Introduction
Garlic (Allium sativum) is a vegetable of the
Allium class of bulb-shaped plants, which has been used for
thousands of years and in numerous cultures as a food and for its
medicinal purposes, dating back >4,000 years (1). Currently, garlic is used to aid in the
prevention of heart disease, high cholesterol and blood pressure
and to boost the immune system (2–4).
However, evidence from epidemiological and experimental
carcinogenesis studies has indicated that certain components of
garlic possess anticancer activity (5). Subsequent to reacting with endogenous
antioxidants, including cysteine and reduced glutathione, the
majority of garlic allyl sulfides, which are absorbed in the
gastrointestinal tract, have also been reported to biotransform to
the corresponding allylmercapto glutathione S-congugate (6). One of these allylmercapto glutathione
S-conjugates, S-allylmercapto-L-cysteine (SAMC), is a water soluble
organosulfur compound that is found in aged garlic extract and is
obtained through the ethanol extraction of sliced garlic bulbs.
SAMC has been reported to exert an inhibitory effect on
tumorigenesis (7), however, the
mechanisms through which this occurs are poorly understood. The
current study investigates the effects of SAMC on the growth of the
SW620 human colorectal carcinoma cell line.
Materials and methods
Cell culture and maintenance
The human colorectal carcinoma SW620 cell line was
obtained from the Peking Union Medical College (Beijing, China),
and maintained in minimal RPMI 1640 medium (Life Technologies,
Carlsbad, CA, USA) containing 10% fetal bovine serum (FBS; Life
Technologies) and 1% antibioticantimycotic (Life Technologies) in a
humidified atmosphere of 5% CO2 at 37°C.
Drug treatment
SAMC (06-284) was provided by Wakunaga
Pharmaceutical Co., Ltd. (Osaka, Japan). According to the
manufacturer’s instructions, sterilized stock solutions of SAMC (5
mM) were freshly prepared in phosphate-buffered saline (PBS), and
refrigerated at 4°C. The SW620 cells were seeded at a density of
1×104 cells/well in 24-well plates and incubated for 24
h. SAMC was dissolved in PBS and added to the culture media at
various concentrations, ranging from 0–450 μM, and the cells were
subsequently incubated for 72 h.
Cell viability assay
The SW620 cells were plated in 96-well plates at a
density of 1×104 cells/well. The cells were incubated
with various concentrations of SAMC (0, 100, 200, 300, 350, 400 and
450 μM) for 12, 24, 48 and 72 h. MTT solution [0.5 g
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide in 100
ml PBS; Sigma, St. Louis, MO, USA] was added to the culture medium
(final concentration, 500 μg/ml) 4 h prior to the completion of the
treatment and the reaction was terminated by the addition of 100 μl
of 10% acidified sodium dodecyl sulfate to the cell culture. The
absorbance value (A) was measured at 570 nm using a multiwell
spectrophotometer (Bio-Rad 550; Bio-Rad, Hercules, CA, USA). The
percentage of cell inhibition was calculated using the following
formula: Inhibitory rate (%) = (1 − A of experiment well/A of
control well) × 100. A dose-survival curve was obtained for each
experiment. The experiments were performed in triplicate.
Apoptosis analysis
Apoptosis of SW620 cells was detected using terminal
deoxynucleotidyl transferase-mediated deoxyribonucleotide
triphosphate-digoxigenin nick-end labeling (TUNEL), following drug
treatment for 72 h. Briefly, the cells were fixed for 1 h at room
temperature and subsequently incubated in permeabilization solution
for 2 min on ice. The subsequent staining was performed according
to the manufacturer’s instructions.
Quantitative polymerase chain reaction
(PCR)
The expression of components of the c-Jun N-terminal
kinase (JNK) and p38 mitogen activated protein kinase (p38)
pathways was assessed using reverse transcription (RT)-PCR and
quantitative-PCR. Briefly, following treatment with SAMC, the cells
were washed with cold PBS and the total RNA from the cells was
isolated using TRIzol reagent (Life Technologies). The RNA was then
reverse transcribed into the complementary (c)DNA for PCR
amplification. The primers used were designed using the software
Primer Premier version 5.0 (Premier Biosoft, Palo Alto, CA, USA)
and synthesized by SBS Genetech Co., Ltd. (Beijing, China). Further
information with regard to the gene-specific primer pairs used is
listed in Table I. The PCR products
were detected by 2.5% agarose gel electrophoresis. Quantitative
RT-PCR analysis was performed according to the protocol detailed in
a previous study (8). Briefly, 500
ng of the total RNA was reverse-transcribed to cDNA using Takara
PCR Thermal Cycler Dice (Takara Biotechnology (Dalian) Co., Ltd.,
Dalian, China) and subsequently the cDNA was subjected to
quantitative-PCR using the Applied Biosystems 7500 PCR device
(Applied Biosystems, Foster City, CA, USA). mRNA expression of the
components of the JNK and p38 pathways was normalized using GAPDH
mRNA and the data were analyzed according to relative gene
expression using the 2−ΔΔCT (Livak) method. The
experiments were performed in triplicate.
 | Table IPrimer sequences used in reverse
transcription-PCR and quantitative PCR assays. |
Table I
Primer sequences used in reverse
transcription-PCR and quantitative PCR assays.
| Gene | Direction | Primer sequence | Tm, °C | Cycle | Fragment size,
bp |
|---|
| RAC1 | F | 5′
AAACCGGTGAATCTGGGCTT 3′ | 60.0 | 30 | 91 |
| R | 5′
AGAACACATCTGTTTGCGGA 3′ | | | |
| DAXX | F | 5′
GCTTAGTTGCATGAAGGCGG 3′ | 60.0 | 30 | 149 |
| R | 5′
AGAATTCCTGCTCAGAAACCGT 3′ | | | |
| ASK1 | F | 5′
CATGTCAACCGGGATGTCCA 3′ | 58.0 | 30 | 169 |
| R | 5′
CTAGACCCGTACTGCTGCTG 3′ | | | |
| MEKK1 | F | 5′
AAGCCTGCCGGTGACTAAC 3′ | 60.0 | 30 | 116 |
| R | 5′
GCATCACCCGGAGGAGAAAT 3′ | | | |
| MKK3 | F | 5′
GAAAGCCTGCCGGTGACTAA 3′ | 60.0 | 30 | 200 |
| R | 5′
TTCCCGTTCTCAGCCTTGAC 3′ | | | |
| JNK | F | 5′
CTGAAGCAGAAGCTCCACCA 3′ | 60.0 | 30 | 159 |
| R | 5′
CACCTAAAGGAGAGGGCTGC 3′ | | | |
| p38 | F | 5′
ATGAAGCTCTCCAACACCCG 3′ | 60.0 | 30 | 205 |
| R | 5′
GCACCTAAAGGAGAGGGCTG 3′ | | | |
| p53 | F | 5′
CAGCCCTCTCCTTTAGGTGC 3′ | 57.5 | 30 | 137 |
| R | 5′
GCTGCTGCTTCTAGACTGCT 3′ | | | |
| Bcl2 | F | 5′
GCTCTCCAACACCCGTACAT 3′ | 60.0 | 30 | 203 |
| R | 5′
GCTGCACCTAAAGGAGAGGG 3′ | | | |
| Bax | F | 5′
AGGGTGTAAAACGCAGCTCA 3′ | 58.7 | 30 | 202 |
| R | 5′
AGGGTGTAAAACGCAGCTCA 3′ | | | |
| β-actin | F | 5′
AATGGGCAGCCGTTAGGAAA 3′ | 60.0 | 30 | 169 |
| R | 5′
GCGCCCAATACGACCAAATC 3′ | | | |
Statistical analysis
Statistical analyses of the data were performed
using a one-way analysis of variance followed by the Tukey-Kramer
honest significant difference (HSD) test for the three sets of
results. P<0.05 was considered to indicate a statistically
significant difference. Statistical analyses were performed using
JMP® Statistical Discovery Software version 4.0 (SAS
Institute, Cary, NC).
Results
SAMC-induced reduction of SW620 cell
viability
Viability of SAMC-treated SW620 cells was initially
assessed using an MTT assay. The results revealed that SAMC
decreased tumor cell viability in a dose- and time-dependent manner
(Fig. 1). However, cell death was
observed when the concentration of SAMC was >450 μM.
Apoptosis analysis
SW620 cells were treated with SAMC at various
concentrations, and subsequently cultured for 72 h. TUNEL is a
common method for detecting DNA fragmentation that results from
apoptotic signaling cascades. DNA fragmentation was labeled in
situ via a TUNEL assay. A significant increase in the
proportion of DNA fragmentation-positive cells was observed
following SAMC treatment, in contrast with the control group
(Fig. 2).
JNK and p38 pathway assay through
PCR
To investigate the roles of the JNK and p38 pathways
in SAMC-induced apoptosis, the mRNA levels of the members of the
JNK and p38 pathways in SAMC treated SW620 cells were determined.
Cellular total RNA was isolated following 24, 48 and 72 h of
treatment with SAMC respectively, and RT-PCR and quantitative PCR
were performed for members of JNK and p38 pathway, including RAC1,
DAXX, MEKK1, ASK1, JNK, MKK3, p38, p53, Bcl-2 and Bax. The PCR
assay demonstrated that following incubation with SAMC, the
specific genes of the JNK and p38 apoptosis pathway, including
RAC1, DAXX, MEKK1, ASK1, JNK, MKK3, p38, p53, Bcl-2 and Bax could
be detected. Predominantly, the gene expression leved showed a
time-lapse increase, however, the gene expression of Bcl-2 showed a
time-lapse decrease (Figs. 3 and
4).
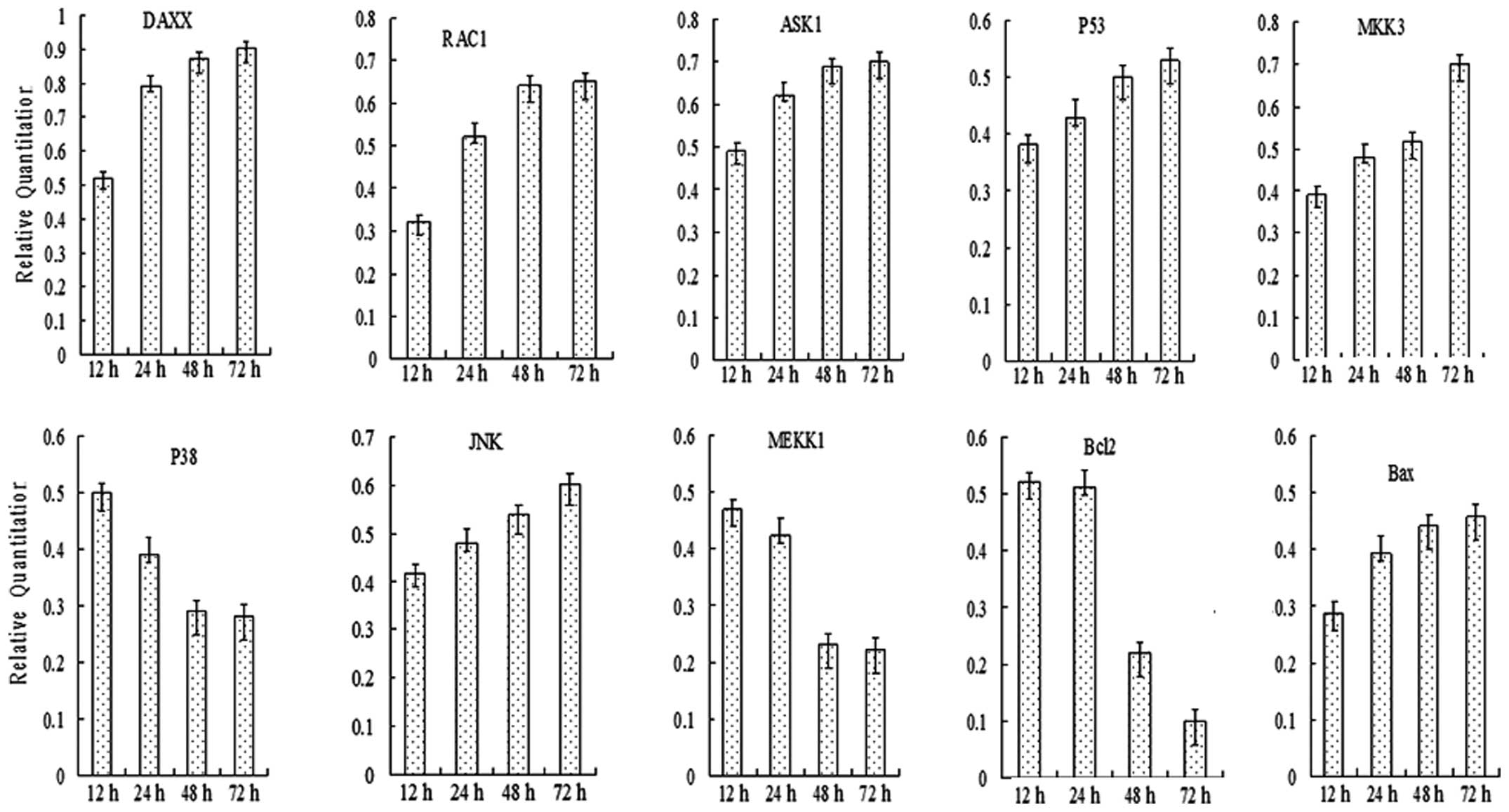 | Figure 4Quantitative polymerase chain reaction
analyses of the expression of JNK and p38 pathway members in SW620
cells at 72 h following treatment with SAMC (400 μM). Expression of
RAC1, DAXX, MEKK1, ASK1, JNK, MKK3, p38, p53, Bcl2 and Bax was
detected, and the gene expression level exhibited a time-lapse
increase. However, the gene expression of Bcl2 exhibited a
time-lapse decrease. JNK, c-Jun N-terminal kinase; SAMC,
S-allylmercaptocysteine; RAC1, Ras-related C3 botulinum toxin
substrate 1; DAXX, death-associated protein 6; MEKK1,
mitogen-activated protein kinase kinase 1; ASK1, Apoptosis
signal-regulating kinase 1; MKK3, mitogen-activated protein kinase
kinase 3; p38, p38 MAP kinase; p53, tumor protein p53, Bcl2, B-cell
lymphoma 2; Bax, Bcl2-like protein 4. |
Discussion
Garlic has been shown to be effective against a
broad spectrum of diseases and SAMC, one of the constituents of
garlic, has been proposed to be responsible for this biological
activity (9). In the current study,
the results clearly demonstrated that SAMC induces apoptosis in the
human colorectal carcinoma cell line SW620 in vitro, which
may explain the antiproliferative activity of garlic. These results
also suggest that the induction of apoptotic cell death by SAMC
occurs via the JNK and p38 pathways that activate p53 and Bax.
Apoptosis is a systematically regulated process that involves the
expression of numerous gene products. Of the major genes that
regulate apoptosis, the antiapoptotic Bcl-2 gene and the
proapoptotic Bax gene are of particular interest. p53 is a tumor
suppressor gene and a sequence-specific transcription factor; p53
activation may occur through a variety of forms of cellular stress
(10). Tumor-derived p53 mutants
that were able to promote cell growth and transformation were the
focus of the majority of initial studies of p53 (11,12)
and subsequently, p53 was determined as an essential mediator of
cell cycle arrest in response to various cellular stresses
(13). Experiments that introduced
p53 into a p53-deficient leukemia cell line >10 years after its
discovery provided the first evidence that p53 could promote
apoptosis (14). The results
obtained by Basu and Haldar indicated that a loss of p53 function
may substitute for elevated Bcl-2 activity in breast cancer cells
as well as suggesting that p53 may be able to downregulate Bcl-2
(15). As it is often highly
dependent on cell context, a common theme of cell regulation is
crosstalk between various signaling pathways. Numerous stimuli
simultaneously activate the JNK and p38 pathways as several
upstream regulators are shared between the two pathways (16). Increased activation of p38
inhibition by JNK has also been observed in mouse models. For
example, increased activation of the JNK pathway accounts for
p38-deficient myoblasts not exiting the cell cycle or proliferating
in differentiation-promoting conditions (17). JNK activation has been identified in
samples from human gastric cancer. Similarly, in a mouse model of
gastric cancer caused by methyl-nitroso-urea treatment, JNK has
been found to control tumor initiation and promotion by affecting
cell proliferation and the production of reactive oxygen species
(ROS) (18). The induction of
apoptosis by numerous types of cellular stress also involves p38.
These effects can be mediated by transcriptional and
post-transcriptional mechanisms, which affect death receptors,
survival pathways or pro- and antiapoptotic Bcl-2 proteins. The
contribution of these various mechanisms to p38-induced apoptosis
is likely to be regulated in a stimulus- and context-dependent
manner (19). p38 activation is
occasionally triggered by apoptotic stimuli through secondary
routes, including the production of ROS. It is likely that this
mechanism is significant in the suppression of p38-mediated tumor
initiation, which triggers apoptosis in response to the expression
of oncogenes that induce ROS in immortalized cells (20). The results presented in the current
study provide a mechanistic basis for the antiproliferative effects
of SAMC and partially elucidate the chemopreventive action of SAMC
that has been reported in previous studies (21,22).
The present results also provide a mechanistic basis for the
previously observed effects of SAMC on human colorectal carcinoma
cells as the JNK and p38 pathways were revealed to regulate the
apoptosis of cells through the activation of the p53 pathway.
In conclusion, the present study provides evidence
that the garlic derivative SAMC inhibits the growth of cancer cells
in vitro by directly activating the p53 pathway. The current
study also provided evidence that SAMC-induced apoptosis is
related, at least in part, to the activation of the JNK and p38
pathways; however, additional signaling mechanisms have yet to be
elucidated. These results indicate that SAMC or related compounds
may provide a novel approach to cancer chemoprevention and therapy,
and may encourage the development of more potent derivatives of
SAMC.
References
|
1
|
Milner JA: Garlic: its anticarcinogenic
and antitumorigenic properties. Nutr Rev. 54:S82–S86. 1996.
|
|
2
|
Dorey K, Barilá D, Gavin AC, Nebreda AR
and Superti-Furga G: Regulation of human c-Abl tyrosine kinase
activity in Xenopus oocytes and acceleration of
progesterone-induced G2/M transition by oncogenic forms. Biol Chem.
380:223–230. 1999.
|
|
3
|
Rahman K and Lowe GM: Garlic and
cardiovascular disease: a critical review. J Nutr. 136(Suppl 3):
736S–740S. 2006.
|
|
4
|
Sodimu O, Joseph PK and Augusti KT:
Certain biochemical effects of garlic oil on rats maintained on
high fat-high cholesterol diet. Experientia. 40:78–80. 1984.
|
|
5
|
Lee Y: Induction of apoptosis by
S-allylmercapto-L-cysteine, a biotransformed garlic derivative, on
a human gastric cancer cell line. Int J Mol Med. 21:765–770.
2008.
|
|
6
|
Medina-Campos ON, Barrera D, Segoviano
Murillo S, et al: S-allylcysteine scavenges singlet oxygen and
hypochlorous acid and protects LLC-PK(1) cells of potassium
dichromate-induced toxicity. Food Chem Toxicol. 45:2030–2039.
2007.
|
|
7
|
Xiao D, Pinto JT, Soh JW, et al: Induction
of apoptosis by the garlic-derived compound S-allylmercaptocysteine
(SAMC) is associated with microtubule depolymerization and c-Jun
NH(2)-terminal kinase 1 activation. Cancer Res. 63:6825–6837.
2003.
|
|
8
|
Chomczynski P: A reagent for the
single-step simultaneous isolation of RNA, DNA and proteins from
cell and tissue samples. Biotechniques. 15:532–534. 536–537.
1993.
|
|
9
|
Qi R and Wang Z: Pharmacological effects
of garlic extract. Trends Pharmacol Sci. 24:62–63. 2003.
|
|
10
|
Ko LJ and Prives C: p53: puzzle and
paradigm. Genes Dev. 10:1054–1072. 1996.
|
|
11
|
Eliyahu D, Raz A, Gruss P, Givol D and
Oren M: Participation of p53 cellular tumor antigen in
transformation of normal embryonic cells. Nature. 312:646–649.
1984.
|
|
12
|
Jenkins JR, Rudge K and Currie GA:
Cellular immortalization by a cDNA clone encoding the
transformation-associated phosphoprotein p53. Nature. 312:651–654.
1984.
|
|
13
|
Kastan MB, Onyekwere O, Sidransky D,
Vogelstein B and Craig RW: Participation of p53 protein in the
cellular response to DNA damage. Cancer Res. 51:6304–6311.
1991.
|
|
14
|
Hemann MT and Lowe SW: The p53-Bcl-2
connection. Cell Death Differ. 13:1256–1259. 2006.
|
|
15
|
Basu A and Haldar S: The relationship
between Bcl2, Bax and p53: consequences for cell cycle progression
and cell death. Mol Hum Reprod. 4:1099–1109. 1998.
|
|
16
|
Cuevas BD, Abell AN and Johnson GL: Role
of mitogen-activated protein kinase kinase kinases in signal
integration. Oncogene. 26:3159–3171. 2007.
|
|
17
|
Perdiguero E, Ruiz-Bonilla V, Gresh L, et
al: Genetic analysis of p38 MAP kinases in myogenesis: fundamental
role of p38alpha in abrogating myoblast proliferation. EMBO J.
26:1245–1256. 2007.
|
|
18
|
Shibata W, Maeda S, Hikiba Y, et al: c-Jun
NH2-terminal kinase 1 is a critical regulator for the development
of gastric cancer in mice. Cancer Res. 68:5031–5039. 2008.
|
|
19
|
Demidov ON, Kek C, Shreeram S, et al: The
role of the MKK6/p38 MAPK pathway in Wip1-dependent regulation of
ErbB2-driven mammary gland tumorigenesis. Oncogene. 26:2502–2506.
2007.
|
|
20
|
Dolado I, Swat A, Ajenjo N, De Vita G,
Cuadrado A and Nebreda AR: p38alpha MAP kinase as a sensor of
reactive oxygen species in tumorigenesis. Cancer Cell. 11:191–205.
2007.
|
|
21
|
Saunders PA, Cooper JA, Roodell MM, et al:
Quantification of active caspase 3 in apoptotic cells. Anal
Biochem. 284:114–124. 2000.
|
|
22
|
Scharfenberg K, Wagner R and Wagner KG:
The cytotoxic effect of ajoene, a natural product from garlic,
investigated with different cell lines. Cancer Lett. 53:103–108.
1990.
|


















