significant predictor of the response to
5-FU. Low TS and DPD expression levels are associated with poor
outcomes in colorectal cancer patients who are treated with surgery
alone, whereas these low expression levels are associated with
improved outcomes in patients who are treated with 5-FU
chemotherapy (20).
The tumor tissue of the patient in the present case
and of a patient in a previous case (14) exhibited markedly higher DPD mRNA
levels compared with those observed in two BTC patients who did not
respond to S-1 treatment (Table I).
Conversely, the use of a 5-FU agent in the absence of CDHP is
likely to exhibit a decrease in the efficacy, with the agent
rapidly becoming inactive due to degradation by the excess DPD
produced by the tumor. In the current study, while no conclusive
evidence was obtained that the S-1 treatment resulted in the
downstaging of the cancer, it is reasonable to speculate that the
CDHP in S-1 was significant, and may have enhanced the antitumor
efficacy of 5-FU through the inhibition of the excess DPD produced
by the tumor, although, additional studies may be required to
confirm this.
In conclusion, the current study reports the case of
a patient with advanced GBC for whom the anticancer agent, S-1, was
effective and a pathological R0 resection was achieved. The results
of the present study indicate that the CDHP in S-1 may enhance the
antitumor effect of 5-FU by inhibiting the excess DPD protein
produced by the tumor. The use of S-1 in patients with GBC warrants
further clinical studies. The present study suggests that the CDHP
in S-1 may have enhanced the antitumor efficacy of 5-FU by
inhibiting the excess DPD produced by the tumor. Analysis of the
intratumoral gene expression levels of DPD may be useful to predict
the efficacy of S-1 treatment.
References
|
1
|
Zhu AX, Hong TS, Hezel AF and Kooby DA:
Current management of gallbladder carcinoma. Oncologist.
15:168–181. 2010.
|
|
2
|
Glimelius B, Hoffman K, Sjödén PO, et al:
Chemotherapy improves survival and quality of life in advanced
pancreatic and biliary cancer. Ann Oncol. 7:593–600. 1996.
|
|
3
|
Ishikawa T, Horimi T, Shima Y, et al:
Evaluation of aggressive surgical treatment for advanced carcinoma
of the gallbladder. J Hepatobiliary Pancreat Surg. 10:233–238.
2003.
|
|
4
|
Shirasaka T, Shimamato Y, Ohshimo H, et
al: Development of a novel form of an oral 5-fluorouracil
derivative (S-1) directed to the potentiation of the tumor
selective cytotoxicity of 5-fluorouracil by two biochemical
modulators. Anticancer Drugs. 7:548–557. 1996.
|
|
5
|
Boku N, Yamamoto S, Fukuda H, et al:
Gastrointestinal Oncology Study Group of the Japan Clinical
Oncology Group: Fluorouracil versus combination of irinotecan plus
cisplatin versus S-1 in metastatic gastric cancer: a randomised
phase 3 study. Lancet Oncol. 10:1063–1069. 2009.
|
|
6
|
Koizumi W, Narahara H, Hara T, et al: S-1
plus cisplatin versus S-1 alone for first-line treatment of
advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet
Oncol. 9:215–221. 2008.
|
|
7
|
Ueno H, Okusaka T, Ikeda M, Takezako Y and
Morizane C: Phase II study of S-1 in patients with advanced biliary
tract cancer. Br J Cancer. 91:1769–1774. 2004.
|
|
8
|
Van Triest B, Pinedo HM, Giaccone G and
Peters GJ: Downstream molecular determinants of response to
5-fluorouracil and antifolate thymidylate synthase inhibitors. Ann
Oncol. 2000 Apr;11(4): 385–91
|
|
9
|
Miyoshi Y, Uemura H, Ishiguro H, et al:
Expression of thymidylate synthase, dihydropyrimidine
dehydrogenase, thymidine phosphorylase, and orotate phosphoribosyl
transferase in prostate cancer. Prostate Cancer Prostatic Dis.
8:260–265. 2005.
|
|
10
|
Diasio RB and Lu Z: Dihydropyrimidine
dehydrogenase activity and fluorouracil chemotherapy. J Clin Oncol.
12:2239–2242. 1994.
|
|
11
|
Kuramochi H, Hayashi K, Uchida K, et al:
Vascular endothelial growth factor messenger RNA expression level
is preserved in liver metastases compared with corresponding
primary colorectal cancer. Clin Cancer Res. 12:29–33. 2006.
|
|
12
|
Manfredi S, Benhamiche AM, Isambert N, et
al: Trends in incidence and management of gallbladder carcinoma: a
population-based study in France. Cancer. 89:757–762. 2000.
|
|
13
|
Grobmyer SR, Lieberman MD and Daly JM:
Gallbladder cancer in the twentieth century: single institution’s
experience. World J Surg. 28:47–49. 2004.
|
|
14
|
Kitajima K, Kobayashi S, Shiba H, et al:
Successful treatment of advanced gallbladder cancer with an
anticancer drug S-1: assessment based on intratumoral gene. Int J
Clin Oncol. 13:545–551. 2008.
|
|
15
|
Bismuth H, Adam R, Lévi F, et al:
Resection of nonresectable liver metastases from colorectal cancer
after neoadjuvant chemotherapy. Ann Surg. 224:509–520. 1996.
|
|
16
|
Alberts SR, Horvath WL, Sternfeld WC, et
al: Oxaliplatin, fluorouracil, and leucovorin for patients with
unresectable liver-only metastases from colorectal cancer: a North
Central Cancer Treatment Group phase II study. J Clin Oncol.
23:9243–9249. 2005.
|
|
17
|
Adam R, Avisar E, Ariche A, et al:
Five-year survival following hepatic resection after neoadjuvant
therapy for nonresectable colorectal. Ann Surg Oncol. 8:347–353.
2001.
|
|
18
|
Morimoto H, Ajiki T, Takase S, et al:
Resection of gallbladder cancer with hepatic metastasis after
chemotherapy with gemcitabine. J Hepatobiliary Pancreat Surg.
15:655–658. 2008.
|
|
19
|
Takita M, Iwasaki E, Hatogai K, et al:
Advanced gallbladder cancer that showed complete response to
gemcitabine plus S-1 chemotherapy. Nihon Shokakibyo Gakkai Zasshi.
108:1263–1270. 2011.(in Japanese).
|
|
20
|
Soong R, Shah N, Salto-Tellez M, et al:
Prognostic significance of thymidylate synthase, dihydropyrimidine
dehydrogenase and thymidine phosphorylase protein expression in
colorectal cancer patients treated with or without
5-fluorouracil-based chemotherapy. Ann Oncol. 19:915–919. 2008.
|