Introduction
Pulmonary epithelioid hemangioendothelioma (PEH) is
the current term for a rare neoplasm originally described by Dail
and Liebow in 1975 as intravascular sclerosing bronchioalveolar
tumor (IVBAT) of the lung (1,2). PEH
is a rare pulmonary neoplasm of vascular origin with fewer than 50
cases reported in the literature (3–6). PEH
typically manifests as multiple bilateral lung nodules that are
usually discovered incidentally in young or middle-aged Caucasian
women, although cases in children and the elderly have also been
reported (4,7). Male, symptomatic, the presence of
cough, hemoptysis, chest pain, multiple unilateral nodules, pleural
effusion, metastases to more than one site and lymph node
metastases are the factors associated with a poor prognosis.
Symptomatic patients and the presence of a pleural effusion are
independent predictors of survival in patients with PEH (7). Epithelial hemangioendothelioma has
also been reported to originate in the liver, head and neck area,
oral mucosa, bone, mediastinum, diaphragm and brain (8,9).
PEH is a rare low-grade malignant vascular tumor
that occurs in the lungs and, due to its rarity, it is easy to
clinically misdiagnose PEH as other lung diseases. In the present
study, four cases of PEH, which were diagnosed and treated at
Shanghai Chest Hospital, Shanghai Jiao Tong University (Shanghai,
China), were observed and analyzed with respect to the clinical
manifestations, imaging findings, histopathological
characteristics, immunohistochemical phenotypes and prognosis. This
study was performed according to the Declaration of Helsinki and
was approved the the ethics committee of Shanghai Chest Hospital,
Shanghai Jiao Tong University (Shanghai, China). Written informed
consent was obtained from all patients.
Case reports
Clinical manifestations
The present study describes four cases of PEH that
were diagnosed at the Chest Hospital Affiliated to Shanghai
Jiaotong University from 2006 to 2013. Two of the cases were
surgical patients at the hospital, while the other two cases were
consultation patients from other hospitals. Case 1 was a
54-year-old male with multiple lung nodules that were revealed
during a preoperative examination for cholecystitis. Case 2 was a
54-year-old female who was admitted for chest tightness and
fatigue, whereby X-ray examination revealed a right pleural
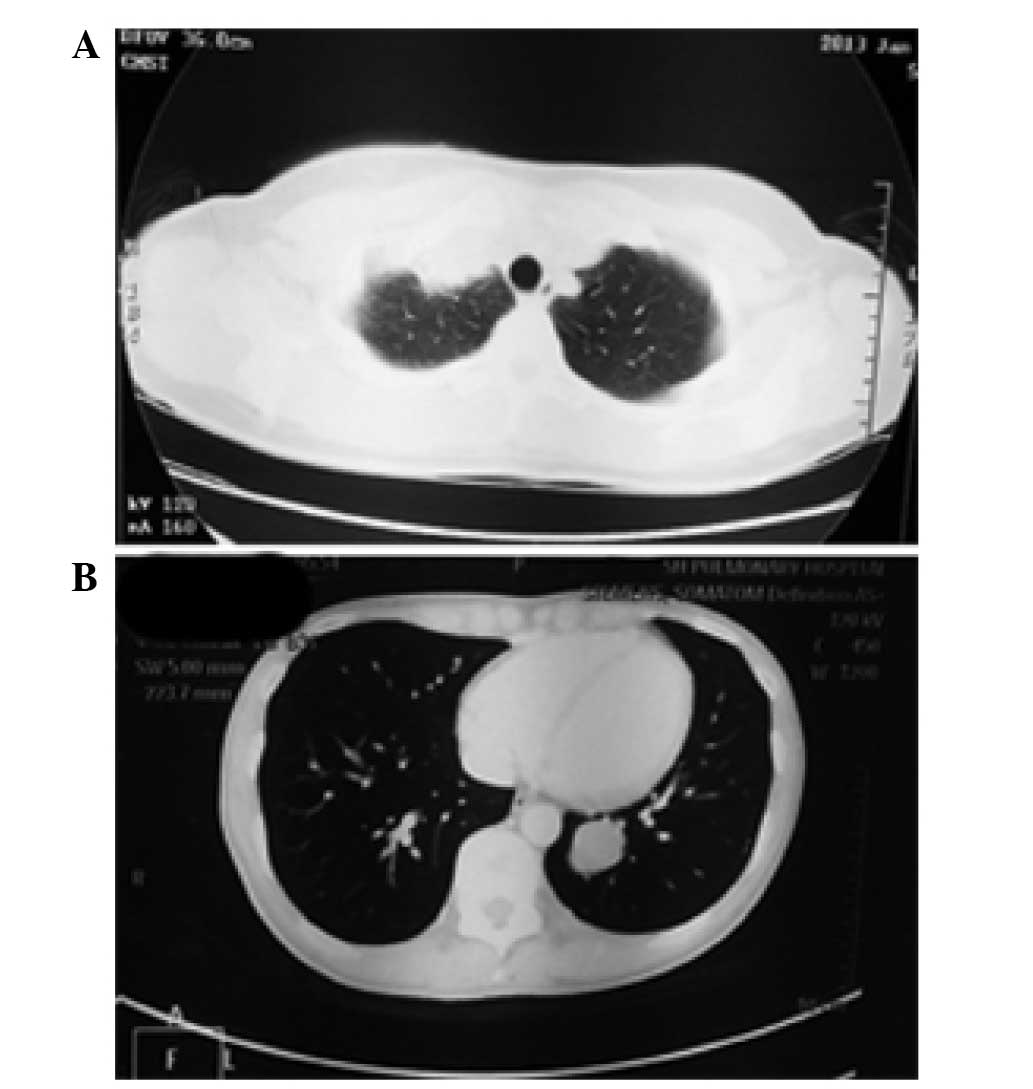
effusion. Case 3 was a 46-year-old female with no obvious symptoms;
yet irregular clumps were found below the pleura of the right upper
lung on a chest computed tomography (CT) during a routine physical
examination (Fig. 1A). Case 4 was a
30-year-old female who was also without obvious symptoms, and
multiple nodules in both lungs were revealed on a chest CT during a
routine physical examination (Fig.
1B). Cases 3 and 4 underwent lobectomy, while cases 1 and 2
underwent pulmonary wedge resection. A seven-year postoperative
follow-up for case 1 showed that the patient’s condition was
stable, with no significant progression. Persistent pleural
effusion was observed in case 2, and the patient received
chemotherapy at another hospital, but succumbed to the disease
after three years. Cases 3 and 4 were six and five months into the
postoperative follow-up period at the time of writing,
respectively. (Table I)
 | Table IClinical data of the four cases of
pulmonary epithelioid hemangioendothelioma. |
Table I
Clinical data of the four cases of
pulmonary epithelioid hemangioendothelioma.
| Case no. | Gender | Age (years) | Clinical
symptoms | Disease site | Imaging findings | Tumor diameter
(cm) | Treatment | Follow-up |
|---|
| 1 | Male | 54 | No obvious
symptom | Left lung | Multiple nodules in
both lungs | 4 nodules:
0.5–1.0 | Wedge resection of
left upper lobe and left lower lobe | 7 years, in stable
condition |
| 2 | Female | 54 | Pleural effusion on
right side for 1 month | Right upper lung | Irregular pleural
thickening at right upper lobe | 2 lesions: 3×2×1,
1×0.5×0.5 | Wedge resection of
right upper lobe with postoperative chemotherapy | Succumbed 3 years
after surgery |
| 3 | Female | 46 | No obvious
symptoms | Right upper lung | Irregular clumps
below the pleura of the right upper lobe | Multiple lesions:
0.3–3 | Right upper
lobectomy, nodular resection in right middle lower lobe and pleural
nodules | Postoperative
follow-up for 6 months |
| 4 | Female | 30 | No obvious
symptoms | Left lower lung | Multiple nodules in
both lungs | 2 nodules: 1.5–3 | Left lower
lobectomy | Postoperative
follow-up for 5 months |
Materials and methods
All of the specimens were fixed in 4% neutral
formalin, embedded in paraffin and stained with hematoxylin and
eosin. Briefly, following deparaffinization, rehydration,
heat-induced epitope retrieval and endogenous peroxidase blocking,
the slides were incubated with primary antibodies for 1 h. The
primary monoclonal antibodies, including mouse anti-human cluster
of differentiation 31 (CD31) (1:200 dilution), mouse anti-human
CD34 (1:200 dilution, mouse anti-human creatine kinase (CK) (1:100
dilution), mouse anti-human CK7 (1:200 dilution), mouse
anti-epithelial membrane antigen (EMA) (1:200 dilution), mouse
anti-human calretinin (1: 200 dilution), mouse anti-human desmin
(1:200 dilution), mouse anti-human thyroid transcription factor 1
(TTF1) (1:200 dilution) and mouse anti-human vimentin (1:200
dilution) and polyclonal rabbit anti-human factor VIII (F8) (1:200
dilution) were all purchased from Dako (Carpinteria, CA, USA). The
specimens were subsequently subjected to the polyclonal goat
anti-rabbit anti-mouse secondary antibody (Dako) (dilution 1:500)
for 30 min and visualized using 3,3′-diaminobenzidine
tetrahydrochloride as chromogen using an Envision system (Dako)
(10).
Case 1
Two specimens from the pulmonary wedge resection of
the left upper lobe were submitted for examination. One gray nodule
was observed in each section, with diameters of 0.6 and 1 cm. Two
other specimens from the pulmonary wedge resection of the left
lower lobe were submitted for examination. One gray nodule was
observed in each specimen, with diameters of 0.5 and 0.9 cm.
Case 2
Two specimens from the pulmonary wedge resection of
the right upper lobe were submitted for examination. Two patchy
thickenings were found on the pleural surface, and the sections
were gray and hard, measuring 3×2×1 cm and 1.5×1×0.5 cm.
Case 3
The right upper lobe specimen was submitted for
examination. A lump 3×2×2.1 cm in size was found in the upper right
tip segment that was pale yellow-gray in color, hard, and invading
into the pleura with pleural adhesions and thickening. Multiple
gray nodules were also found in the middle and lower segments of
the examined right lung lobe and right pleura, 0.3–0.5 cm in
diameter.
Case 4
A specimen of the left lower lobe was submitted for
examination. A lump 3×3×2.5 cm in size was found in the basal
segment of the left lower lobe that was gray-yellow, hard,
well-defined and located 0.5 cm from the pleura. At 2 cm distant
from the lump in the basal segment, another lump 1.5×1.5×1.4 cm in
size was observed that was also gray-yellow, hard, well-defined and
located 1 cm from the pleura.
Microscopy
The tumors were composed of the tumor cells arranged
in short cords and nests with degenerated stromal mucoid. The tumor
cells were medium in size, polygonal or spindle shaped, with
unclear cell boundaries. The cytoplasm was abundant and
eosinophilic, and the nuclei were round, with small nucleoli
showing mild or moderate atypia. The lumen or vacuolization
containing one or more erythrocytes was commonly observed in the
cytoplasm of the tumor cells (Fig. 2A
and B). In case 4, the tumor cells were abundant in some areas,
with obvious atypia; the tumor cells were arranged in solid nests
and pseudoglandular structures, growing toward the surrounding
alveoli and filling the alveolar space. The tumor cells in other
areas formed papillary structures in the blood vessels (Fig. 2C). In case 3, the tumor cells were
growing in multiple small nodules, with abundant cells surrounding
the nodules and sclerosis at the nodular centers that was similar
to hyaline degeneration or necrosis, and calcifications were
observed in the necrotic center (Fig.
2D).
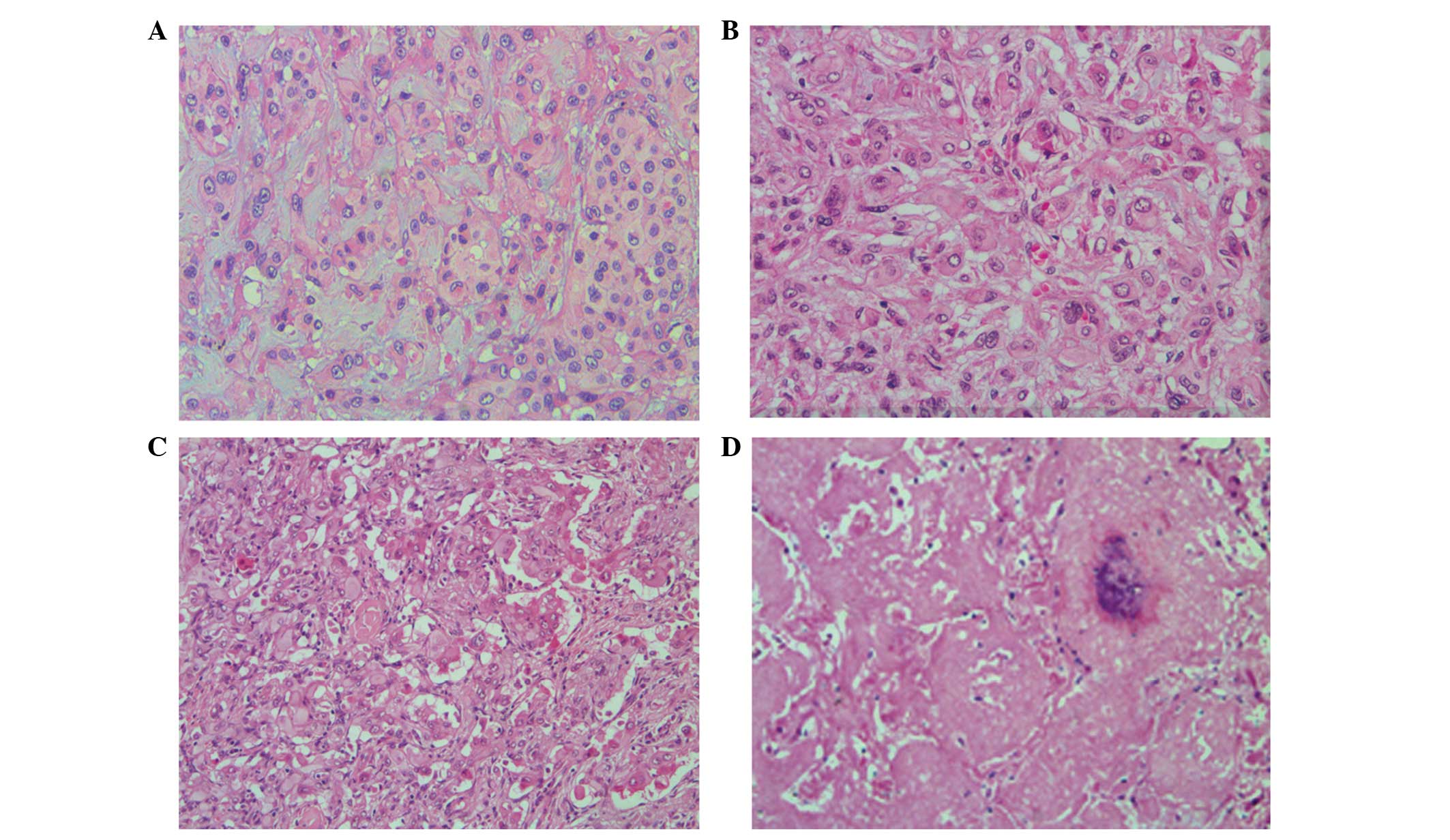 | Figure 2Histological examination
characteristics of pulmonary epithelioid hemangioendothelioma
(hematoxylin and eosin staining) (A, case 1; B, case 2; C, case 3;
D, case 4). (A) Neoplasms are composed of short cords and nested
tumor cells, and interstitial mucus degeneration (magnification,
×200). (B) Typically, the lumen or cavity in the tumor cytoplasm
contain single or multiple erythrocytes (magnification, ×200). (C)
Tumor cells are rich in certain areas, with marked atypia. The
tumor cells are arranged in solid nests and duct-like structures,
and form papillary structures in the blood vessels (magnification,
×200). (D) Tumor cells show multiple small nodules and local
cerebral calcification at the necrotic center (magnification,
×100). |
Immunohistochemistry
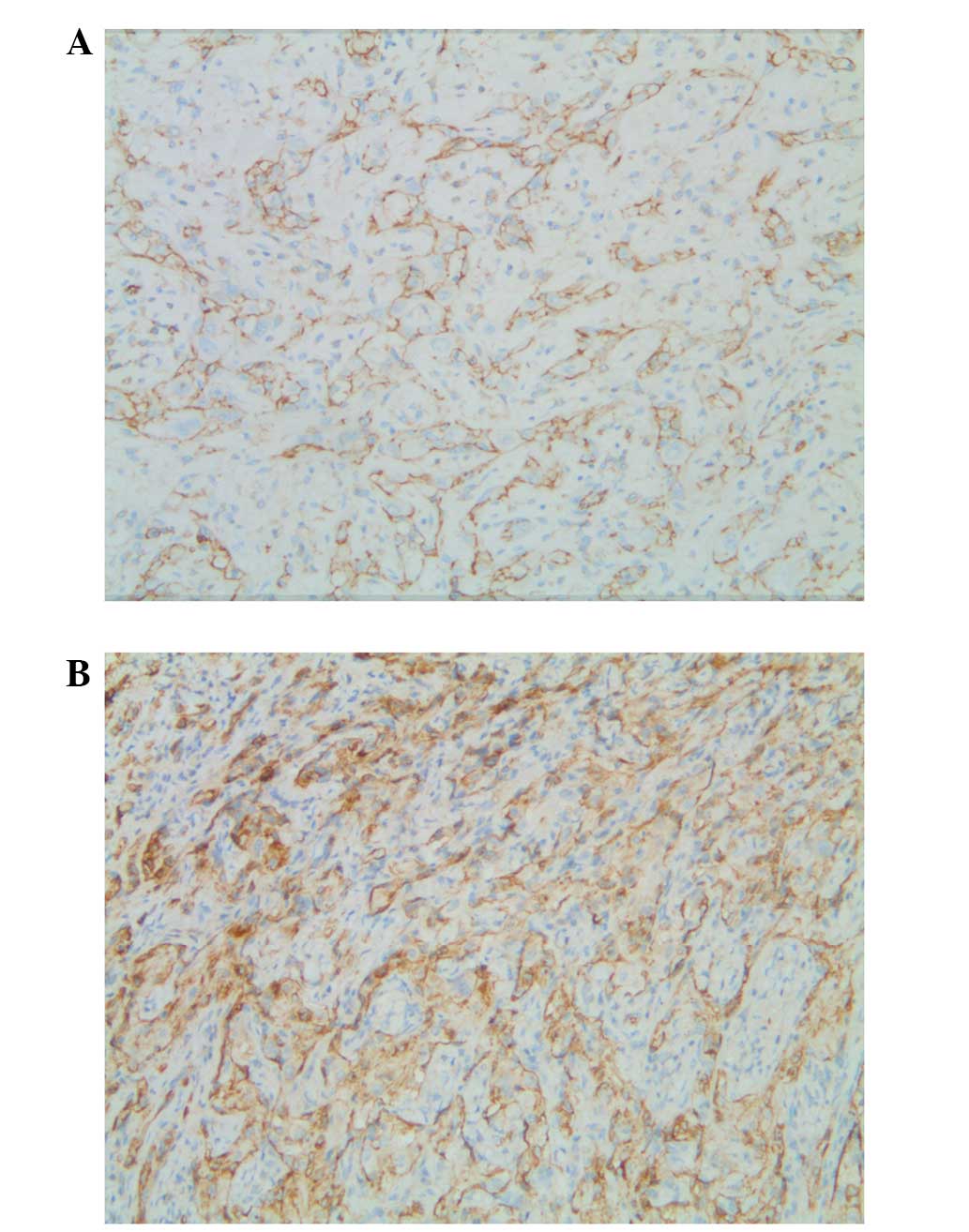
The tumor cells of all four cases were positive for
CD31, F8 and vimentin; CD34 expression was positive in three cases;
and EMA was focally positive in one case. The tumor cells of all
four cases were negative for other markers, including TTF1, CK,
CK7, calretinin and desmin (Fig. 3A and
B). The pathological diagnosis was PEH in each case.
Discussion
Epithelioid hemangioendothelioma is a low-grade
malignant vascular tumor that was previously considered an
intermediate vascular tumor, but was reclassified as a low-grade
angiosarcoma in the 2002 WHO classification (11). It often occurs in the superficial
and deep soft tissues of the extremities, and cases occurring in
lungs are rare and often multifocal (2,12). PEH
was first reported by Dail and Liebow (13).
PEH often occurs in middle-aged women, with a mean
patient age of 40 years old. With no obvious symptoms, patients are
often diagnosed on physical examination. For patients with
symptoms, chest tightness, shortness of breath and difficulty
breathing after exertion can occur; the tumors grow slowly,
manifesting as chronic processes clinically, and pleural effusion
is the first sign of pleural invasion (7,14–16).
Among the four cases of PEH in this study, three cases were
initially identified during physical examination and one case
showed a right pleural effusion as the first sign.
The imaging of PEH is mainly characterized by
multiple pulmonary nodules, rare solitary lesions and nodule
diameters of 1–2 cm, although a diameter of >5 cm has also been
reported (9,15). Calcifications and ossifications have
occasionally been found in the lesions. If the lesions have invaded
the pleura, changes such as pleural thickening and pleural effusion
can occur (17). The four cases in
this study all showed multiple lung nodules or lesions.
Most commonly, PEH presents as bilateral or
unilateral multiple pulmonary nodules with clear boundaries,
diameters of 0.3–2 cm, and pale gray or brown coloration. These can
invade the pleura and cause pleural effusion or nodular thickening.
Calcifications or ossifications can occur in the center of the
nodules (18,19).
The intralesional vascular structure was unclear,
and the tumors formed short, cord-like strips with solid nest
structures by the eosinophilic endothelial cells, which were round
or slightly fusiform. The stroma was light blue hyaline mucus with
hyaline degeneration in certain cases. The tumor cells showed
abundant, ill-defined cytoplasm and intracytoplasmic lumina or
vacuoles, which at times contained single or multiple intraluminal
red blood cells. These red blood cells were evidence of the
differentiation of tumor cells into vascular endothelial cells. The
tumor cells had mild to moderate atypia, with nuclear
vacuolization, inconspicuous nucleoli and rare or missing mitoses.
Some tumor nodule centers showed hardening and an acellular zone
accompanied by coagulation necrosis, calcification and
ossification. The abundant tumor cells surrounding the nodules
broke into the alveolar cavity in papillary or polypoid structures.
A number of the atypical morphologies were observed in
approximately one-third of cases, which were manifested as the
obvious atypia in tumor cells, including a mitotic count of
>1/10 per high-powered field, fusiform cells and accompanying
necrosis. These lesions were highly invasive, and tumors at this
phase are malignant epithelioid hemangioendotheliomas, with a
continuation of the morphology of epithelioid angiosarcoma
(4,8,20,21).
PEH expresses a variety of vascular antigens, such
as CD31, CD34, F8, friend leukemia integration 1 transcription
factor, Ulex europaeus agglutinin type 1 and FKBNP12 (22,23).
Among these, F8 was highly specific, but its sensitivity was the
lowest; CD31 was relatively specific and highly sensitive, globally
expressed in 90% of the cases; CK or EMA was focally expressed in
25–30% of the cases. In the present study, all four cases expressed
CD31 and F8 (100%), three cases expressed CD34 (75%), one case was
focally positive for EMA (25%) and no case expressed CK (0%).
Due to the fact that PEH is rare, clinically, it is
easily confused with a variety of benign and malignant lung
diseases (24). As PEH shows
multiple lung nodules on imaging, it is often misdiagnosed as
peripheral lung cancer with lung metastasis. The differential
diagnosis of PEH includes chronic granulomatous disease, amyloid
nodules, hamartoma, primary or metastatic lung cancer, malignant
mesothelioma and angiosarcoma (25).
Chronic granulomatous diseases include tuberculosis,
sarcoidosis and fungal granuloma. These diseases may present as
multiple unilateral or bilateral pulmonary nodules on imaging.
However, tuberculosis is positive in the clinical tuberculin test,
and the effectiveness of anti-TB treatment can contribute to its
identification. Among patients with active sarcoidosis, 80% show an
increased level of angiotensin converting enzyme, and sarcoidosis
is characterized by multiple mediastinal lymph nodes and hilar
lymphadenopathy, with or without pulmonary nodular lesions. These
are all pathologically expressed as epithelioid nodular
hyperplasia. Sarcoidosis can show typical caseous necrosis, and
sarcoidosis is characterized by concentric granulomas, implying
that the outer layer arrangement of the collagen granuloma is
onion-like, without necrosis or with only focal necrosis. Fungal
granulomas show refraction spores inside and outside of
multinucleated giant cells and histiocytes. Therefore, they may be
clearly pathologically distinguished from PEH (26).
Patients with pulmonary amyloid nodules also show no
obvious symptoms, and they are usually elderly patients with single
or multiple pulmonary lesions found on physical examination,
presenting similar clinical and radiological manifestations to PEH.
Pathologically, nodular amyloidosis shows an irregular structure on
microscopy, with homogeneous powder staining. Additionally, the
nodules are surrounded by foreign body giant cells phagocytizing
the amyloid, with occasional focal ossification and calcification.
No tumor cells differentiated from mild or moderate atypical
vascular endothelial cells, as in PEH, are observed. Congo red and
methyl violet starch staining can confirm the diagnosis (27).
Chest imaging of hamartoma typically reveals a
solitary and well-defined nodule, while multiple lesions are rare.
Pathomorphologically, the nodule is mainly comprised of lobulated
mature cartilage, and retraction of the bronchiolar epithelium may
be observed. When a large mucus cartilage-like stroma of PEH
appears, it must be distinguished from the true cartilage component
of hamartoma. However, based on the findings of different imaging
studies, its pathomorphology and immunohistochemical markers, it is
not difficult to distinguish hamartoma from PEH.
The metastasis of primary lung adenocarcinoma within
the lungs or the metastasis of extrapulmonary tumors to the lungs
is the most common reason for the occurrence of multiple nodules in
the lungs and, thus, it is not difficult to explain why PEH is
often misdiagnosed as these tumor types. In addition, the nested
and corded arrangement of the tumor cells and the myxoid stroma in
PEH are also easily misdiagnosed as adenocarcinoma or mucinous
adenocarcinoma on biopsy and during intraoperative freezing.
However, the atypia of the tumor cells in primary or metastatic
lung cancer are obvious, with clear mitoses. If the
immunohistochemistry shows the expression of the epithelial cell
marker and is negative for the vascular endothelial marker, such
tumors can be differentiated from PEH.
In the case of PEH invading the pleura or a pleural
effusion, PEH must be distinguished from malignant pleural
mesothelioma. The tumor cells of the latter are characterized by
bidirectional differentiation, with the expression of the
epithelial markers CK and CK7, the mesenchymal marker vimentin, and
mesothelial cell markers, such as calretinin, Wilms tumor 1, D2–40
and CK5/6. In addition, malignant pleural mesothelioma cells are
negative for vascular endothelial marker (CD31 and CD34)
expression.
Angiosarcoma is common in skin and rare in the
lungs, and tends to present as a large solitary mass. Tumor
vascular lumina, luminal lining epithelioid malignant cells, highly
atypical tumor cells and mitoses are commonly observed under the
microscope, and large lesions with spindle cells are also
observable. Some PEHs may show certain atypical morphologies that
are similar to epithelioid angiosarcoma, forming a morphological
continuity with epithelioid angiosarcoma (28).
When the lesions of PEH inside one lung are
relatively limited, surgery is the preferred treatment; however,
when the lesions have invaded both lungs and cannot be completely
resected, postoperative chemotherapy may be required, according to
the condition of the patient. The average survival time of patients
with asymptomatic pulmonary nodules is 15 years, and this can be
>25 years in the best cases (12,13).
Patients whose lung lesions were completely removed by surgery have
shown long remissions or have been cured. Patients whose lesions
were not completely removed and showed invasion into the airway,
blood vessels and pleura showed poor prognosis; patients at
advanced stages often died of respiratory failure (7,9). It
has been reported that the survival rate for 40% of patients with
PEH is less than five years (12,29).
Since the prognosis for patients with PEH varies widely, long-term
follow-up is necessary. For the four cases in the present study,
one patient showed no recurrence within a seven-year follow-up, one
patient died three years after the surgery, and the other two
patients are five and six months into the postoperative follow-up
period, respectively.
In conclusion, the clinicopathological features of
four PEH cases were investigated and the associated literature was
reviewed. This revealed PEH to be a rare yet diverse form of
malignant vasular tumor with varying patient prognoses. The results
of this study may improve our understanding of PEH. However,
studies with a larger sample size are required in order to provide
a more comprehensive understanding, which would improve the
clinical treatment and prognosis for patients with PEH.
References
|
1
|
Dail DH and Liebow AA: Intravascular
bronchioalveolar tumor. Am J Pathol. 78:6–7. 1975.
|
|
2
|
Anagnostou V, Mossa E, Mihas S, Lepouras A
and Tiniakos DG: Epithelioid haemangioendothelioma of the lung
presenting with pulmonary nocardiosis. In Vivo. 21:1123–1126.
2007.
|
|
3
|
Robinson AA, Tolentino LF, Uyanne J,
Melrose R and Calhoun CC: Malignant epithelioid
hemangioendothelioma of the lip: a case report and comprehensive
literature review. J Oral Maxillofac Surg. 72:695–701. 2014.
|
|
4
|
Leleu O, Lenglet F, Clarot C, Kleinmann P
and Jounieaux V: Pulmonary epithelioid haemangioendothelioma:
reports of three cases and a review of the literature. Rev Mal
Respir. 27:778–783. 2010.
|
|
5
|
Cronin P and Arenberg D: Pulmonary
epithelioid hemangioendothelioma: an unusual case and a review of
the literature. Chest. 125:789–793. 2004.
|
|
6
|
Darbari A, Singh D, Singh PK and Bharadwaj
M: Pulmonary epithelioid hemangioendothelioma: A rare pulmonary
tumor in differential diagnosis of bronchogenic carcinoma. Lung
India. 27:37–38. 2010.
|
|
7
|
Amin RM, Hiroshima K, Kokubo T, et al:
Risk factors and independent predictors of survival in patients
with pulmonary epithelioid haemangioendothelioma. Review of the
literature and a case report. Respirology. 11:818–825. 2006.
|
|
8
|
Weiss SW, Ishak KG, Dail DH, Sweet DE and
Enzinger FM: Epithelioid hemangioendothelioma and related lesions.
Sem Diag Pathol. 3:259–287. 1986.
|
|
9
|
Kitaichi M, Nagai S, Nishimura K, Itoh H,
Asamoto H, Izumi T and Dail DH: Pulmonary epithelioid
haemangioendothelioma in 21 patients, including three with partial
spontaneous regression. Eur Respir J. 12:89–96. 1998.
|
|
10
|
Yang S, Sun R, Zhou Z, Zhou J, Liang J and
Mu H: Expression of Amyloid-β Protein and Amyloid-β Precursor
Protein After Primary Brain-Stem Injury in Rats. Am J Forensic Med
Pathol. Jun 19–2014.(Epub ahead of print).
|
|
11
|
Harb H and Habil I: Frequency and profile
of induced abortions: hospital based study in tertiary hospitals in
Egypt. J Prev Med Hyg. 54:159–162. 2013.
|
|
12
|
Erasmus JJ, McAdams HP and Carraway MS: A
63-year old woman with weight loss and multiple lung nodules.
Chest. 111:236–238. 1997.
|
|
13
|
Dail DH, Liebow AA, Gmelich JT, et al:
Intravascular, bronchiolar, and alveolar tumor of the lung (IVBAT).
An analysis of twenty cases of a peculiar sclerosing endothelial
tumor. Cancer. 51:452–464. 1983.
|
|
14
|
Liao QL, Chen XD, Wang ZC, Wang W and Lai
RQ: Pulmonary epithelioid hemangioendothelioma: a
clinicopathological analysis. Zhonghua Jie He He Hu Xi Za Zhi.
34:187–191. 2011.(In Chinese).
|
|
15
|
Xu JH and Chen LR: Pulmonary epithelioid
hemangioendothelioma accompanied by bilateral multiple calcified
nodules in lung. Diagn Pathol. 6:212011.
|
|
16
|
Rosengarten D, Kramer MR, Amir G, Fuks L
and Berkman N: Pulmonary epithelioid hemangioendothelioma. Isr Med
Assoc J. 13:676–679. 2011.
|
|
17
|
Bahrami A, Allen TC and Cagle PT:
Pulmonary epithelioid hemangioendothelioma mimicking mesothelioma.
Pathol Int. 58:730–734. 2008.
|
|
18
|
Sardaro A, Bardoscia L, Petruzzelli MF,
Nikolaou A, Detti B and Angelelli G: Pulmonary epithelioid
hemangioendothelioma presenting with vertebral metastases: a case
report. J Med Case Rep. 8:2012014.
|
|
19
|
Al-Shraim M, Mahboub B, Neligan PC,
Chamberlain D and Ghazarian D: Primary pleural epithelioid
haemangioendothelioma with metastases to the skin. A case report
and literature review. J Clin Pathol. 58:107–109. 2005.
|
|
20
|
Weissferdt A and Moran CA: Primary
vascular tumors of the lungs: a review. Ann Diagn Pathol.
14:296–308. 2010.
|
|
21
|
Díaz R, Segura A, Calderero V, Cervera I,
Aparicio J, Jordá MV and Pellín L: Central nervous system
metastases of a pulmonary epitheloid haemangioendothelioma. Eur
Respir J. 23:483–486. 2004.
|
|
22
|
Białas M, Papla B and Bulanda A:
Immunohistochemical investigation of selected endothelial markers
in pulmonary epithelioid haemangioendothelioma. Pol J Pathol.
62:236–240. 2011.
|
|
23
|
Traweek ST, Kandalaft PL, Mehta P and
Battifora H: The human hematopoietic progenitor cell antigen (CD34)
in vascular neoplasia. Am J Clin Pathol. 96:25–31. 1991.
|
|
24
|
Molina Palma MI, Cervantes Góngora JA,
García de la Torre E, Conde Pérez de la Blanca I and Ramírez
Tortosa CL: Primary intraoral epithelioid hemangioendothelioma.
Case report and review of the literature. Acta Otorrinolaringol
Esp. 53:215–218. 2002.(In Spanish).
|
|
25
|
Otani K, Ishikawa T, Aizawa Y, Fujise K,
Koyama T, Ohkusa T and Tajiri H: A long-term survival case of liver
epithelioid hemangioendothelioma with multiple lung metastases that
regressed by long-term administration of interleukin-2. Nihon
Shokakibyo Gakkai Zasshi. 109:2097–2102. 2012.
|
|
26
|
Jinghong X and Lirong C: Pulmonary
epithelioid hemangioendothelioma accompanied by bilateral multiple
calcified nodules in lung. Diagn Pathol. 6:212011.(In
Japanese).
|
|
27
|
Reich S, Ringe H, Uhlenberg B, Fischer B
and Varnholt V: Epithelioid hemangioendothelioma of the lung
presenting with pneumonia and heart rhythm disturbances in a
teenage girl. J Pediatr Hematol Oncol. 32:274–276. 2010.
|
|
28
|
Pálföldi R, Radács M, Csada E, Molnár Z,
Pintér S, Tiszlavicz L, Molnár J, Valkusz Z, Somfay A and Gálfi M:
Pulmonary epithelioid haemangioendothelioma studies in vitro and in
vivo: new diagnostic and treatment methods. In Vivo. 27:221–225.
2013.
|
|
29
|
Bagan P, Hassan M, Le Pimpec Barthes F, et
al: Prognostic factors and surgical indications of pulmonary
epithelioid. hemangioendothelioma: a review of the literature. Ann
Thorac Surg. 82:2010–2013. 2006.
|